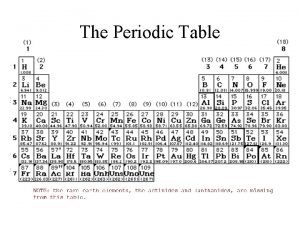
Metals Properties of Metals Nonmetals Reactions of Metals













































- Slides: 74

Metals Properties of Metals & Non-metals Reactions of Metals & Their Compounds Reactivity Series Unreactive Behaviour of Aluminium Rusting & Prevention Alloys Uses of Metals Extraction of Metals Recycling – Advantages & Disadvantages

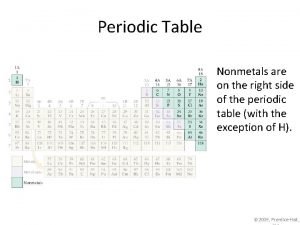
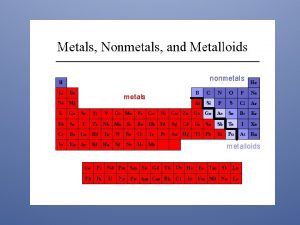
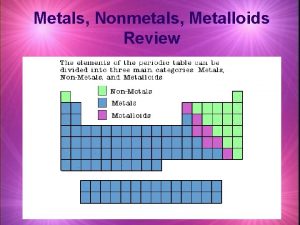
n Elements are classified into metals or nonmetals. n Metals lose electrons to form positive ions. Thus, they behave as reducing agents. n Non-metals gain electrons to form negative ions. Thus, they behave as oxidising agents.

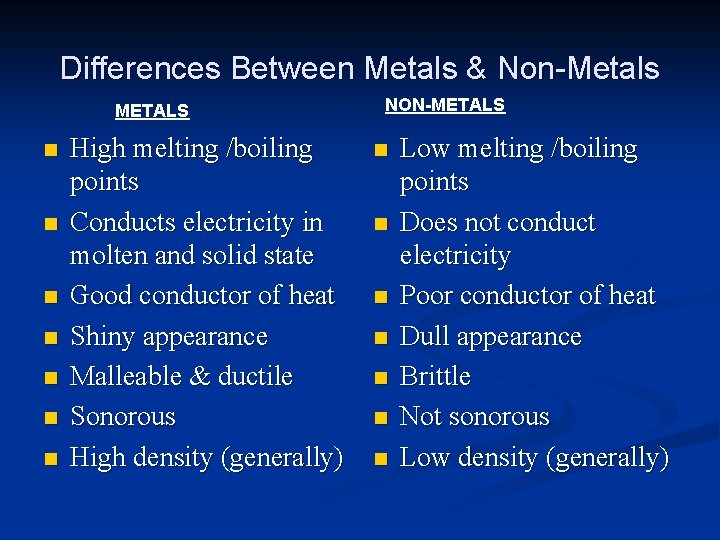
Differences Between Metals & Non-Metals METALS n n n n High melting /boiling points Conducts electricity in molten and solid state Good conductor of heat Shiny appearance Malleable & ductile Sonorous High density (generally) NON-METALS n n n n Low melting /boiling points Does not conduct electricity Poor conductor of heat Dull appearance Brittle Not sonorous Low density (generally)

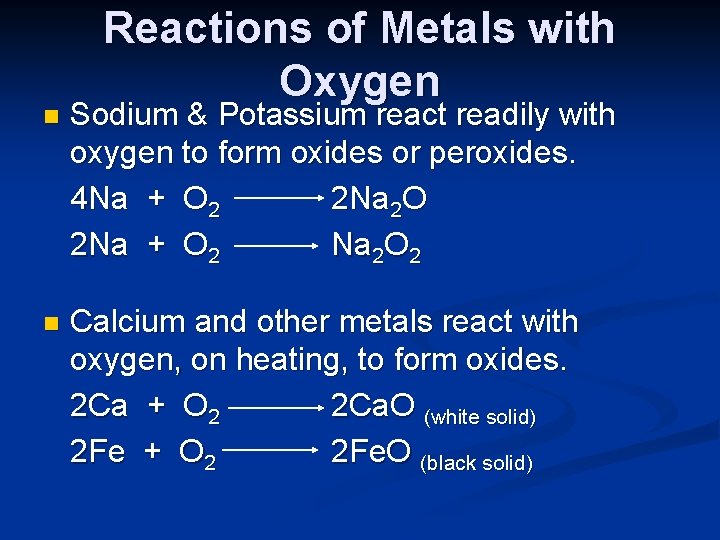
Reactions of Metals with Oxygen n Sodium & Potassium react readily with oxygen to form oxides or peroxides. 4 Na + O 2 2 Na 2 O 2 Na + O 2 Na 2 O 2 n Calcium and other metals react with oxygen, on heating, to form oxides. 2 Ca + O 2 2 Ca. O (white solid) 2 Fe + O 2 2 Fe. O (black solid)

4 Al + 3 O 2 2 Cu + O 2 n 2 Al 2 O 3 (white solid) 2 Cu. O (black solid) Gold is unreactive and does not react with oxygen on heating.

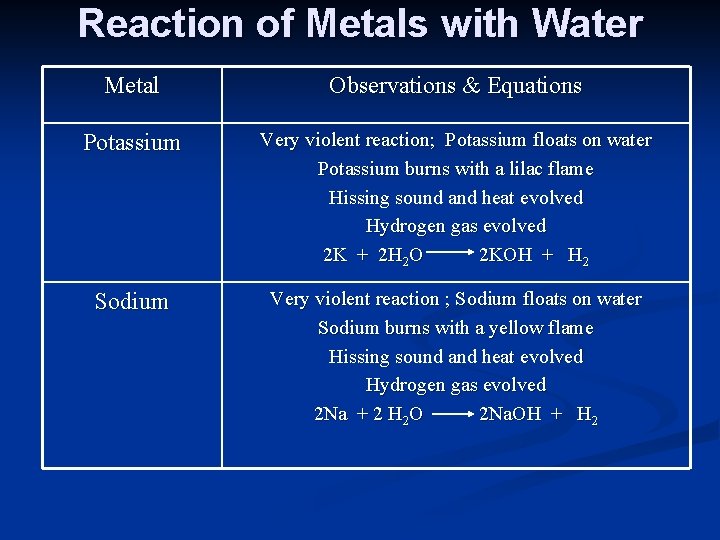
Reaction of Metals with Water Metal Observations & Equations Potassium Very violent reaction; Potassium floats on water Potassium burns with a lilac flame Hissing sound and heat evolved Hydrogen gas evolved 2 K + 2 H 2 O 2 KOH + H 2 Sodium Very violent reaction ; Sodium floats on water Sodium burns with a yellow flame Hissing sound and heat evolved Hydrogen gas evolved 2 Na + 2 H 2 O 2 Na. OH + H 2

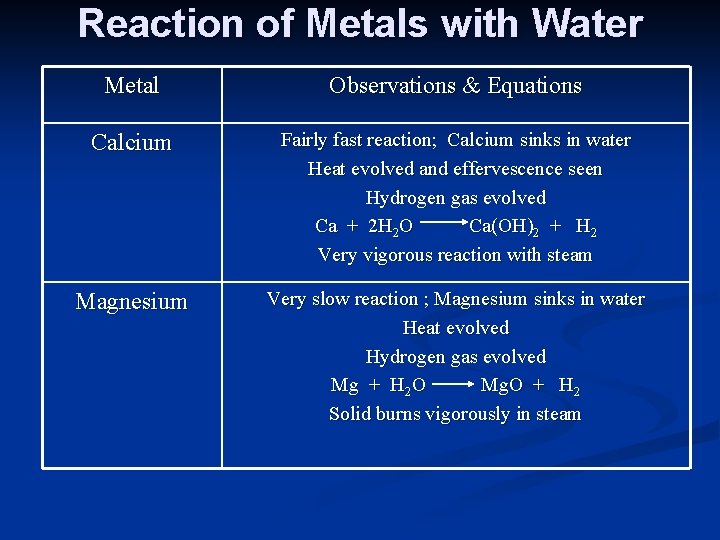
Reaction of Metals with Water Metal Observations & Equations Calcium Fairly fast reaction; Calcium sinks in water Heat evolved and effervescence seen Hydrogen gas evolved Ca + 2 H 2 O Ca(OH)2 + H 2 Very vigorous reaction with steam Magnesium Very slow reaction ; Magnesium sinks in water Heat evolved Hydrogen gas evolved Mg + H 2 O Mg. O + H 2 Solid burns vigorously in steam

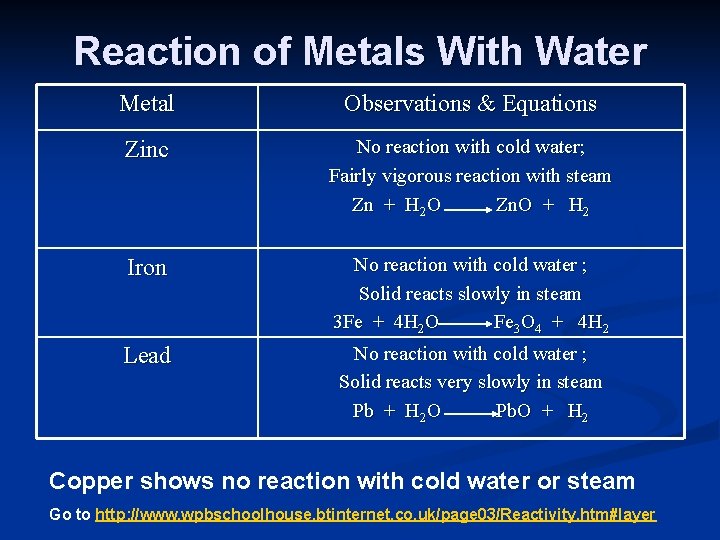
Reaction of Metals With Water Metal Observations & Equations Zinc No reaction with cold water; Fairly vigorous reaction with steam Zn + H 2 O Zn. O + H 2 Iron No reaction with cold water ; Solid reacts slowly in steam 3 Fe + 4 H 2 O Fe 3 O 4 + 4 H 2 Lead No reaction with cold water ; Solid reacts very slowly in steam Pb + H 2 O Pb. O + H 2 Copper shows no reaction with cold water or steam Go to http: //www. wpbschoolhouse. btinternet. co. uk/page 03/Reactivity. htm#layer

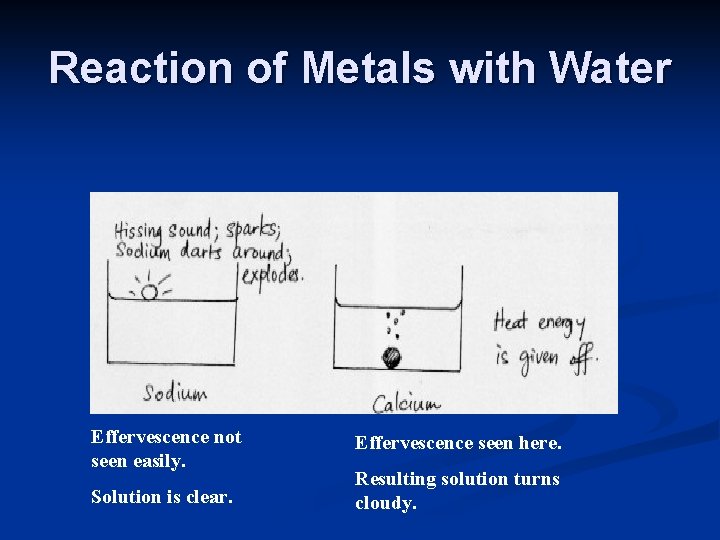
Reaction of Metals with Water Effervescence not seen easily. Solution is clear. Effervescence seen here. Resulting solution turns cloudy.

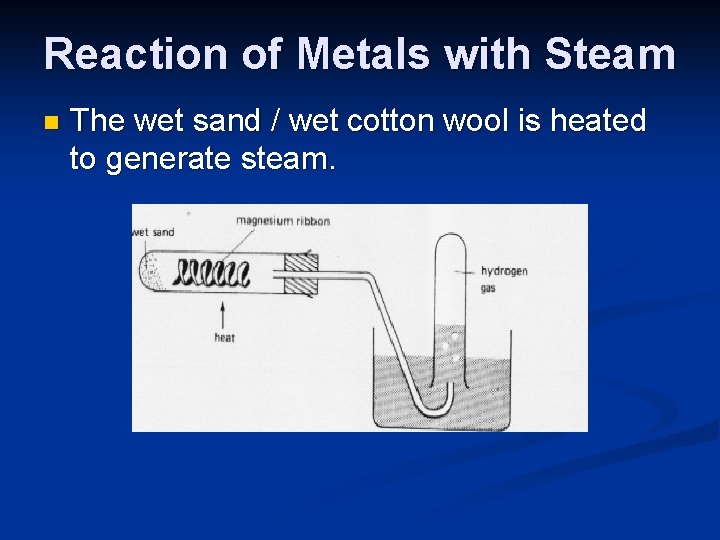
Reaction of Metals with Steam n The wet sand / wet cotton wool is heated to generate steam.

n Potassium floats on water. Sparks formed. Catches fire. Hissing sound. Heat evolved. What would you expect to see if universal indicator were added to the solution? Ans : Green Universal Indicator turns purple. The resulting solution is alkaline.

Calcium sinks in water. Effervescence seen. Heat evolved. White insoluble solid formed as product. The resulting solution turns chalky. What is the white solid? n Ans : Calcium hydroxide

Answers to Questions 1. Al, Zn, Fe, Sn, Pb 2. Sodium

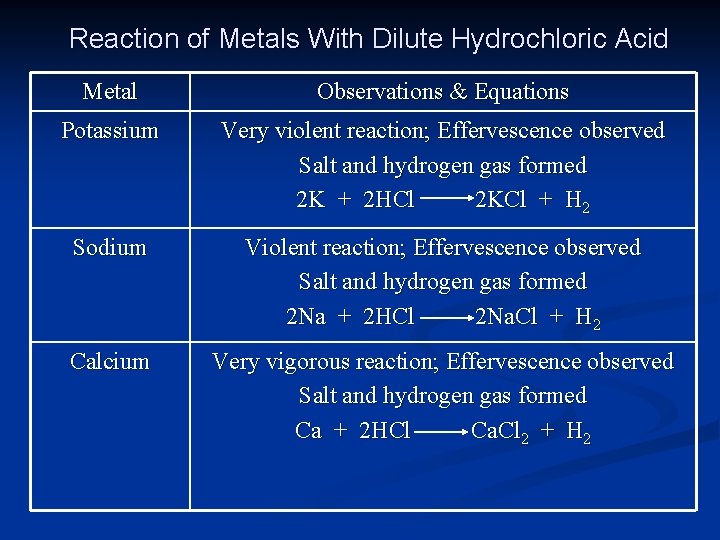
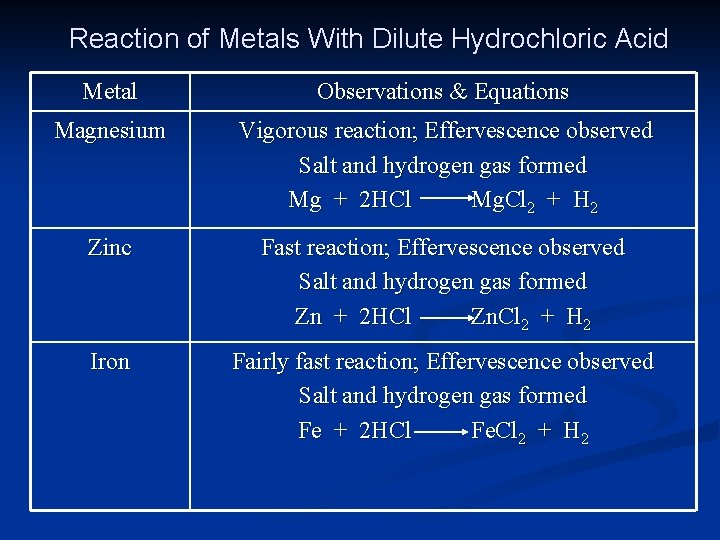
Reaction of Metals With Dilute Hydrochloric Acid Metal Observations & Equations Potassium Very violent reaction; Effervescence observed Salt and hydrogen gas formed 2 K + 2 HCl 2 KCl + H 2 Sodium Violent reaction; Effervescence observed Salt and hydrogen gas formed 2 Na + 2 HCl 2 Na. Cl + H 2 Calcium Very vigorous reaction; Effervescence observed Salt and hydrogen gas formed Ca + 2 HCl Ca. Cl 2 + H 2

Reaction of Metals With Dilute Hydrochloric Acid Metal Observations & Equations Magnesium Vigorous reaction; Effervescence observed Salt and hydrogen gas formed Mg + 2 HCl Mg. Cl 2 + H 2 Zinc Fast reaction; Effervescence observed Salt and hydrogen gas formed Zn + 2 HCl Zn. Cl 2 + H 2 Iron Fairly fast reaction; Effervescence observed Salt and hydrogen gas formed Fe + 2 HCl Fe. Cl 2 + H 2

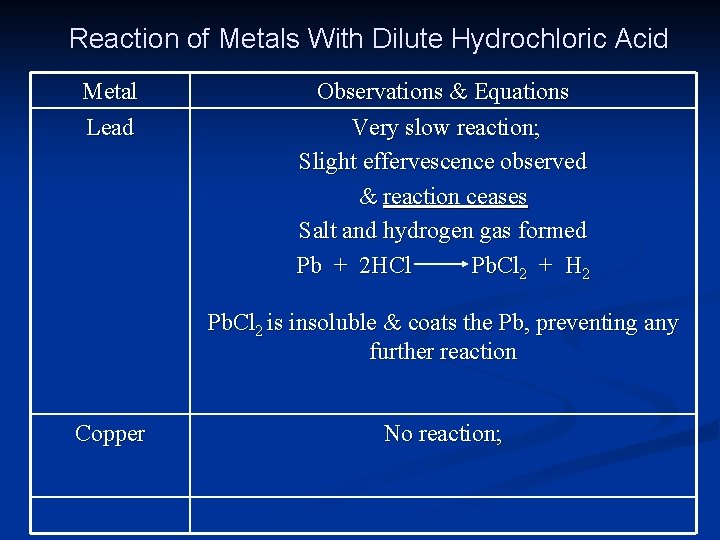
Reaction of Metals With Dilute Hydrochloric Acid Metal Lead Observations & Equations Very slow reaction; Slight effervescence observed & reaction ceases Salt and hydrogen gas formed Pb + 2 HCl Pb. Cl 2 + H 2 Pb. Cl 2 is insoluble & coats the Pb, preventing any further reaction Copper No reaction;

Answers to Questions 1. 2. 3. Zinc (Copper shows no reaction with dilute acids) Iron (Transition element – Copper not acceptable as it does not react with dilute acids) The insoluble lead (II) chloride formed coats the lead, preventing any further reaction.

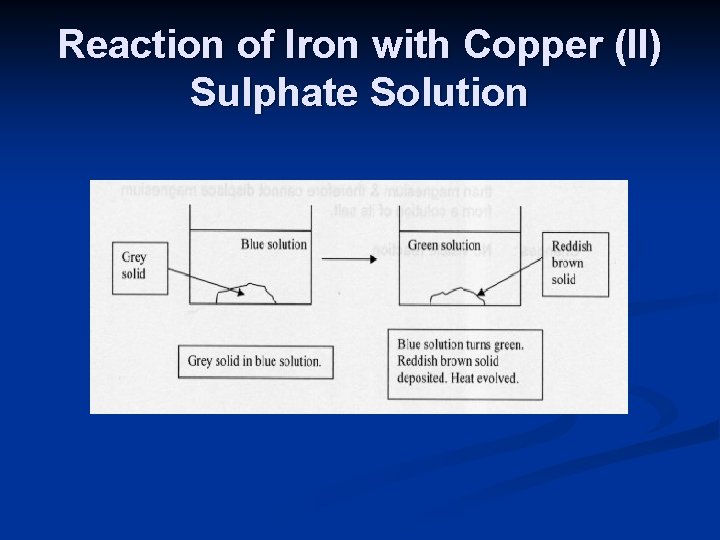
Metal Displacement Reactions n A more reactive metal displaces a less reactive metal from a solution of its salt. eg. 1 : When iron is added to copper (II) sulphate solution , iron displaces copper from its solution. This occurs as iron is more reactive than the metal present in solution i. e. copper (II) ions.

Reaction of Iron with Copper (II) Sulphate Solution

n Changes : n Blue solution turns pale green due to presence of iron (II) ions. n A reddish brown solid / deposit is formed due to metallic copper formed. n The mixture turns warm as heat is given off during the exothermic reaction n Fe + Cu. SO 4 Fe. SO 4 + Cu

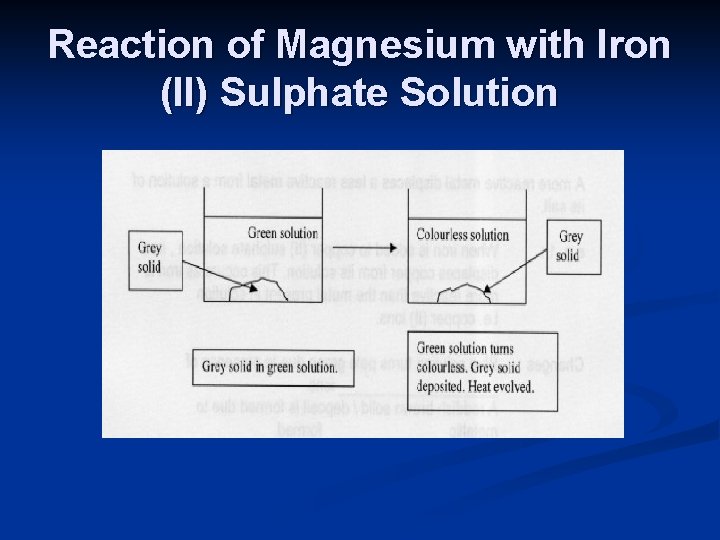
n e. g. 2 : When magnesium is added to iron (II) sulphate solution , magnesium displaces iron from its solution. This occurs as magnesium is more reactive than the metal present in solution i. e. iron (II) ions.

Reaction of Magnesium with Iron (II) Sulphate Solution

Changes : n Pale green solution turns colourless due to formation of magnesium ions. n A grey solid / deposit is formed due to metallic iron formed. n The mixture turns warm as heat is given off during the exothermic reaction. n Mg + Fe. SO 4 Mg. SO 4 + Fe

n e. g. 3 : When copper metal is added to magnesium (II) sulphate solution , no reaction occurs as copper does not displace magnesium from a solution of its ions. This is because copper is less reactive than magnesium & therefore cannot displace magnesium from a solution of its salt. Changes : No visible reaction.

Copper added to Aqueous Silver Nitrate

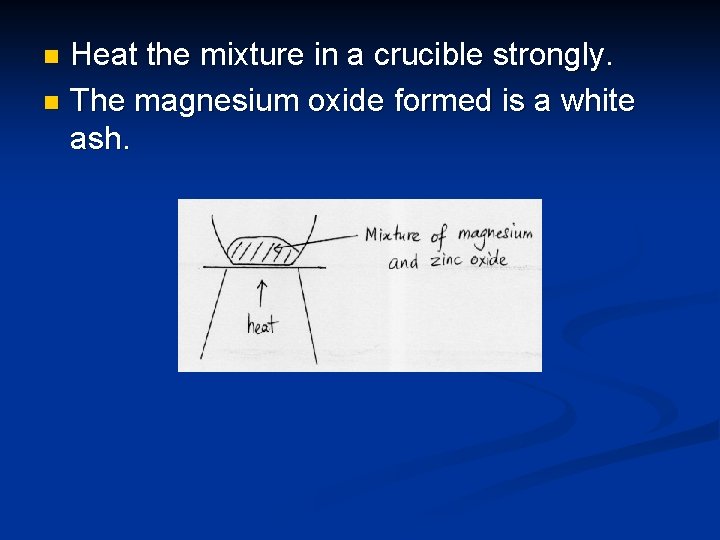
Displacement of metals from metal oxides This type of reaction can also be classified as a redox reaction. n Here, a more reactive metal can displace a less reactive metal from its oxide. n Both the metal & metal oxide are heated strongly in a crucible. e. g. Mg + Zn. O Mg. O + Zn e. g. Cu + Zn. O no reaction n

Heat the mixture in a crucible strongly. n The magnesium oxide formed is a white ash. n

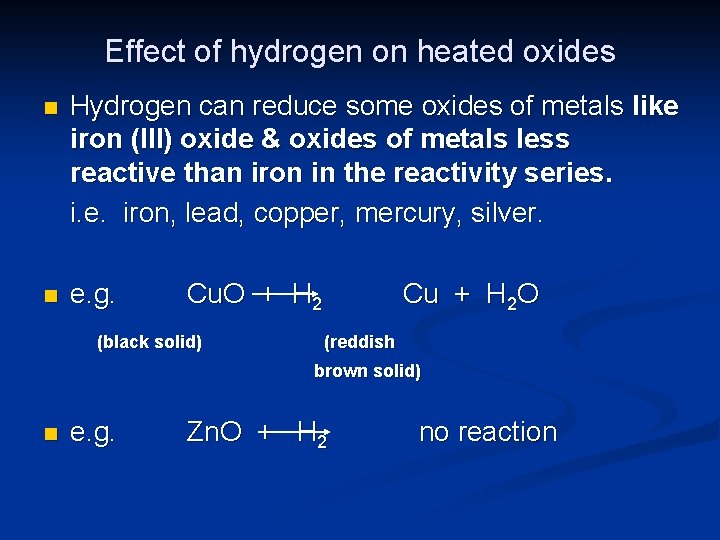
Effect of hydrogen on heated oxides n Hydrogen can reduce some oxides of metals like iron (III) oxide & oxides of metals less reactive than iron in the reactivity series. i. e. iron, lead, copper, mercury, silver. n e. g. Cu. O + H 2 (black solid) Cu + H 2 O (reddish brown solid) n e. g. Zn. O + H 2 no reaction

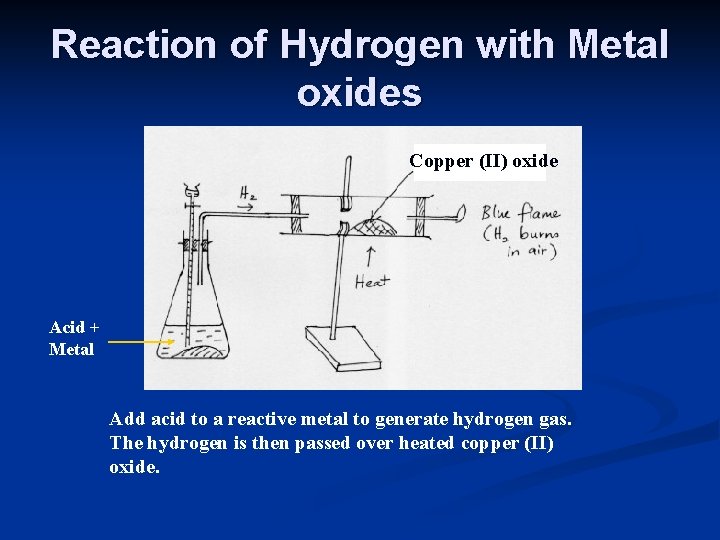
Reaction of Hydrogen with Metal oxides Copper (II) oxide Acid + Metal Add acid to a reactive metal to generate hydrogen gas. The hydrogen is then passed over heated copper (II) oxide.

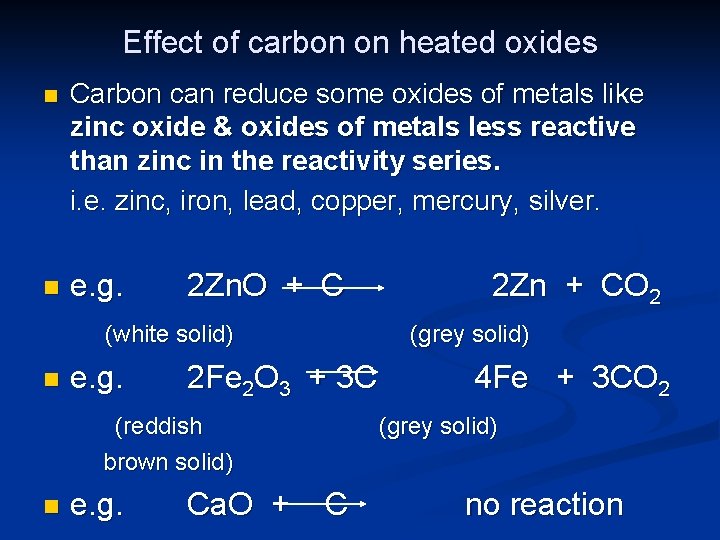
Effect of carbon on heated oxides n Carbon can reduce some oxides of metals like zinc oxide & oxides of metals less reactive than zinc in the reactivity series. i. e. zinc, iron, lead, copper, mercury, silver. n e. g. 2 Zn. O + C (white solid) n e. g. (grey solid) 2 Fe 2 O 3 + 3 C (reddish brown solid) n e. g. Ca. O + 2 Zn + CO 2 4 Fe + 3 CO 2 (grey solid) C no reaction

Answers to Questions 1. a) b) B, C, A Zinc

Reactions of metal carbonates n n n Potassium and Sodium carbonate (& ammonium carbonate) are soluble in water. All other metal carbonates (excluding group I metal carbonates) are insoluble in water. Potassium carbonate and sodium carbonate are stable compounds and therefore, cannot be decomposed by heating. This is because potassium and sodium are very reactive metals & form very stable compounds. heat e. g. Na 2 CO 3 no reaction

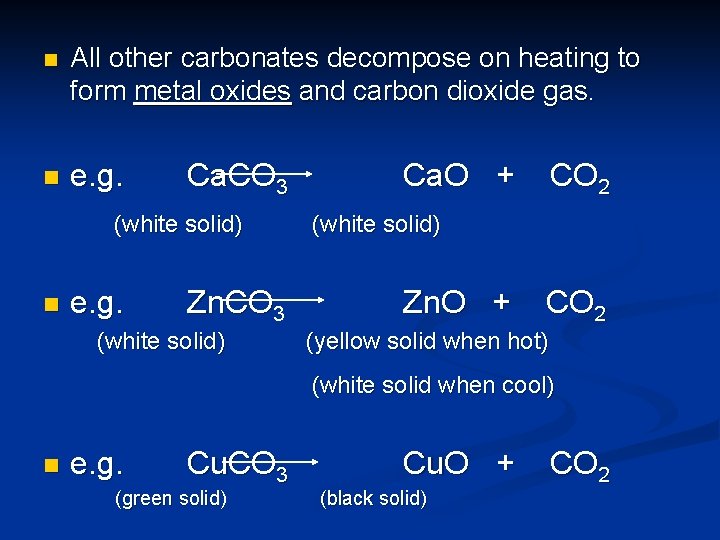
n All other carbonates decompose on heating to form metal oxides and carbon dioxide gas. n e. g. Ca. CO 3 (white solid) n e. g. Zn. CO 3 (white solid) Ca. O + CO 2 (white solid) Zn. O + CO 2 (yellow solid when hot) (white solid when cool) n e. g. Cu. CO 3 (green solid) Cu. O + (black solid) CO 2

Answers to Questions 1. a) b) W, Y, X Sodium / Potassium 2. a) P is Carbon dioxide Bubble the gas through limewater. If the gas is carbon dioxide, a white ppt is formed in limewater. White solid turns yellow when hot. On cooling, yellow solid turns white. b)

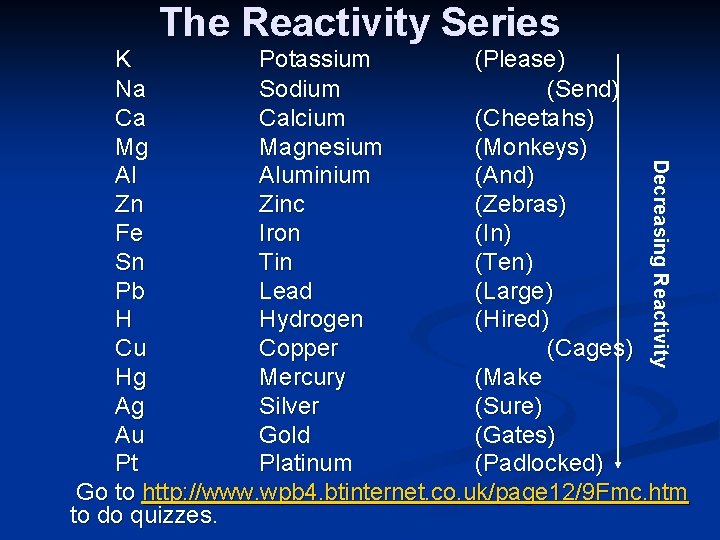
The Reactivity Series Decreasing Reactivity K Potassium (Please) Na Sodium (Send) Ca Calcium (Cheetahs) Mg Magnesium (Monkeys) Al Aluminium (And) Zn Zinc (Zebras) Fe Iron (In) Sn Tin (Ten) Pb Lead (Large) H Hydrogen (Hired) Cu Copper (Cages) Hg Mercury (Make Ag Silver (Sure) Au Gold (Gates) Pt Platinum (Padlocked) Go to http: //www. wpb 4. btinternet. co. uk/page 12/9 Fmc. htm to do quizzes.

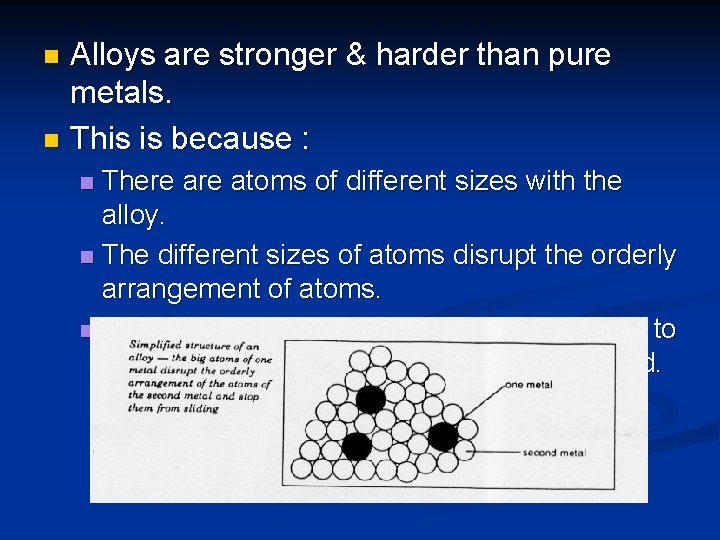
Alloys are mixtures of two or more metals. Sometimes, non-metals are present in the mixture. n Examples of alloys: n Stainless steel ( Fe, C, Cr & Ni) n Bronze ( Cu & Sn) n Brass ( Cu & Zn) n

Alloys are stronger & harder than pure metals. n This is because : n There atoms of different sizes with the alloy. n The different sizes of atoms disrupt the orderly arrangement of atoms. n This makes it difficult for the layers of atoms to slide over each other when a force is applied. n

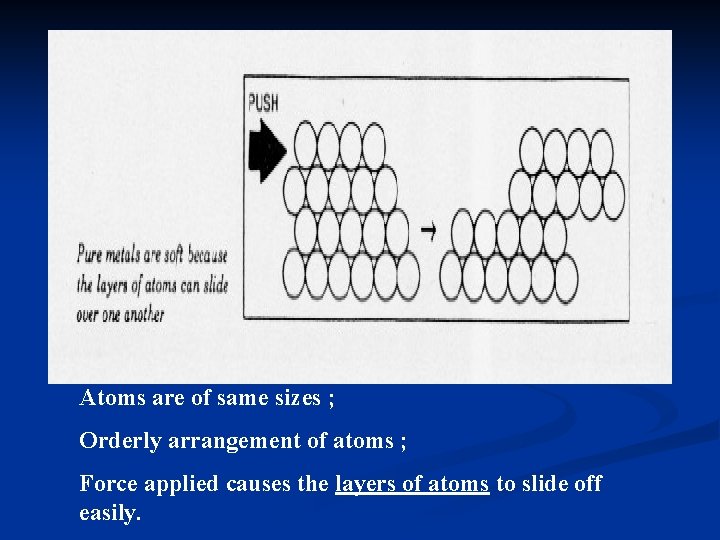
Atoms are of same sizes ; Orderly arrangement of atoms ; Force applied causes the layers of atoms to slide off easily.

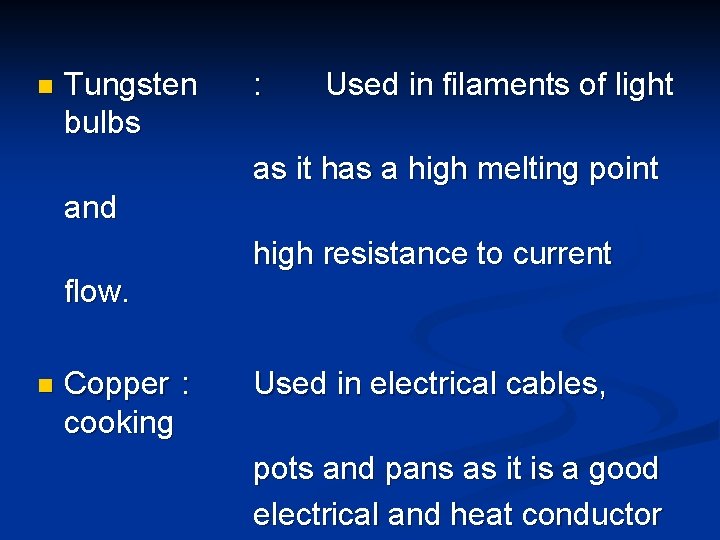
Uses of Metals & Alloys n Tin : metal Used in soldering wires or pieces of together due to its low melting point. n Zinc : where metals Used in galvanising of steel or iron, zinc corrodes in place of less reactive like iron. n Nickel : Used in making of engines of aircraft as it has a high melting and boiling point. It

n Tungsten bulbs : Used in filaments of light as it has a high melting point and high resistance to current flow. n Copper : cooking Used in electrical cables, pots and pans as it is a good electrical and heat conductor

n Aluminium is used in: Fuel tanks of vehicles/ aircraft due to its low density; n Cooking pots & pans due to its low density, good heat conductivity & its resistance to corrosion. n Soft drink cans due to its low density & corrosion resistance; n Parts of motor vehicles, bodies of buses due to its low density; n Reflectors in car headlights, big lamps at stadium as it is a good reflector of light. n Duralumin is an alloy of aluminium. It is strong, light, hard & used in aircraft construction. It is n

n n n Alloys of Copper : Brass : Made of copper & zinc; Used to make pins of electric plugs, ornaments & door handles as it is corrosion resistance. Bronze : Made of copper & tin; Stronger than brass & more corrosion resistant. Used to make propellors of ships as it is hard & corrosion resistant ; cooling

n Alloys of iron : n Mild Steel silicon, : 99% iron, 1% manganese & 0. 2% carbon. Used in buses, cars, ships and steel rods in the construction industry as it is strong & hard. n Stainless Steel : Made up of iron, carbon, chromium & nickel.

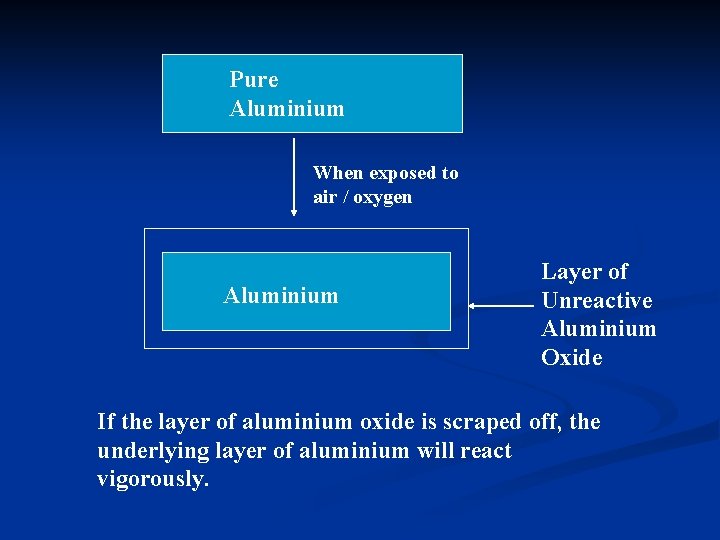
Unreactive Behaviour of Aluminium : Aluminium is a fairly reactive metal in the reactivity series. However, it appears unreactive. n This is due to the formation of a protective layer of aluminium oxide when pure aluminium is exposed to oxygen in air. 4 Al + 3 O 2 2 Al 2 O 3 n n The aluminium oxide formed is very unreactive and does not allow the aluminium atoms beneath to react with

Pure Aluminium When exposed to air / oxygen Aluminium Layer of Unreactive Aluminium Oxide If the layer of aluminium oxide is scraped off, the underlying layer of aluminium will react vigorously.

Rusting & Rust Prevention n Iron and steel can corrode when exposed to oxygen in air and moisture. The product of corrosion is a reddish-brown solid called rust. Hence, this process is called rusting. n All other metals that can corrode DO NOT form rust & therefore, cannot undergo rusting. n Rust is actually hydrated iron (III) oxide.

n Conditions for rusting to occur : a) oxygen b) moisture / water n Rusting occurs faster if salts are dissolved in the water into which iron is placed i. e. salt water increases the rate of rusting.

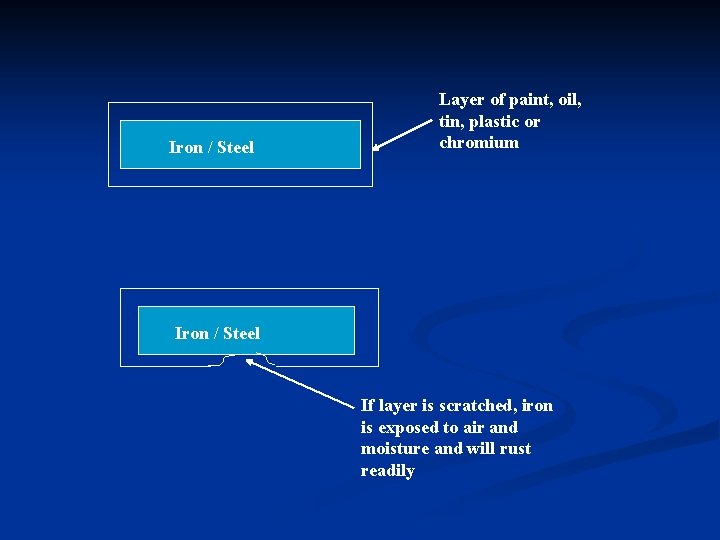
n Rust Prevention : Methods of rust prevention are : Coating with paint n Coating with oil / grease n Coating with plastic n Tin plating n Chromium plating n Galvanising iron / steel (Coating iron / steel with a layer of zinc) n

n Paint, oil / grease, plastic, tin and chromium are substances that are unreactive or less reactive than iron / steel. When these substances are coated onto iron / steel, they form a protective layer. n As long as the layer is not scratched off, the iron / steel is protected. If the layer is scratched, the iron / steel is exposed to oxygen and moisture, and will start to rust.

Iron / Steel Layer of paint, oil, tin, plastic or chromium Iron / Steel If layer is scratched, iron is exposed to air and moisture and will rust readily

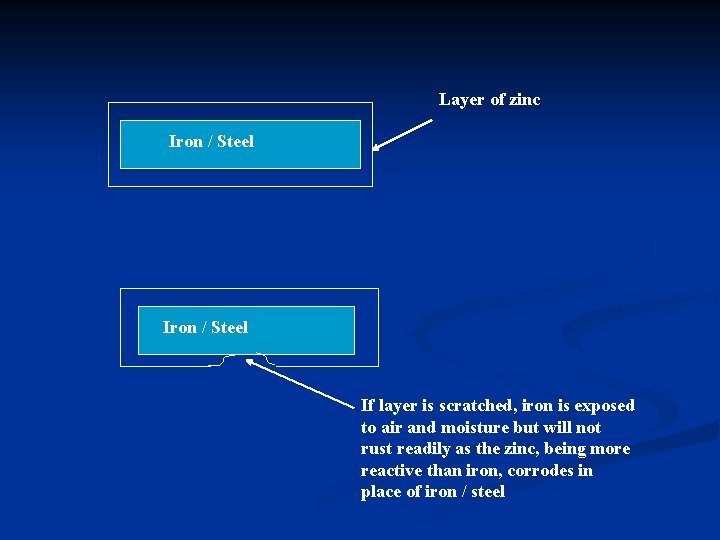
n The process of coating iron / steel with a layer of zinc is called galvanising. n When iron / steel is coated with a layer of zinc, the iron is protected against rusting. The zinc, being reactive than iron, corrodes more readily than iron. Thus, it corrodes in place of iron / steel (corrodes preferentially). This process is called ‘sacrificial protection’.

Layer of zinc Iron / Steel If layer is scratched, iron is exposed to air and moisture but will not rust readily as the zinc, being more reactive than iron, corrodes in place of iron / steel

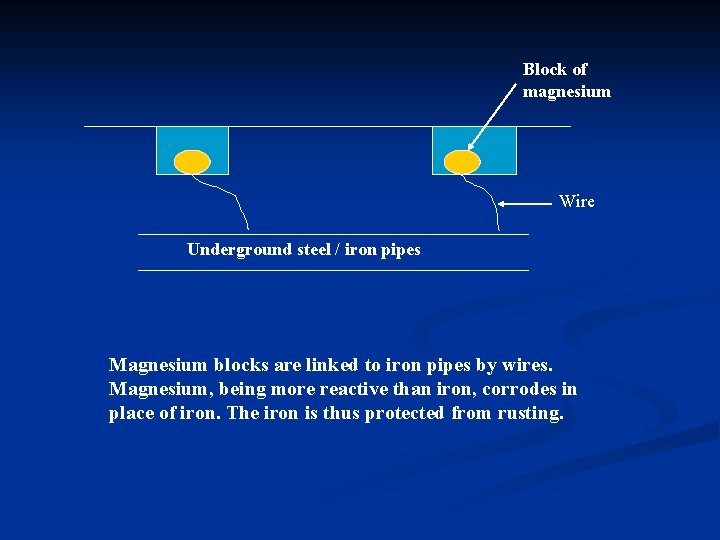
n Applications of Sacrificial Protection : i) Underground / underwater steel pipes tend to rust easily. To prevent rusting, pieces or blocks of magnesium are attached or connected to the pipes by wire. In this way, the magnesium, being more reactive than steel, corrodes in place of steel. The steel is protected until the magnesium corrodes completely. The

Block of magnesium Wire Underground steel / iron pipes Magnesium blocks are linked to iron pipes by wires. Magnesium, being more reactive than iron, corrodes in place of iron. The iron is thus protected from rusting.

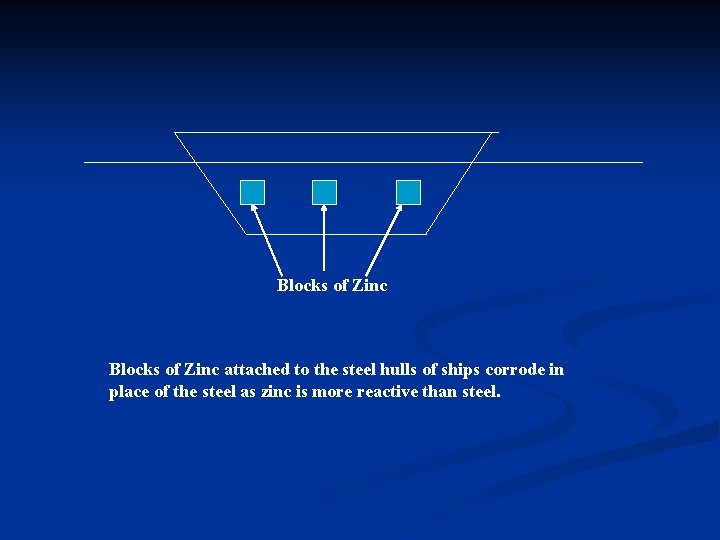
ii) The hulls of ships are made of steel and can corrode easily as the hulls are constantly exposed to water and oxygen (and salt). n To prevent this from happening, blocks of zinc are attached on the sides of the hull. n The zinc, being more reactive than steel, corrodes in place of steel. These zinc blocks have to be replaced regularly.

Blocks of Zinc attached to the steel hulls of ships corrode in place of the steel as zinc is more reactive than steel.

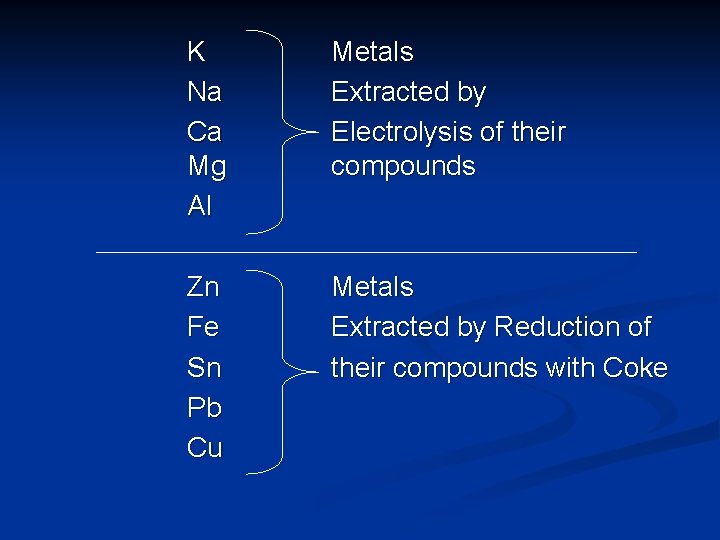
Extraction of Metals n Metals can be extracted from their ores by : a) Reduction with coke/carbon. b) Electrolysis. n The more reactive metals will form very stable compounds which cannot be easily extracted by reduction using coke.

K Na Ca Mg Al Metals Extracted by Electrolysis of their compounds Zn Fe Sn Pb Cu Metals Extracted by Reduction of their compounds with Coke

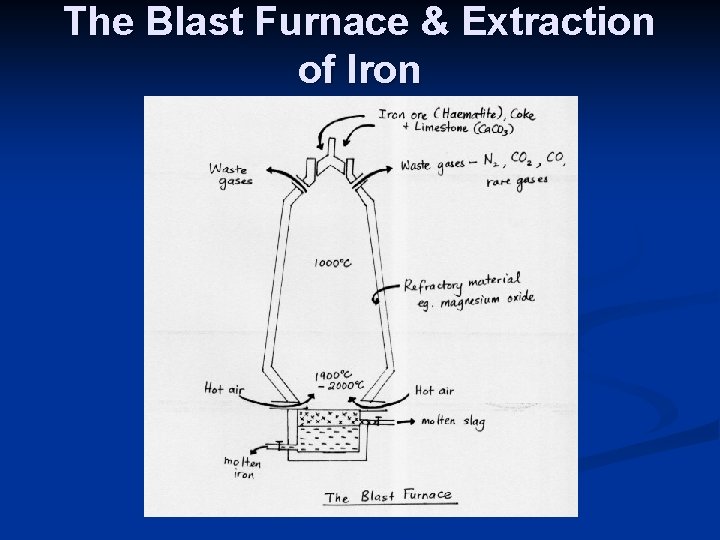
Extraction of Iron n n Coke, limestone & roasted Haematite (Iron Ore) are added at the top of the furnace. Roasted iron ore is essentially iron (III) oxide, Fe 2 O 3 and is the source of iron. The coke acts as a reducing agent and forms carbon monoxide, which is a more powerful reducing agent. The reducing agent converts iron (III) oxide to iron. The limestone, Ca. CO 3 removes impurities from the iron formed. The main impurity is sand (impure silicon dioxide).

n Hot air is then blown in through the mixture from the bottom of the furnace. The hot air reacts with the coke to form carbon dioxide. This is an exothermic reaction, where heat is given off to the surroundings. C + O 2 ------- CO 2 n The carbon dioxide formed is then reduced by coke to form carbon monoxide gas. CO 2 + C ------- 2 CO

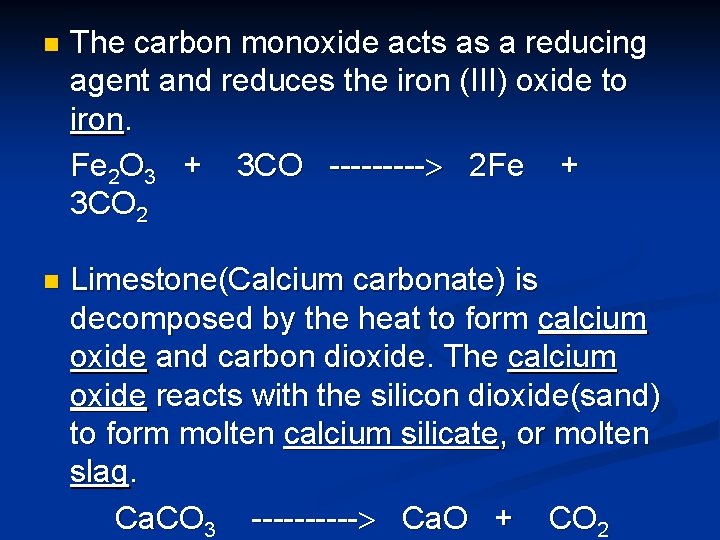
n The carbon monoxide acts as a reducing agent and reduces the iron (III) oxide to iron. Fe 2 O 3 + 3 CO ----- 2 Fe + 3 CO 2 n Limestone(Calcium carbonate) is decomposed by the heat to form calcium oxide and carbon dioxide. The calcium oxide reacts with the silicon dioxide(sand) to form molten calcium silicate, or molten slag. Ca. CO 3 ----- Ca. O + CO 2

The molten slag floats on top of the iron. It can be run off through pipes at the bottom of the furnace. n The waste gases that leave the top of the furnace are nitrogen gas, carbon dioxide, carbon monoxide, rare gases & oxides of nitrogen. n The molten iron that collects at the bottom of the furnace is run off into moulds and cooled to form long bars. This iron is called ‘pig iron’ or ‘cast iron’. It contains impurities. A large amount of impurities n

The Blast Furnace & Extraction of Iron

Conversion of iron to steel Pig or cast iron still has non-metallic impurities and sand present. n Oxygen is blown into molten cast iron and carbon impurities burn in oxygen to form gaseous carbon dioxide. n Limestone is also added in to remove sand. Slag is formed in this process. n After purification, carbon is added in to the molten iron in required quantities to form steel i. e. low carbon steel or high carbon steel. n

Differences between low carbon & high carbon steel Low carbon steel High carbon steel More malleable & Less malleable & ductile; Is brittle Soft; Less strong Stronger & hard

Recycling of Metals n 1. Advantages of Recycling Metals : n Social : Recycling creates jobs. n About 103, 000 people were employed in recycling processing & manufaturing jobs in USA in 1991. n

n n Economic : Recycling conserves energy. Much less energy is needed to make recycled materials into new products, compared to beginning the process again with new raw materials. Recovered materials have already been refined and processed once, so manufacturing the second time around is usually much cleaner and less energyintensive than the first. By recycling a ton of materials, at least $187 worth of electricity, petroleum, natural gas and coal are conserved, even after accounting for the energy used to collect and transport the materials.

n n n Recycling reduces the cost of disposing trash in landfills or solid waste incinerators. Revenues are earned by the sales of recycled materials. In addition, less has to be spent on disposal of waste. Recycling reduces the cost of manufacturing for manufacturers Recycling provides manufacturing industries with less expensive sources of raw materials. Therefore, the long-term advantage for consumers is that the cost of products and packaging would be less.

n Recycling allows the extraction of valuable metals thereby conserving raw materials n Recycling is a channel whereby valuable metals are extracted from waste / disposed materials, i. e. gold from electrical contacts in old computers , platinum from catalytic converters from motorcars.

n n n Environmental : Recycling reduces pollution Recovered materials have been refined and processed once, and therefore will generate less pollution. Recycling conserves natural resources. This is the main reason for recycling materials. Recycling of materials will reduce the rate at which our natural resources are being used up.

Recycling reduces the number of dumping grounds n By recycling metals, the number of piles of rusty metal being dumped on wasteland is reduced. n Recycling reduces the chances of leaching of metals into soil and river water n Recycling prevents abandoned metal objects from being corroded by air and rain. The metals can leach into the soil and river water, thus causing contamination of water. n

n n Disadvantages of recycling : Environmental : Recycling can pollute the environment Recycling can damage the environment. During smelting, metal fumes can be released into the air and this can pollute the surroundings. Less valuable metals are thrown away and thus cause pollution in the soil and waterways.

Answers to Questions 1. To conserve natural resources. 2. Advantage : n Provides jobs. n Reduces energy consumption in processing of recovered materials. n Reduces cost of disposal of trash n Reduces cost of manufacturing n Enables extraction of valuable metals i. e. conservation of natural resources / raw materials

Reduces pollution as processed materials generate less pollution during manufacturing. n Reduces no. of dumping grounds & leaching of metals into soil. n Disadvantages : n Causes pollution as metal fumes are released into the atmosphere during smelting. n NOTE : Elaborate on your answers for clear understanding of examiners.
 In reactions to form ionic compounds metals generally
In reactions to form ionic compounds metals generally Is solid at room temperature
Is solid at room temperature Properties of metals nonmetals and semimetals
Properties of metals nonmetals and semimetals Metals vs nonmetals vs metalloids
Metals vs nonmetals vs metalloids Optical properties of metals and nonmetals
Optical properties of metals and nonmetals Compare the properties of metals and nonmetals
Compare the properties of metals and nonmetals Metals vs metalloids and nonmetals
Metals vs metalloids and nonmetals Metals vs nonmetals
Metals vs nonmetals Metals nonmetals and metalloids difference
Metals nonmetals and metalloids difference Non metals in the periodic table
Non metals in the periodic table Periodic table divided in metals nonmetals and metalloids
Periodic table divided in metals nonmetals and metalloids Metals nonmetals and metalloids
Metals nonmetals and metalloids Periodic table metals nonmetals metalloids noble gases
Periodic table metals nonmetals metalloids noble gases Periodic table of elements regents
Periodic table of elements regents Periodic table metals nonmetals and metalloids color
Periodic table metals nonmetals and metalloids color Labeled periodic table
Labeled periodic table Chapter 4 metals and nonmetals
Chapter 4 metals and nonmetals Semi metals periodic table
Semi metals periodic table What is a nonmetal element
What is a nonmetal element Metal examples
Metal examples Metals nonmetals semimetals
Metals nonmetals semimetals Poem about metals nonmetals and metalloids
Poem about metals nonmetals and metalloids Metals vs nonmetals vs metalloids
Metals vs nonmetals vs metalloids Least reactive non-metal
Least reactive non-metal When a metal reacts with a nonmetal the metal will
When a metal reacts with a nonmetal the metal will Used of metals
Used of metals Compare metals nonmetals and metalloids
Compare metals nonmetals and metalloids Elements and their properties section 2 nonmetals
Elements and their properties section 2 nonmetals Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Redox reactions examples
Redox reactions examples Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Alkaline earth metal
Alkaline earth metal Ferrous material
Ferrous material Grade 7 ns term 2
Grade 7 ns term 2 Metals and non metals grade 5
Metals and non metals grade 5 Mechanical properties of metals ppt
Mechanical properties of metals ppt Elements and their properties section 1 metals
Elements and their properties section 1 metals Alkali metal melting point
Alkali metal melting point The two rows of elements that seem to be disconnected
The two rows of elements that seem to be disconnected Periodic table element families
Periodic table element families Section 4 metallic bonds and the properties of metals
Section 4 metallic bonds and the properties of metals Reactivity series
Reactivity series The physical properties of metals include luster and
The physical properties of metals include luster and Section 4 metallic bonds and the properties of metals
Section 4 metallic bonds and the properties of metals Metallic bonding
Metallic bonding Alkaline earth metals group
Alkaline earth metals group Extensive vs intensive properties
Extensive vs intensive properties Chemical properties of citric acid
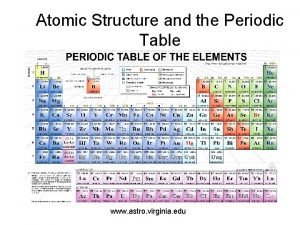
Chemical properties of citric acid Atomic structure of periodic table
Atomic structure of periodic table Covalent bonds are formed between nonmetals true or false
Covalent bonds are formed between nonmetals true or false Where are metals found on the periodic table
Where are metals found on the periodic table Compounds formed between metal and nonmetals
Compounds formed between metal and nonmetals What do 2 nonmetals make
What do 2 nonmetals make Solid nonmetals are
Solid nonmetals are Why do nonmetals rarely lose electrons
Why do nonmetals rarely lose electrons Compound of two nonmetals
Compound of two nonmetals How do covalent bonds form
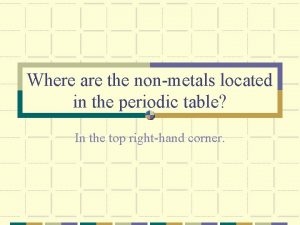
How do covalent bonds form Where are the nonmetals located on the periodic table?
Where are the nonmetals located on the periodic table? Biochemistry
Biochemistry Microplate method for blood grouping
Microplate method for blood grouping Chemsheets as 1017
Chemsheets as 1017 Addition of hydrogen halides to alkynes
Addition of hydrogen halides to alkynes How to identify types of chemical reactions
How to identify types of chemical reactions Homogenous solution
Homogenous solution Cellular respiration equation
Cellular respiration equation What are the two sets of reactions in photosynthesis
What are the two sets of reactions in photosynthesis Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes A balanced chemical reaction obeys the law of
A balanced chemical reaction obeys the law of Examples of double replacement reactions
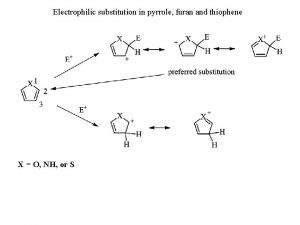
Examples of double replacement reactions Pyrrole nucleophilic substitution
Pyrrole nucleophilic substitution Redox reactions ncea level 2
Redox reactions ncea level 2 Chapter 9 chemical reactions
Chapter 9 chemical reactions Predicting precipitation reactions
Predicting precipitation reactions