Section 4 Metallic Bonds and the Properties of

Section 4: Metallic Bonds and the Properties of Metals form crystal lattices and can be modeled as cations surrounded by a “sea” of freely moving valence electrons. K What I Know W What I Want to Find Out L What I Learned

• 7(D) Describe the nature of metallic bonding and apply theory to explain metallic properties such as thermal and electrical conductivity, malleability, and ductility. • 2(I) Communicate valid conclusions supported by the data through methods such as lab reports, labeled drawings, graphs, journals, summaries, oral reports, and technology–based reports. • 3(A) In all fields of science, analyze, evaluate, and critique scientific explanations by using empirical evidence, logical reasoning, and experimental and observational testing, including examining all sides of scientific evidence of those scientific explanations, so as to encourage critical thinking by the student. Copyright © Mc. Graw-Hill Education Metallic Bonds and the Properties of Metals

Essential Questions • What are the characteristics of a metallic bond? • How does the electron sea model account for the physical properties of metals? • What are alloys, and how can they be categorized? Copyright © Mc. Graw-Hill Education Metallic Bonds and the Properties of Metals
Vocabulary Review New • physical property • • Copyright © Mc. Graw-Hill Education electron sea model delocalized electron metallic bond alloy Metallic Bonds and the Properties of Metals
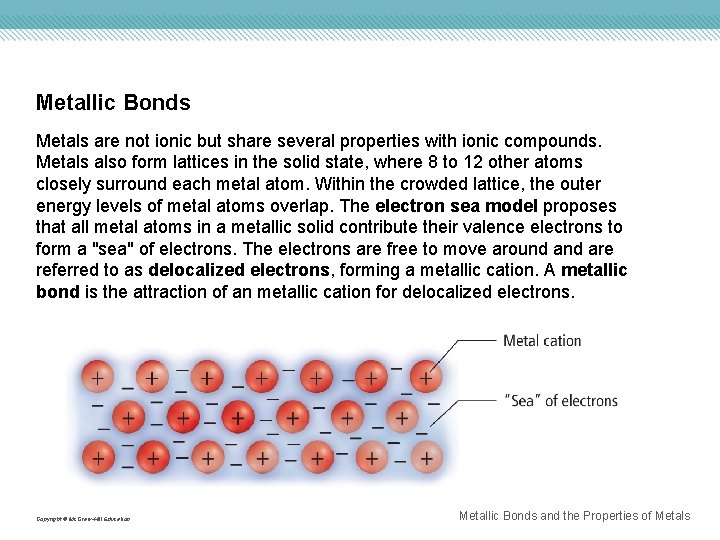
Metallic Bonds Metals are not ionic but share several properties with ionic compounds. Metals also form lattices in the solid state, where 8 to 12 other atoms closely surround each metal atom. Within the crowded lattice, the outer energy levels of metal atoms overlap. The electron sea model proposes that all metal atoms in a metallic solid contribute their valence electrons to form a "sea" of electrons. The electrons are free to move around are referred to as delocalized electrons, forming a metallic cation. A metallic bond is the attraction of an metallic cation for delocalized electrons. Copyright © Mc. Graw-Hill Education Metallic Bonds and the Properties of Metals
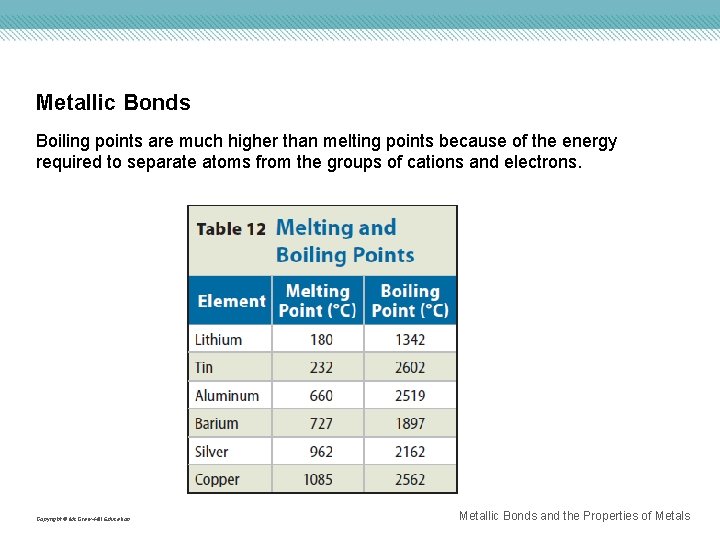
Metallic Bonds Boiling points are much higher than melting points because of the energy required to separate atoms from the groups of cations and electrons. Copyright © Mc. Graw-Hill Education Metallic Bonds and the Properties of Metals
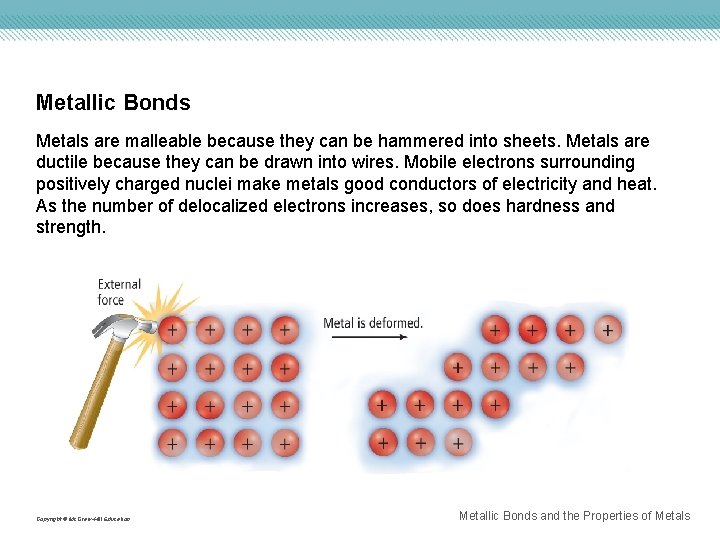
Metallic Bonds Metals are malleable because they can be hammered into sheets. Metals are ductile because they can be drawn into wires. Mobile electrons surrounding positively charged nuclei make metals good conductors of electricity and heat. As the number of delocalized electrons increases, so does hardness and strength. Copyright © Mc. Graw-Hill Education Metallic Bonds and the Properties of Metals

Metal Alloys An alloy is a mixture of elements that has metallic properties. • Example: Stainless steel, brass, cast iron The properties of alloys differ from the elements they contain. • Example: Steel is iron mixed with at least one other element. Some properties of iron are present, like magnetism, but steel is stronger than iron. Substitutional alloys are formed when some atoms in the original metallic solid are replaced by other metals of similar atomic structure. Interstitial alloys are formed when small holes in a metallic crystal are filled with smaller atoms. Copyright © Mc. Graw-Hill Education Metallic Bonds and the Properties of Metals
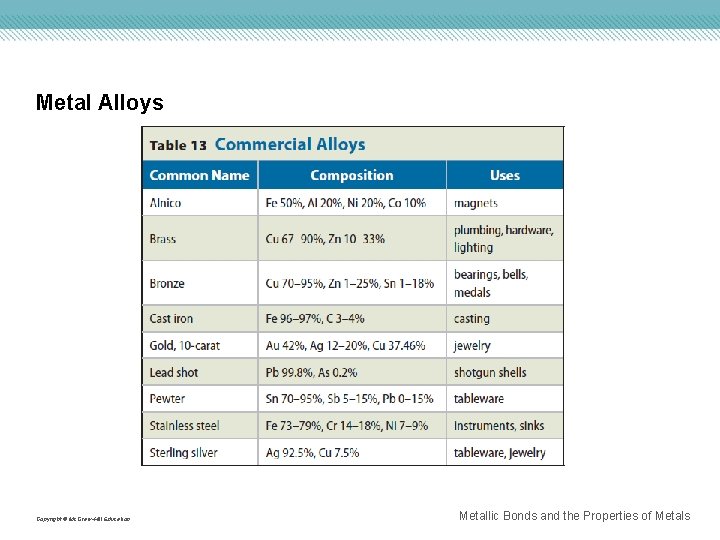
Metal Alloys Copyright © Mc. Graw-Hill Education Metallic Bonds and the Properties of Metals
Review Essential Questions • What are the characteristics of a metallic bond? • How does the electron sea model account for the physical properties of metals? • What are alloys, and how can they be categorized? Vocabulary • electron sea model • metallic bond • delocalized electron Copyright © Mc. Graw-Hill Education • alloy Metallic Bonds and the Properties of Metals
- Slides: 10