Chapter 8 Alkenes and Alkynes II Addition Reactions
- Slides: 51

Chapter 8 Alkenes and Alkynes II: Addition Reactions

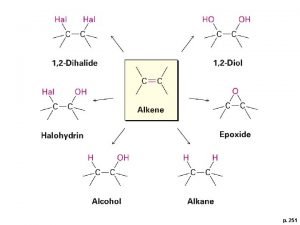
ALKENES Addition Reactions 1. Addition of H 2 2. Addition of HX 3. Addition of ICl 7. Polymerization 8. Oxymercuration. Demercuration 4. Addition of H 2 SO 4 9. Hydroboration Oxydation 5. 10. Oxydation with Os. O 4 and Addition of H 2 O/H+ 6. Addition of X 2 7. Halohydrin Formation KMn. O 4 11. Ozonolysis

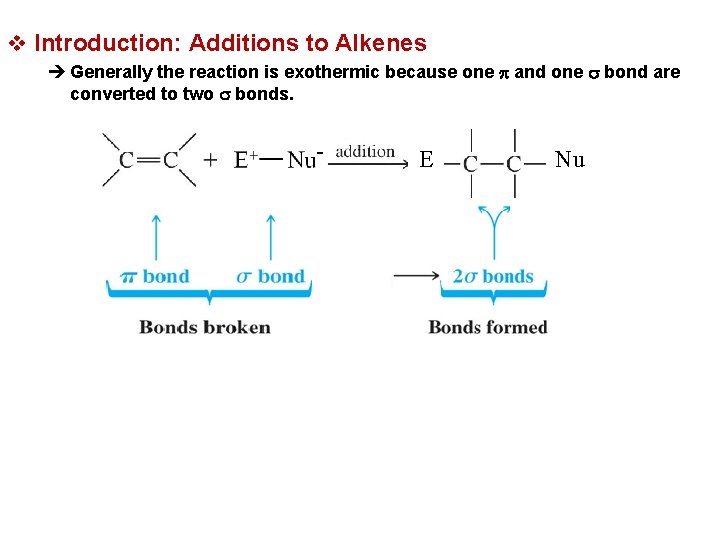
v Introduction: Additions to Alkenes è Generally the reaction is exothermic because one p and one s bond are converted to two s bonds. E Nu

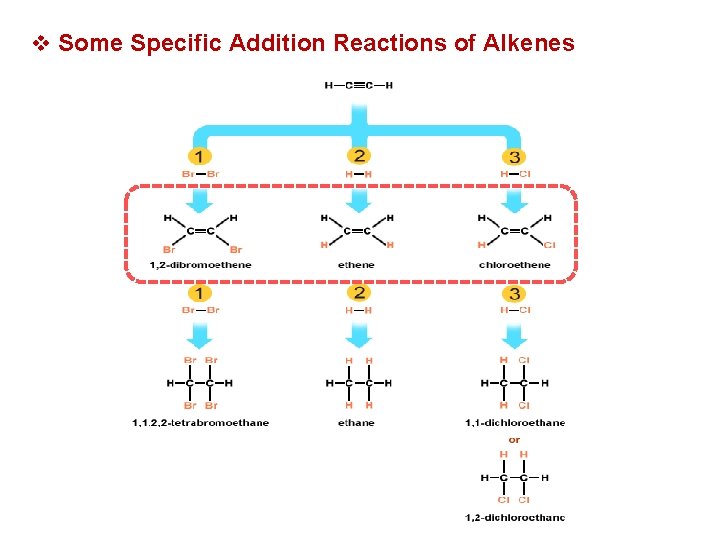
v Some Specific Addition Reactions of Alkenes

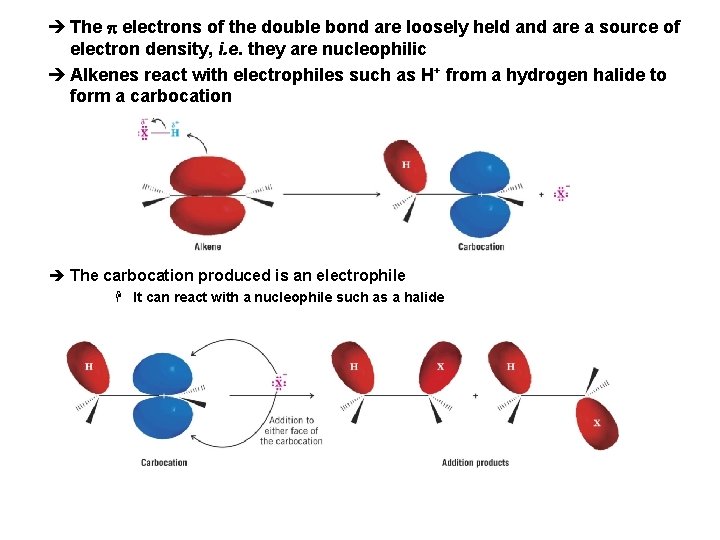
è The p electrons of the double bond are loosely held and are a source of electron density, i. e. they are nucleophilic è Alkenes react with electrophiles such as H+ from a hydrogen halide to form a carbocation è The carbocation produced is an electrophile H It can react with a nucleophile such as a halide

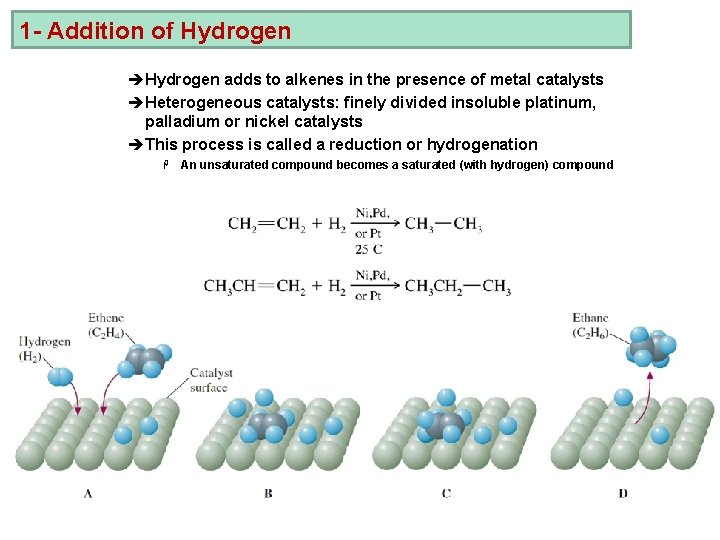
1 - Addition of Hydrogen èHydrogen adds to alkenes in the presence of metal catalysts èHeterogeneous catalysts: finely divided insoluble platinum, palladium or nickel catalysts èThis process is called a reduction or hydrogenation H An unsaturated compound becomes a saturated (with hydrogen) compound

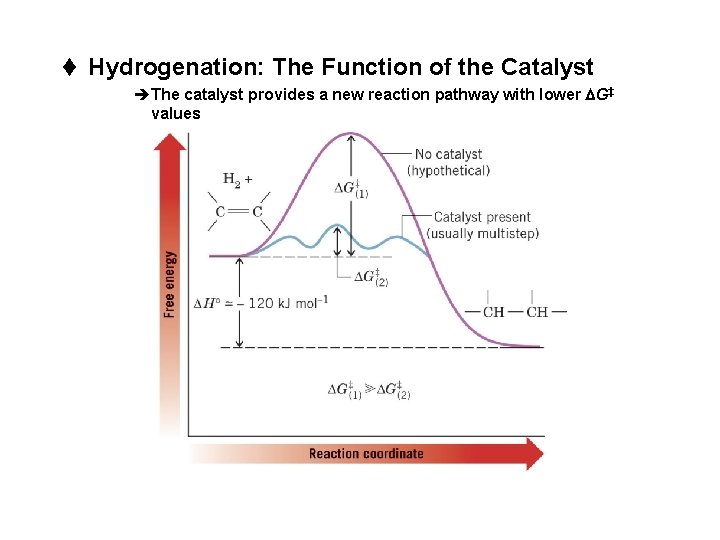
t Hydrogenation: The Function of the Catalyst èThe catalyst provides a new reaction pathway with lower DG‡ values

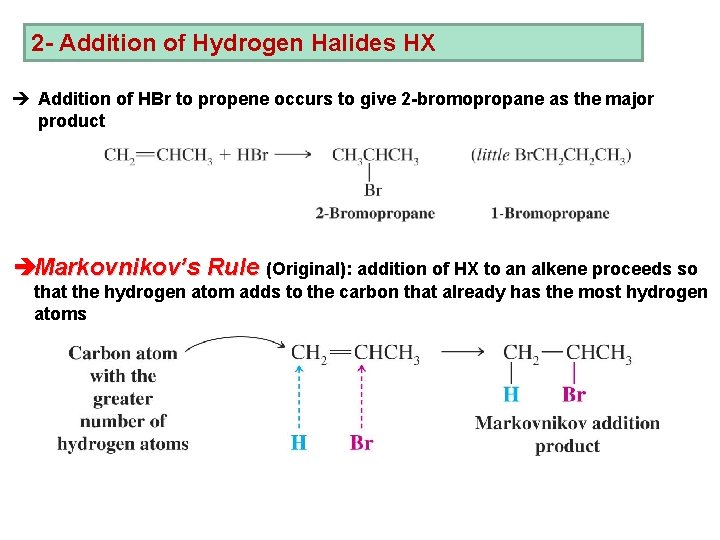
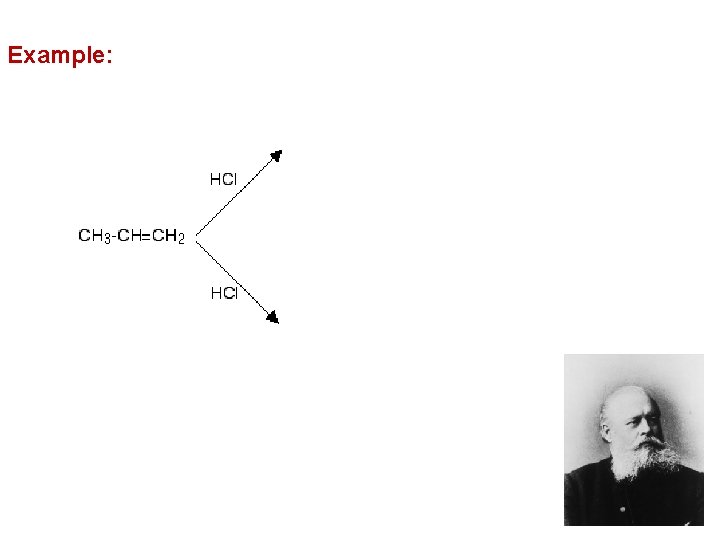
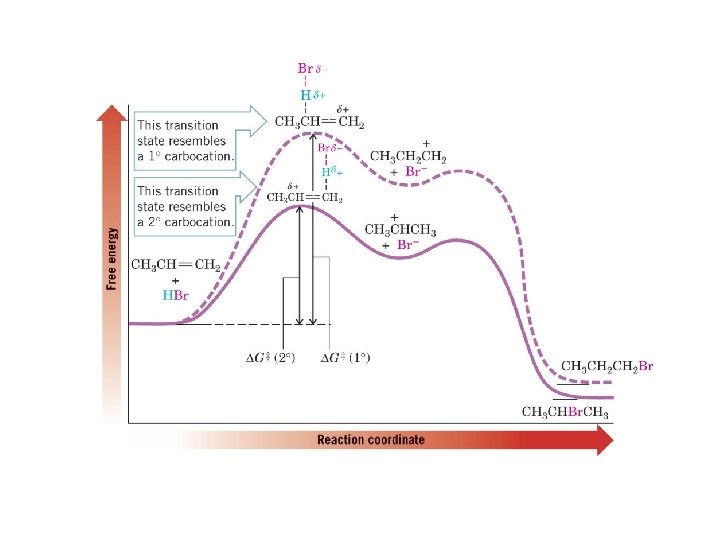
2 - Addition of Hydrogen Halides HX è Addition of HBr to propene occurs to give 2 -bromopropane as the major product èMarkovnikov’s Rule (Original): addition of HX to an alkene proceeds so that the hydrogen atom adds to the carbon that already has the most hydrogen atoms

Example:

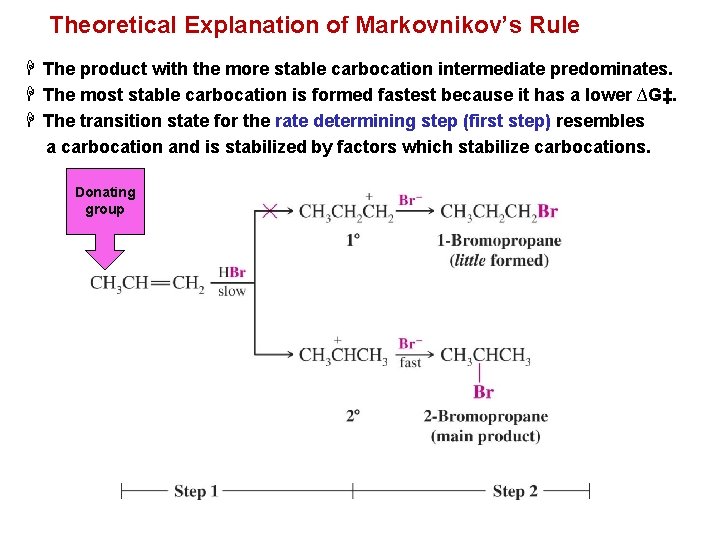
Theoretical Explanation of Markovnikov’s Rule H The product with the more stable carbocation intermediate predominates. H The most stable carbocation is formed fastest because it has a lower ∆G‡. H The transition state for the rate determining step (first step) resembles a carbocation and is stabilized by factors which stabilize carbocations. Donating group

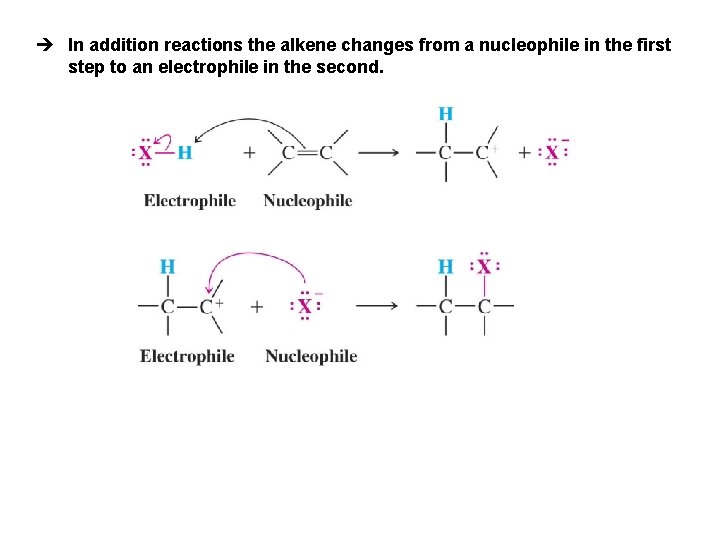
è In addition reactions the alkene changes from a nucleophile in the first step to an electrophile in the second.

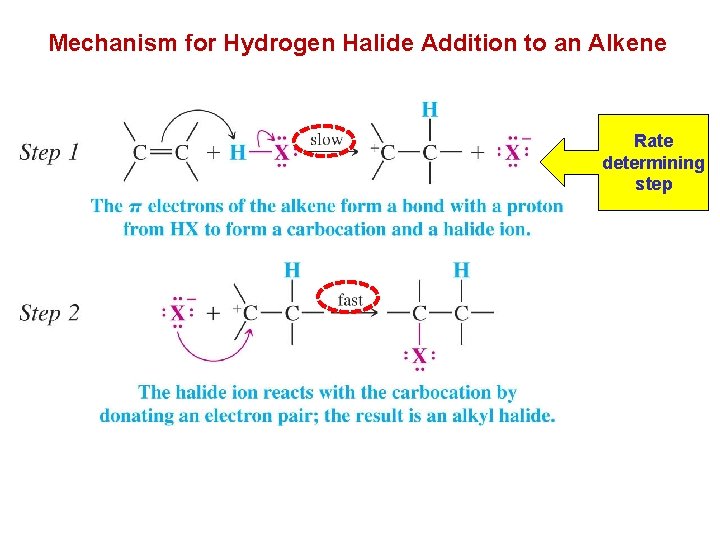
Mechanism for Hydrogen Halide Addition to an Alkene Rate determining step

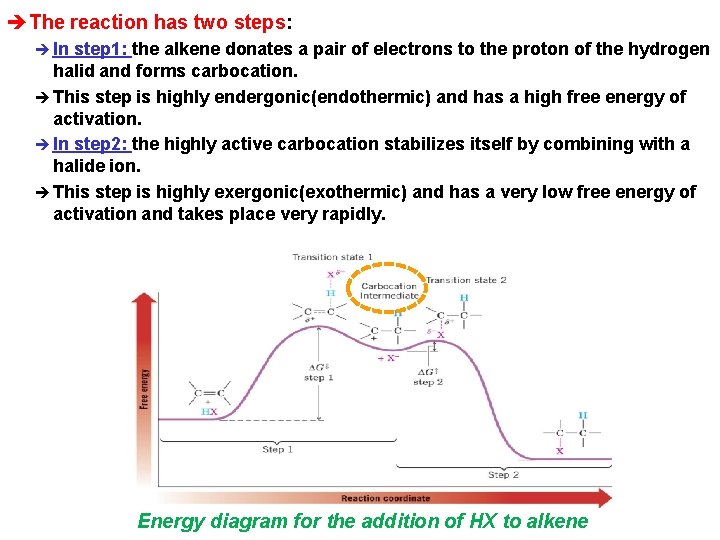
èThe reaction has two steps: è In step 1: the alkene donates a pair of electrons to the proton of the hydrogen halid and forms carbocation. è This step is highly endergonic(endothermic) and has a high free energy of activation. è In step 2: the highly active carbocation stabilizes itself by combining with a halide ion. è This step is highly exergonic(exothermic) and has a very low free energy of activation and takes place very rapidly. Energy diagram for the addition of HX to alkene


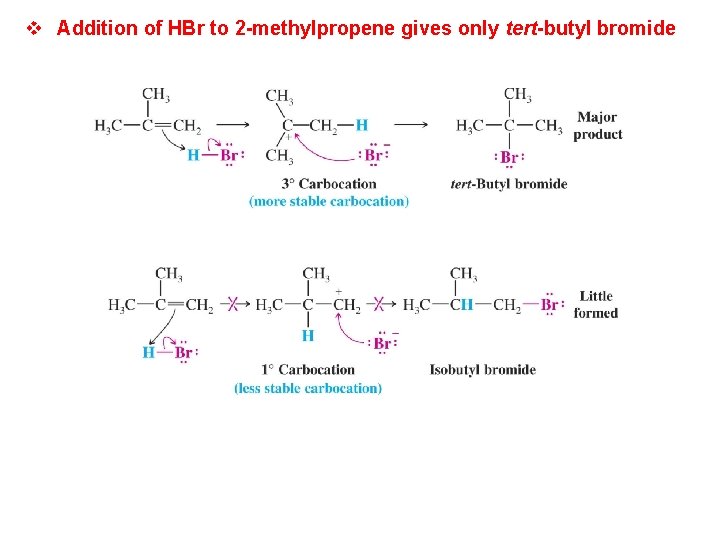
v Addition of HBr to 2 -methylpropene gives only tert-butyl bromide

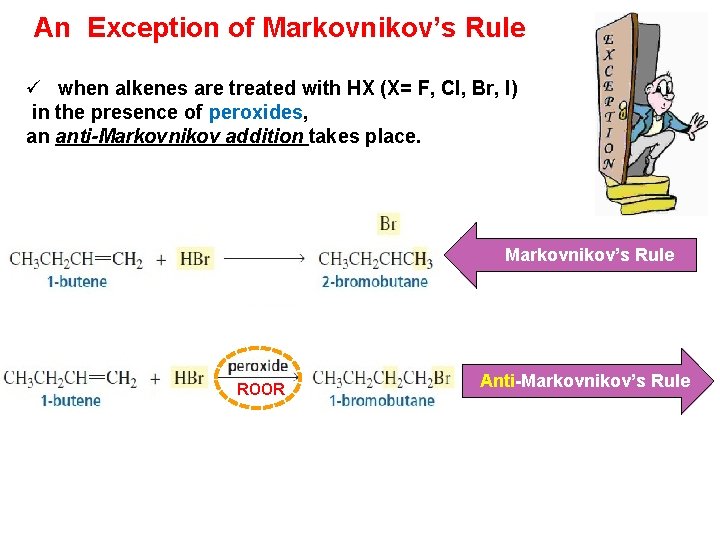
An Exception of Markovnikov’s Rule ü when alkenes are treated with HX (X= F, Cl, Br, I) in the presence of peroxides, an anti-Markovnikov addition takes place. Markovnikov’s Rule ROOR Anti-Markovnikov’s Rule

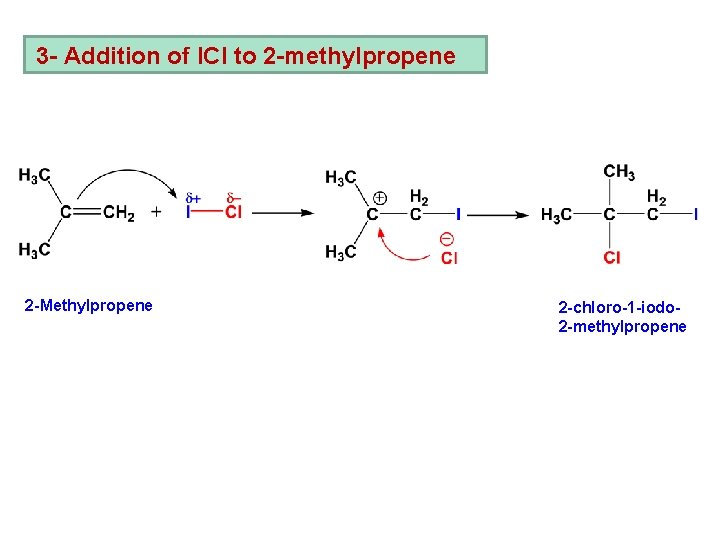
3 - Addition of ICl to 2 -methylpropene 2 -Methylpropene 2 -chloro-1 -iodo 2 -methylpropene

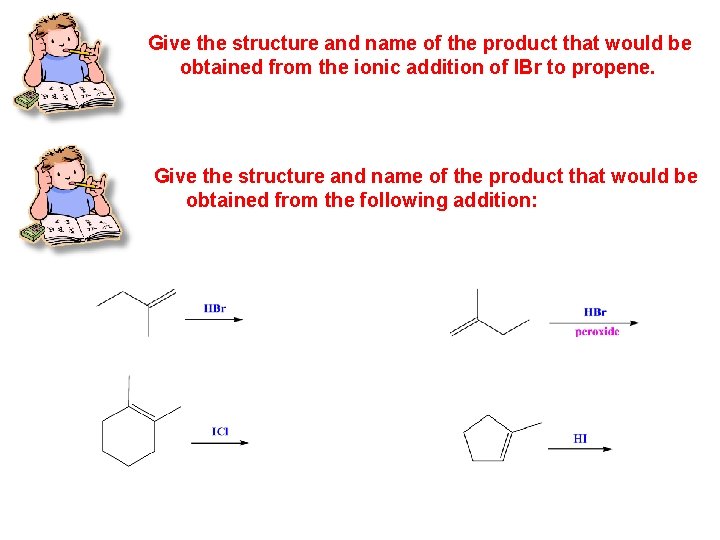
Give the structure and name of the product that would be obtained from the ionic addition of IBr to propene. Give the structure and name of the product that would be obtained from the following addition:

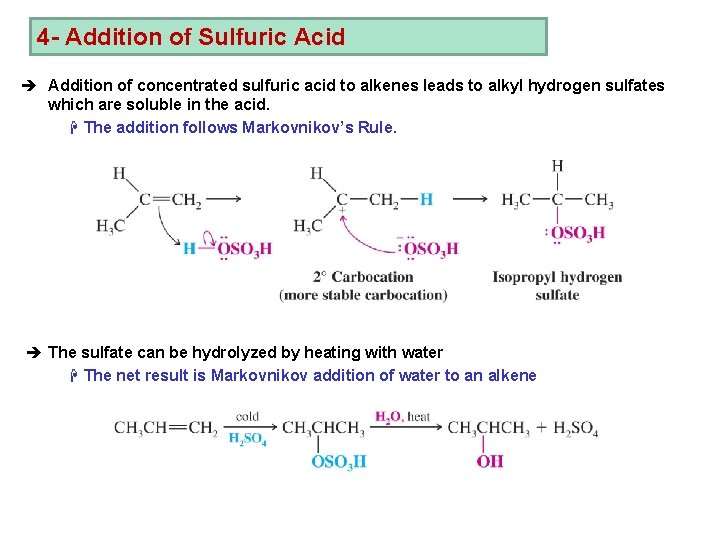
4 - Addition of Sulfuric Acid è Addition of concentrated sulfuric acid to alkenes leads to alkyl hydrogen sulfates which are soluble in the acid. H The addition follows Markovnikov’s Rule. è The sulfate can be hydrolyzed by heating with water H The net result is Markovnikov addition of water to an alkene

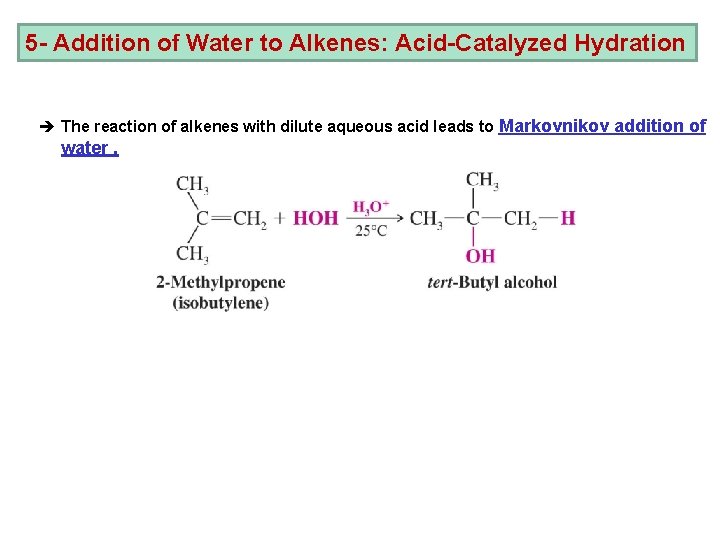
5 - Addition of Water to Alkenes: Acid-Catalyzed Hydration è The reaction of alkenes with dilute aqueous acid leads to Markovnikov addition of water.

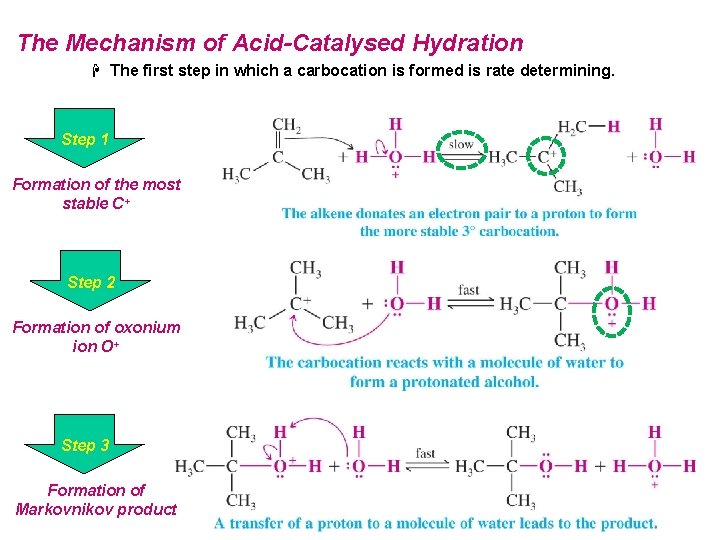
The Mechanism of Acid-Catalysed Hydration H The first step in which a carbocation is formed is rate determining. Step 1 Formation of the most stable C+ Step 2 Formation of oxonium ion O+ Step 3 Formation of Markovnikov product

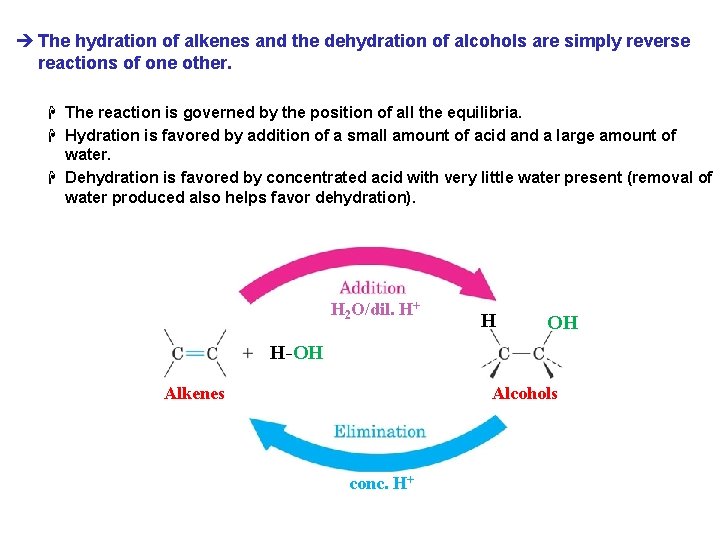
è The hydration of alkenes and the dehydration of alcohols are simply reverse reactions of one other. H The reaction is governed by the position of all the equilibria. H Hydration is favored by addition of a small amount of acid and a large amount of water. H Dehydration is favored by concentrated acid with very little water present (removal of water produced also helps favor dehydration). H 2 O/dil. H+ H OH H-OH Alkenes Alcohols conc. H+

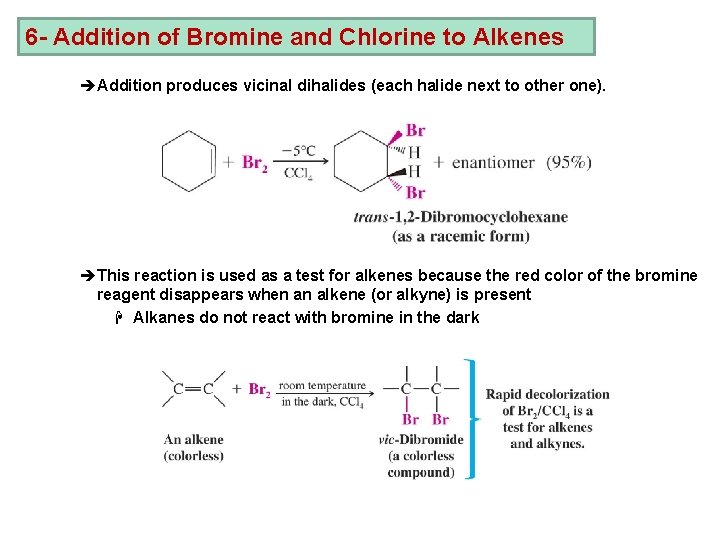
6 - Addition of Bromine and Chlorine to Alkenes èAddition produces vicinal dihalides (each halide next to other one). èThis reaction is used as a test for alkenes because the red color of the bromine reagent disappears when an alkene (or alkyne) is present H Alkanes do not react with bromine in the dark

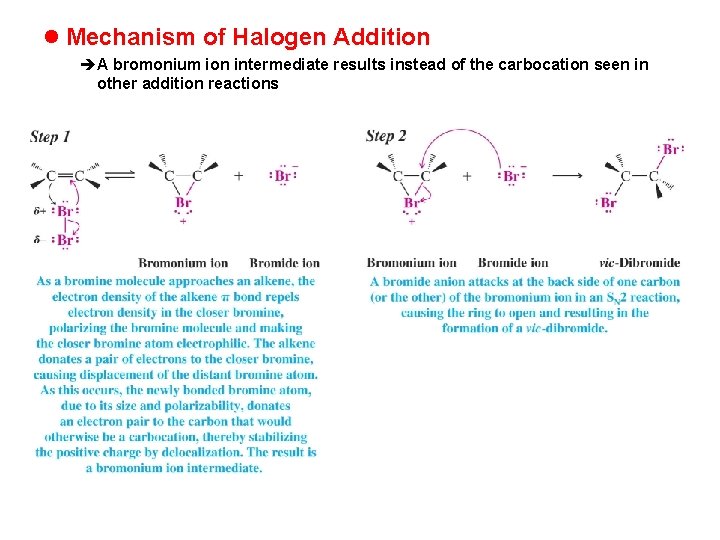
l Mechanism of Halogen Addition èA bromonium ion intermediate results instead of the carbocation seen in other addition reactions

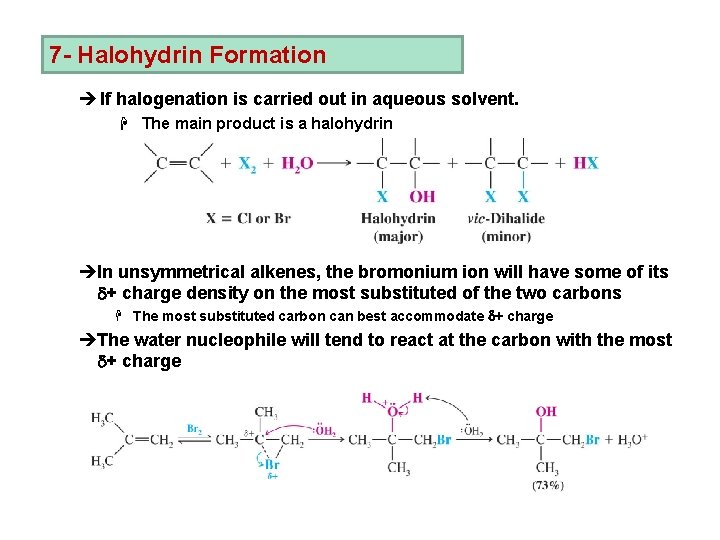
7 - Halohydrin Formation è If halogenation is carried out in aqueous solvent. H The main product is a halohydrin èIn unsymmetrical alkenes, the bromonium ion will have some of its d+ charge density on the most substituted of the two carbons H The most substituted carbon can best accommodate d+ charge èThe water nucleophile will tend to react at the carbon with the most d+ charge

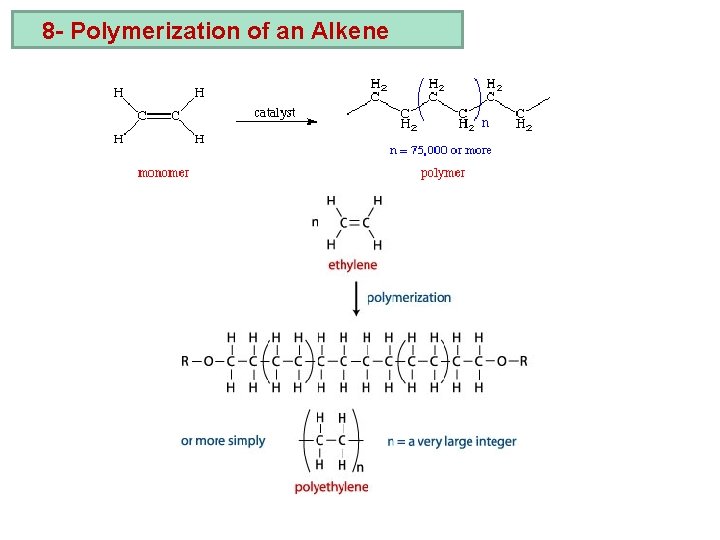
8 - Polymerization of an Alkene

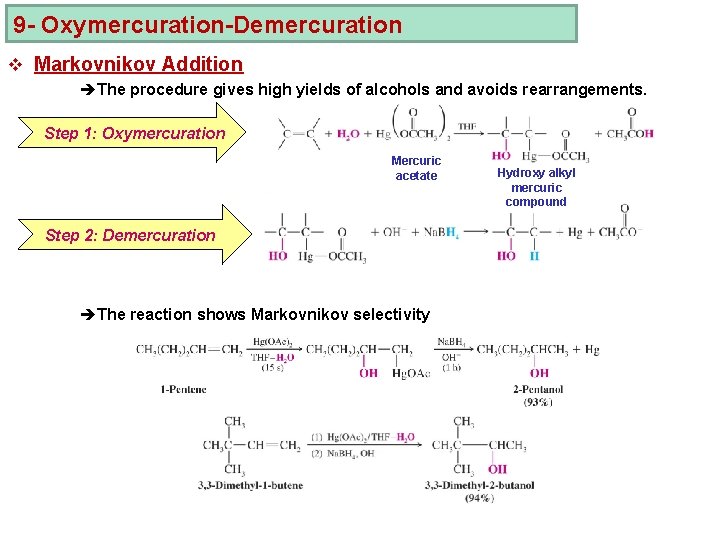
9 - Oxymercuration-Demercuration v Markovnikov Addition èThe procedure gives high yields of alcohols and avoids rearrangements. Step 1: Oxymercuration Mercuric acetate Step 2: Demercuration èThe reaction shows Markovnikov selectivity Hydroxy alkyl mercuric compound

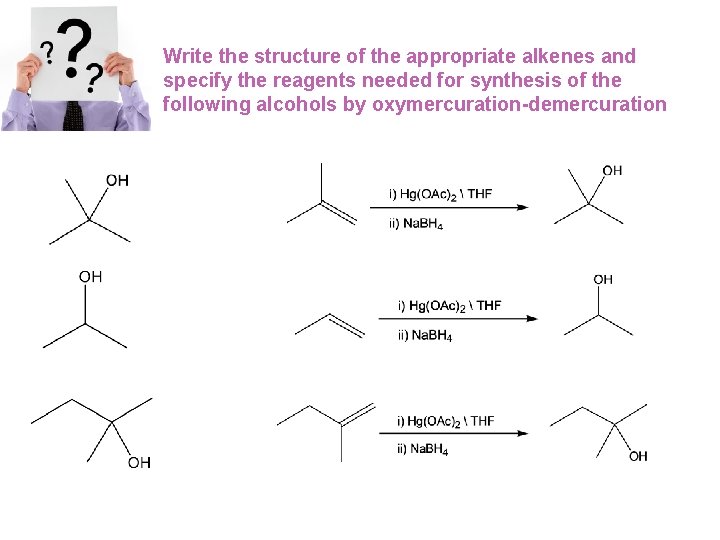
Write the structure of the appropriate alkenes and specify the reagents needed for synthesis of the following alcohols by oxymercuration-demercuration

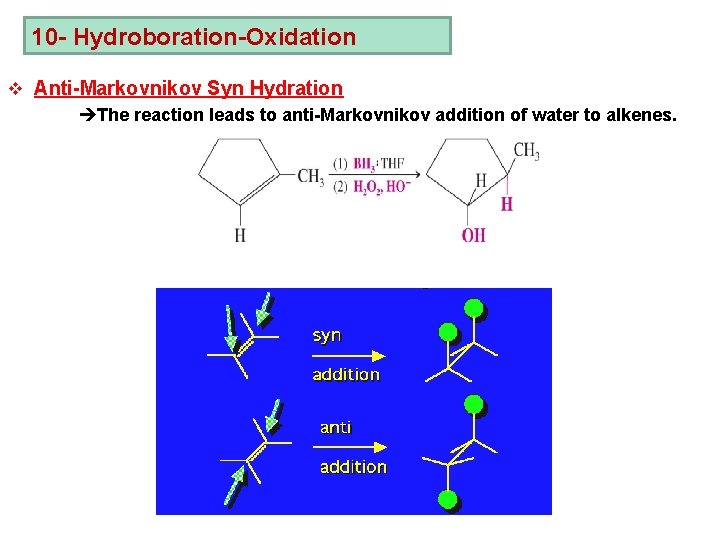
10 - Hydroboration-Oxidation v Anti-Markovnikov Syn Hydration èThe reaction leads to anti-Markovnikov addition of water to alkenes.

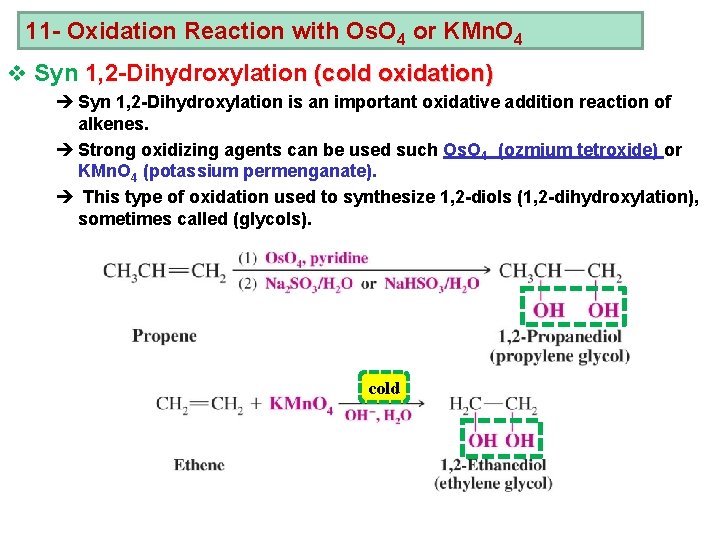
11 - Oxidation Reaction with Os. O 4 or KMn. O 4 v Syn 1, 2 -Dihydroxylation (cold oxidation) è Syn 1, 2 -Dihydroxylation is an important oxidative addition reaction of alkenes. è Strong oxidizing agents can be used such Os. O 4 (ozmium tetroxide) or KMn. O 4 (potassium permenganate). è This type of oxidation used to synthesize 1, 2 -diols (1, 2 -dihydroxylation), sometimes called (glycols). cold

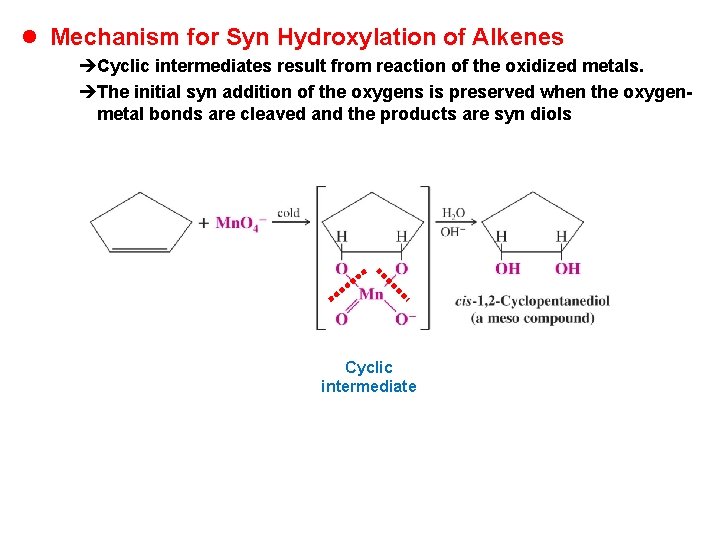
l Mechanism for Syn Hydroxylation of Alkenes èCyclic intermediates result from reaction of the oxidized metals. èThe initial syn addition of the oxygens is preserved when the oxygenmetal bonds are cleaved and the products are syn diols Cyclic intermediate

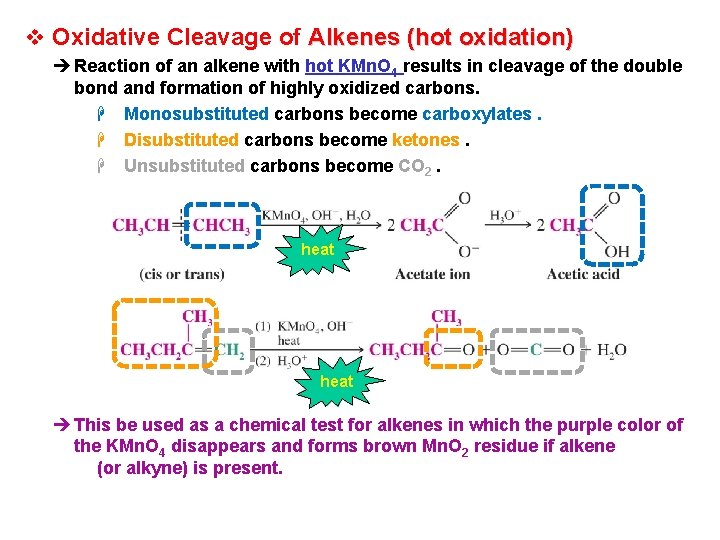
v Oxidative Cleavage of Alkenes (hot oxidation) è Reaction of an alkene with hot KMn. O 4 results in cleavage of the double bond and formation of highly oxidized carbons. H Monosubstituted carbons become carboxylates. H Disubstituted carbons become ketones. H Unsubstituted carbons become CO 2. heat è This be used as a chemical test for alkenes in which the purple color of the KMn. O 4 disappears and forms brown Mn. O 2 residue if alkene (or alkyne) is present.

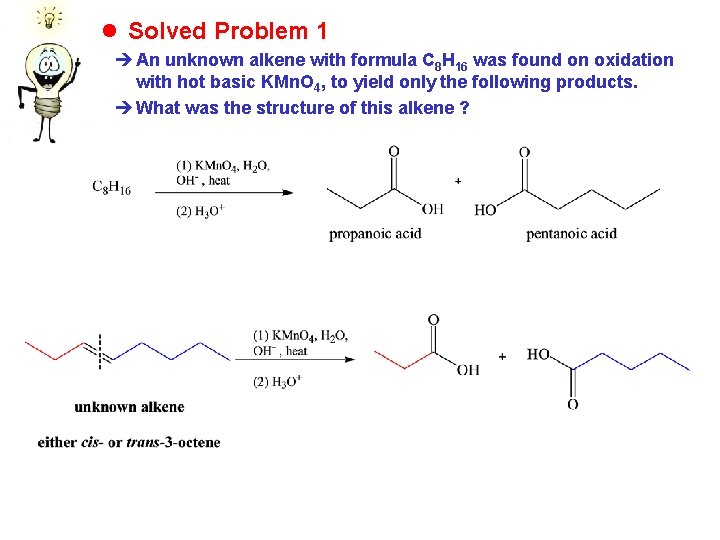
l Solved Problem 1 è An unknown alkene with formula C 8 H 16 was found on oxidation with hot basic KMn. O 4, to yield only the following products. è What was the structure of this alkene ?

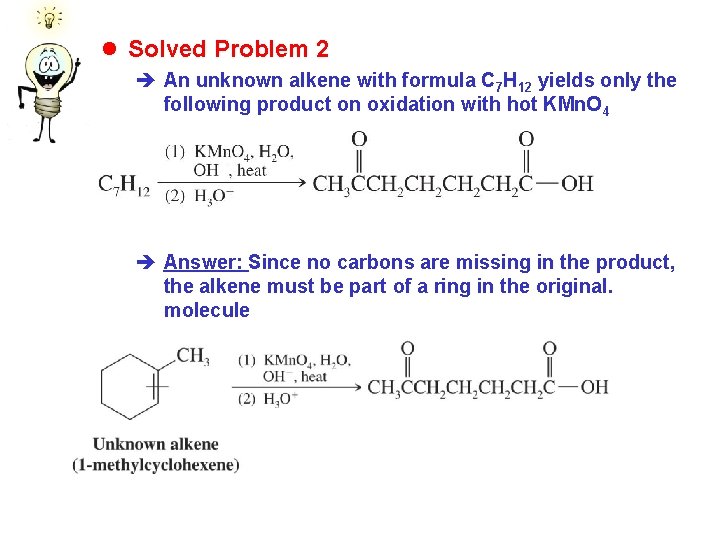
l Solved Problem 2 è An unknown alkene with formula C 7 H 12 yields only the following product on oxidation with hot KMn. O 4 è Answer: Since no carbons are missing in the product, the alkene must be part of a ring in the original. molecule

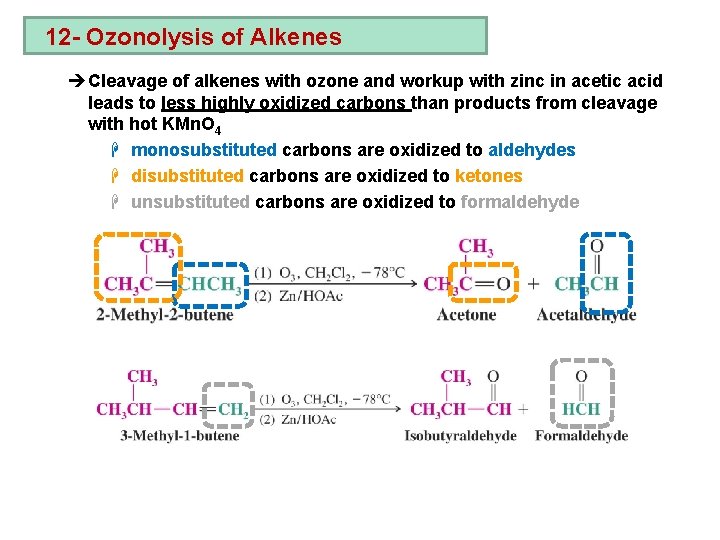
12 - Ozonolysis of Alkenes èCleavage of alkenes with ozone and workup with zinc in acetic acid leads to less highly oxidized carbons than products from cleavage with hot KMn. O 4 H monosubstituted carbons are oxidized to aldehydes H disubstituted carbons are oxidized to ketones H unsubstituted carbons are oxidized to formaldehyde

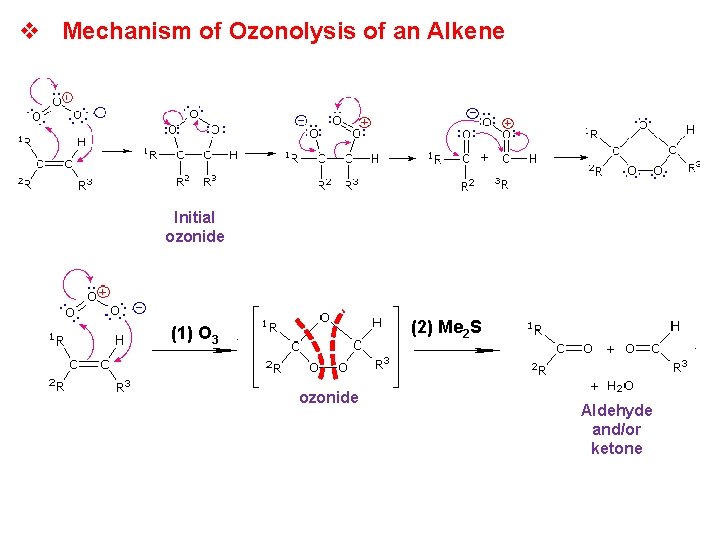
v Mechanism of Ozonolysis of an Alkene Initial ozonide (2) Me 2 S (1) O 3 ozonide Aldehyde and/or ketone

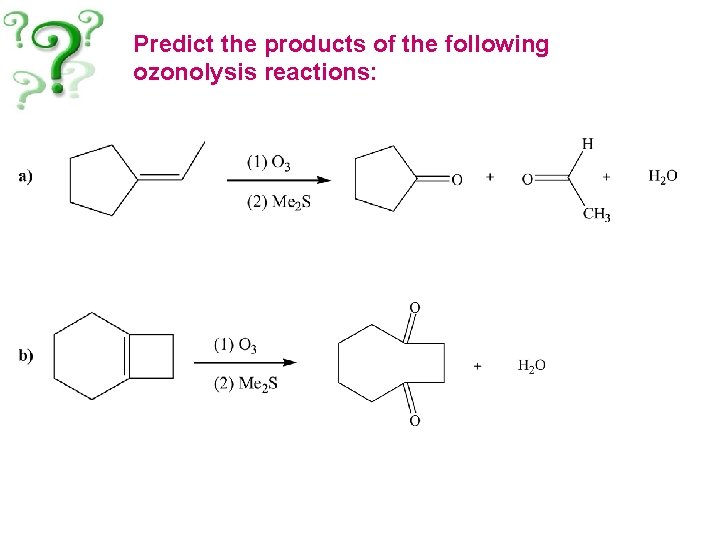
Predict the products of the following ozonolysis reactions:

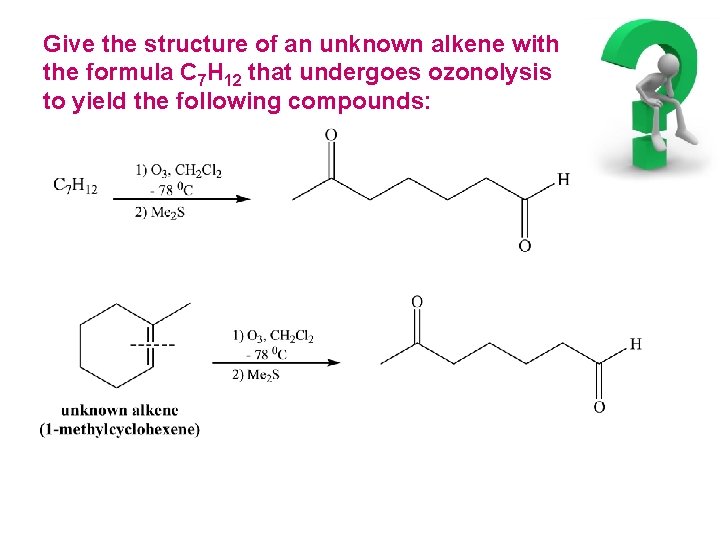
Give the structure of an unknown alkene with the formula C 7 H 12 that undergoes ozonolysis to yield the following compounds:

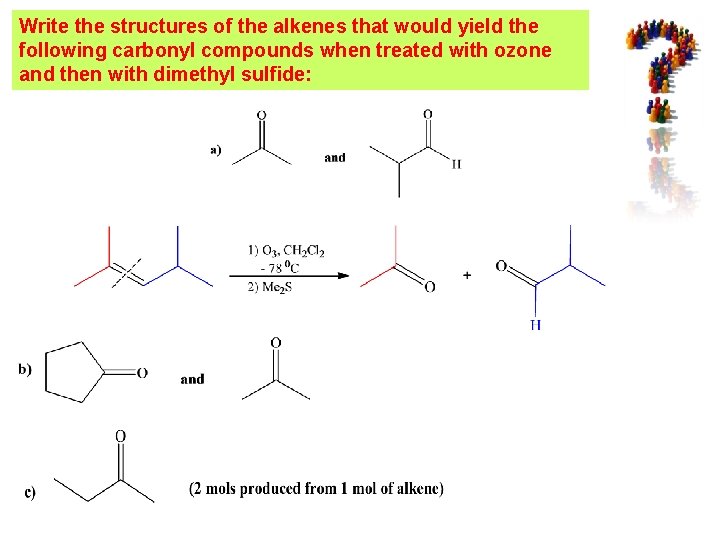
Write the structures of the alkenes that would yield the following carbonyl compounds when treated with ozone and then with dimethyl sulfide:

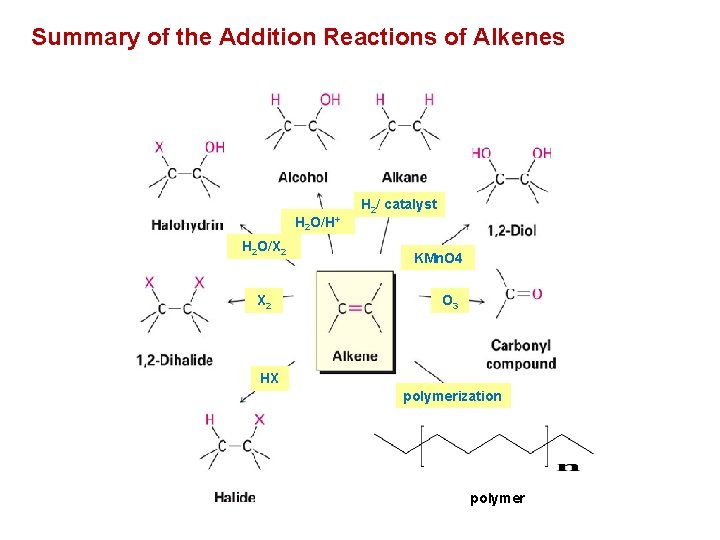
Summary of the Addition Reactions of Alkenes H 2 O/H+ H 2 O/X 2 H 2/ catalyst KMn. O 4 O 3 HX polymerization polymer

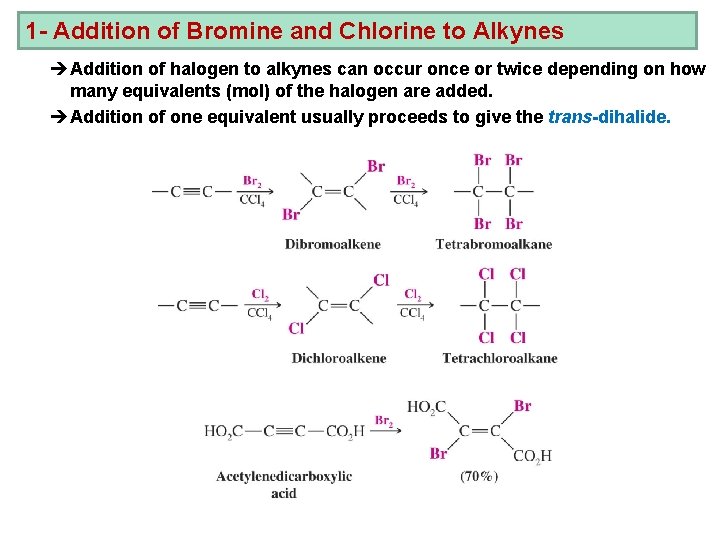
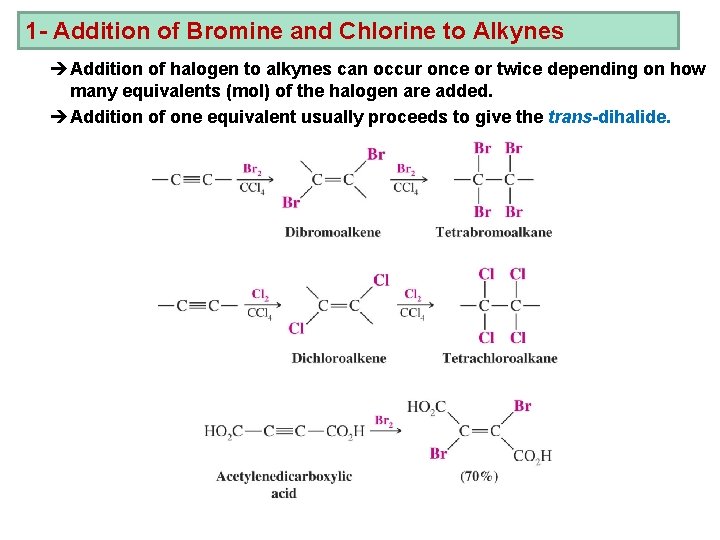
1 - Addition of Bromine and Chlorine to Alkynes èAddition of halogen to alkynes can occur once or twice depending on how many equivalents (mol) of the halogen are added. èAddition of one equivalent usually proceeds to give the trans-dihalide.

ALKYNES Addition Reactions 1. Addition of X 2 2. Addition of HX 3. Ozonolysis 4. Catalytic Hydrogenation

1 - Addition of Bromine and Chlorine to Alkynes èAddition of halogen to alkynes can occur once or twice depending on how many equivalents (mol) of the halogen are added. èAddition of one equivalent usually proceeds to give the trans-dihalide.

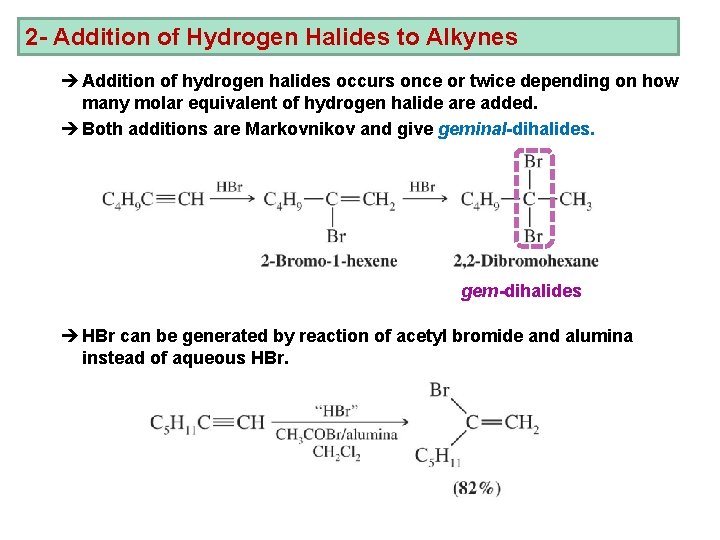
2 - Addition of Hydrogen Halides to Alkynes è Addition of hydrogen halides occurs once or twice depending on how many molar equivalent of hydrogen halide are added. è Both additions are Markovnikov and give geminal-dihalides. gem-dihalides è HBr can be generated by reaction of acetyl bromide and alumina instead of aqueous HBr.

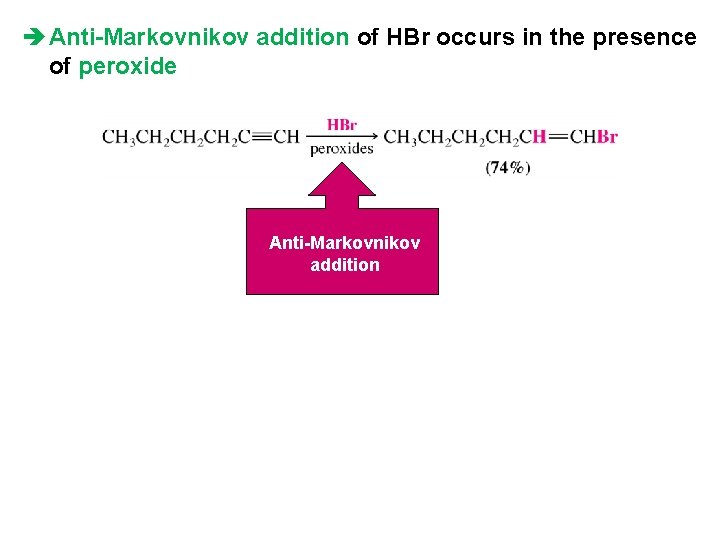
è Anti-Markovnikov addition of HBr occurs in the presence of peroxide Anti-Markovnikov addition

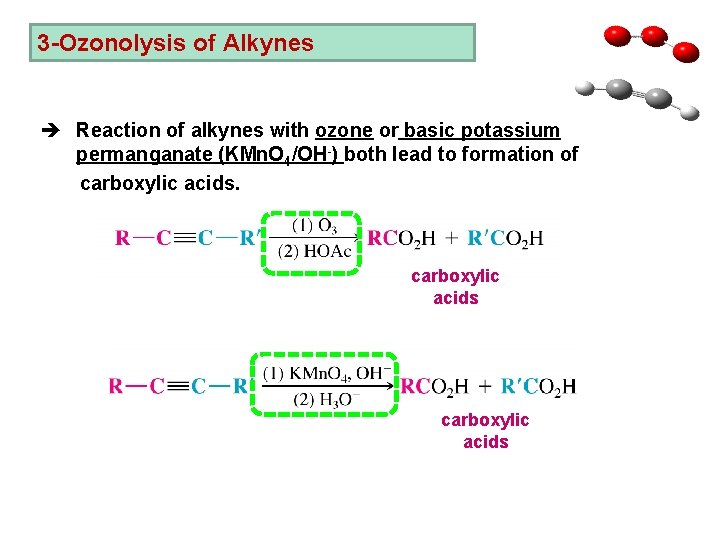
3 -Ozonolysis of Alkynes è Reaction of alkynes with ozone or basic potassium permanganate (KMn. O 4/OH-) both lead to formation of carboxylic acids

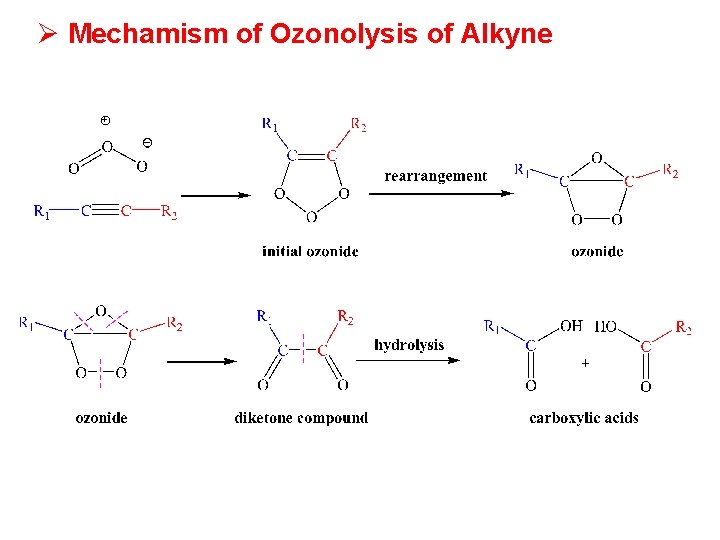
Ø Mechamism of Ozonolysis of Alkyne

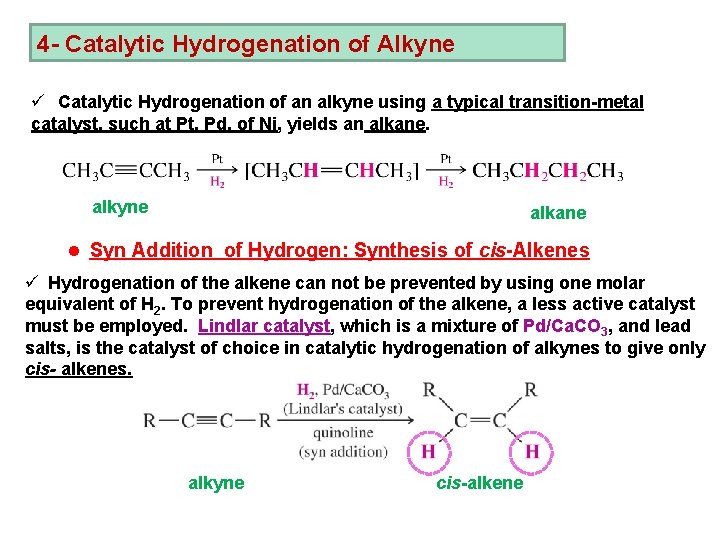
4 - Catalytic Hydrogenation of Alkyne ü Catalytic Hydrogenation of an alkyne using a typical transition-metal catalyst, such at Pt, Pd, of Ni, yields an alkane. alkyne alkane l Syn Addition of Hydrogen: Synthesis of cis-Alkenes ü Hydrogenation of the alkene can not be prevented by using one molar equivalent of H 2. To prevent hydrogenation of the alkene, a less active catalyst must be employed. Lindlar catalyst, which is a mixture of Pd/Ca. CO 3, and lead salts, is the catalyst of choice in catalytic hydrogenation of alkynes to give only cis- alkenes. alkyne cis-alkene

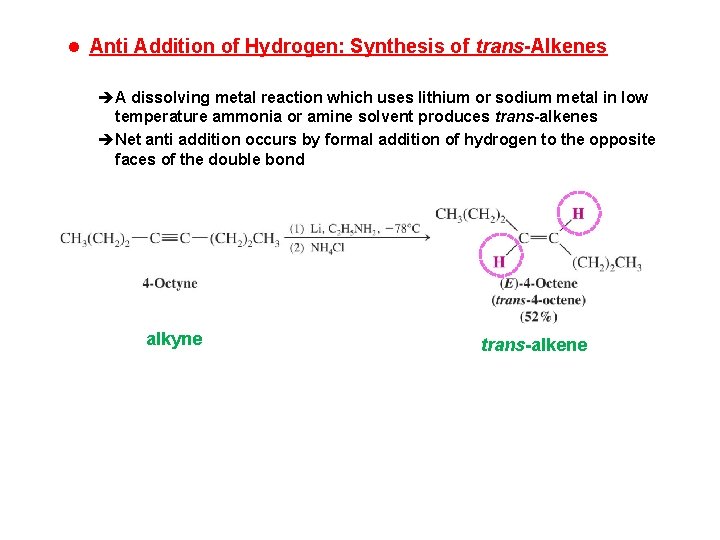
l Anti Addition of Hydrogen: Synthesis of trans-Alkenes èA dissolving metal reaction which uses lithium or sodium metal in low temperature ammonia or amine solvent produces trans-alkenes èNet anti addition occurs by formal addition of hydrogen to the opposite faces of the double bond alkyne trans-alkene

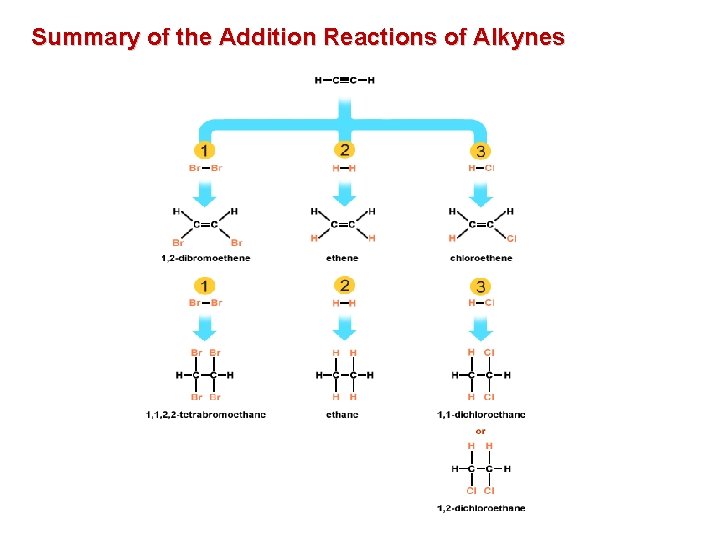
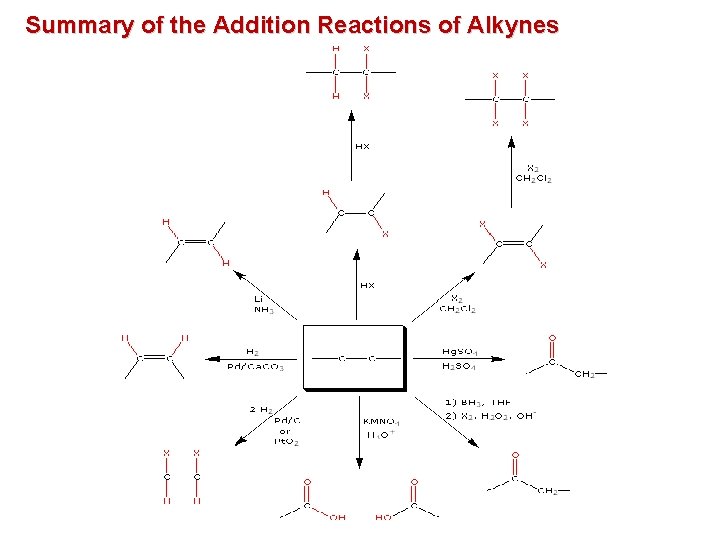
Summary of the Addition Reactions of Alkynes

Summary of the Addition Reactions of Alkynes
 Alkanes alkenes alkynes
Alkanes alkenes alkynes Alkenes reactions and synthesis
Alkenes reactions and synthesis Reactions with alkynes
Reactions with alkynes Chemsheets reactions of halogenoalkanes 2
Chemsheets reactions of halogenoalkanes 2 Is acid catalyzed hydration syn or anti
Is acid catalyzed hydration syn or anti Addition of halogens to alkenes
Addition of halogens to alkenes Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Example of oxidation reduction reaction
Example of oxidation reduction reaction Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Types of reactions
Types of reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Halogenation of alkynes
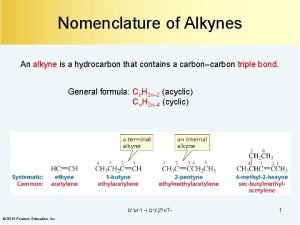
Halogenation of alkynes Triple bond nomenclature
Triple bond nomenclature Mercury catalyzed hydration of alkynes
Mercury catalyzed hydration of alkynes Mercury catalyzed hydration of alkynes
Mercury catalyzed hydration of alkynes General molecular formula of alkene
General molecular formula of alkene Ozonolysis of alkynes
Ozonolysis of alkynes Alkynes structural formula
Alkynes structural formula Preparation of alkynes
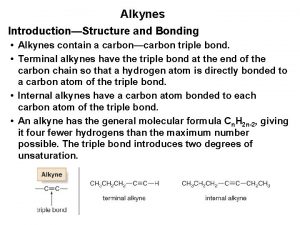
Preparation of alkynes Alkynes
Alkynes Dihydroxylation
Dihydroxylation First 10 members of alkynes
First 10 members of alkynes Claisen adapter
Claisen adapter Combustion reaction of alkanes
Combustion reaction of alkanes Physical properties of alkenes
Physical properties of alkenes Addition polymerization
Addition polymerization Uses of alkanes
Uses of alkanes Rco3h reaction
Rco3h reaction Physical properties of alkenes
Physical properties of alkenes Hybridization of alkenes
Hybridization of alkenes Name the following alkenes
Name the following alkenes Alkyne prefix
Alkyne prefix Bromine water test
Bromine water test Cooh
Cooh E or z configuration
E or z configuration Alkenes introduction
Alkenes introduction Chapter 10 chapter assessment chemical reactions answers
Chapter 10 chapter assessment chemical reactions answers Chapter 9 chemical reactions
Chapter 9 chemical reactions Chapter 8 review chemical equations and reactions section 2
Chapter 8 review chemical equations and reactions section 2 Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Balancing equations chapter 8
Balancing equations chapter 8 Oxidation reduction reactions chapter 19 review
Oxidation reduction reactions chapter 19 review Chapter 9 chemical reactions
Chapter 9 chemical reactions Chapter 9 chemical reactions answers
Chapter 9 chemical reactions answers Chapter 20 worksheet redox
Chapter 20 worksheet redox Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Chapter 11 chemical reactions practice problems
Chapter 11 chemical reactions practice problems Are kc and kp equal
Are kc and kp equal Chapter 19 review oxidation-reduction reactions answers
Chapter 19 review oxidation-reduction reactions answers Chapter 19 chemical reactions simple word equations
Chapter 19 chemical reactions simple word equations 5 types of chemical reactions
5 types of chemical reactions Chapter 4 reactions in aqueous solutions
Chapter 4 reactions in aqueous solutions