Chapter 17 Elements and their Properties Properties of

























- Slides: 46

Chapter 17 Elements and their Properties

Properties of Metals • In the periodic table, metals are elements found to the left of the stair-step line.

Properties of Metals • good conductors of heat and electricity • all but one are solid at room temperature. • reflect light~luster. Typically shiny • malleable -hammered or rolled into sheets. • ductile -drawn into wires

Ionic Bonding in Metals metals – one to three electrons in their outer energy levels. chemical reactions – metals tend to give up electrons easily because of the strength of charge of the protons in the nucleus.

Ionic Bonding in Metals metals combine with nonmetals, – the atoms of the metals tend to lose electrons to the atoms of nonmetals, forming ionic bonds. – Both become more chemically stable when they form ions.

Metallic Bonding In metallic bonding – positively charged metallic ions are surrounded by a cloud of electrons – Outer-level electrons are NOT held tightly to the nucleus of an atom. – the electrons move freely among many positively charged ions.

Metallic Bonding metallic bonding – explains many of the properties of metals. metal – When hammered into sheets or drawn into a wire, it does not break because the ions are in layers that slide past one another without losing their attraction to the electron cloud. – good conductors of electricity because the outer-level electrons are weakly held.

The Alkali Metals Group 1 of the periodic table – one electron in its outer energy level (most reactive of all the metals) – don’t occur in nature in their elemental form and are stored in substances that are unreactive, such as an oil.

The Alkaline Earth Metals alkaline earth metal – two electrons in its outer energy level (still highly reactive) These electrons are given up when an alkaline earth metal combines with a nonmetal

Transition Elements Transition elements are those elements in Groups 3 through 12 in the periodic table. Transition elements are familiar because they often occur in nature as uncombined elements

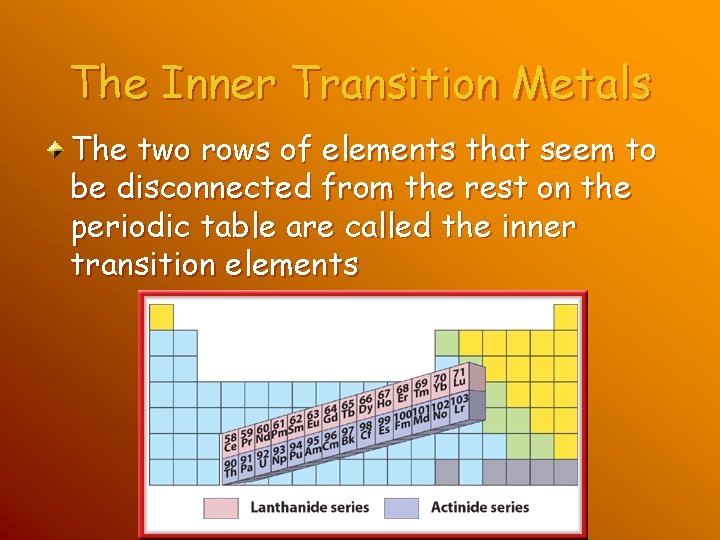
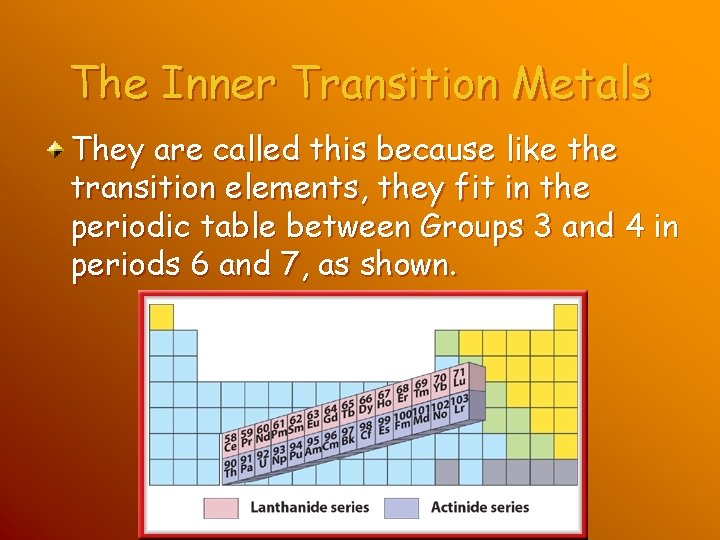
The Inner Transition Metals The two rows of elements that seem to be disconnected from the rest on the periodic table are called the inner transition elements

The Inner Transition Metals They are called this because like the transition elements, they fit in the periodic table between Groups 3 and 4 in periods 6 and 7, as shown.

The Lanthanides The first row includes a series of elements with of 58 to 71. These elements are called the lanthanide series because they follow the element lanthanum.

The Actinides The second row of inner transition metals includes elements with atomic numbers ranging from 90 to 103. These elements are called the actinide series because they follow the element actinium. All of the actinides are radioactive and unstable. Thorium and uranium are the actinides found in the Earth’s crust in usable quantities.

Properties of Nonmetals ü Periodic table üall nonmetals except hydrogen are found at the right of the stair-step line.

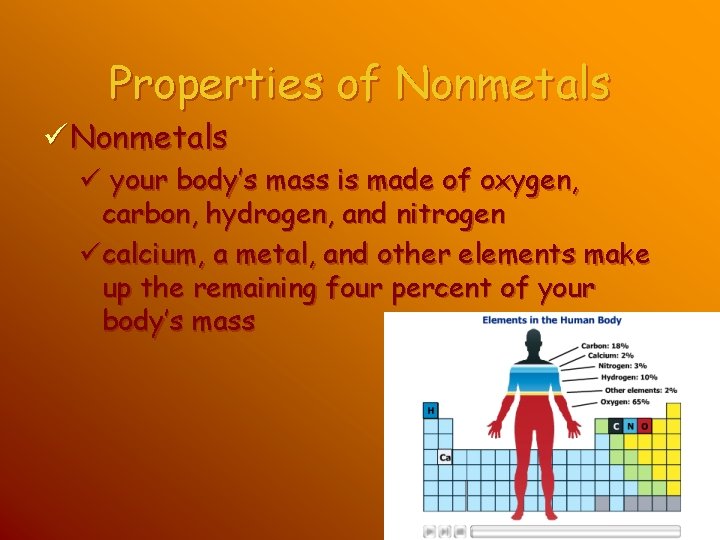
Properties of Nonmetals ü your body’s mass is made of oxygen, carbon, hydrogen, and nitrogen ücalcium, a metal, and other elements make up the remaining four percent of your body’s mass

Properties of Nonmetals ü Nonmetals • elements that usually are gases or brittle solids at room temperature • don’t conduct heat or electricity well, and generally they are not shiny. • as a group, nonmetals are poor conductors of heat and electricity. ü form both ionic and covalent compounds. ü gain electrons from metals, the nonmetals become negative ions in ionic compounds. ü atoms of nonmetals usually share electrons to form covalent compounds.

Hydrogen ü Hydrogen atoms in the universe, üabout 90 percent of them are hydrogen. üfound mostly in the compound water ü Water üwhen broken down into its elements, hydrogen becomes a gas made up of diatomic molecules.

Hydrogen ü Diatomic molecule ütwo atoms of the same element in a covalent bond

Hydrogen ü highly reactive. üa single electron, which the atom shares when it combines with other nonmetals. ücan gain an electron when it combines with alkali and alkaline earth metals. üforms hydrides.

The Halogens ü Halogen lights ücontain small amounts of bromine or iodine. üThese elements, as well as fluorine, chlorine, and astatine, are called halogens and are in Group 17.

The Halogens üvery reactive in their elemental form ütheir compounds have many uses.

The Halogens ü Halogen ü seven electrons in its outer energy level ü only one electron is needed to complete this energy level. üif a halogen gains an electron from a metal, an ionic compound, called a salt is formed.

The Halogens ü Gaseous state üform reactive diatomic covalent molecules ücan be identified by their distinctive colors. üchlorine is greenish yellow, bromine is reddish orange, and iodine is violet.

The Noble Gases ü The noble gases üexist as isolated atoms üstable~ their outermost energy levels are full. üno naturally occurring noble gas compounds are known

The Noble Gases Noble gases stability what makes them useful Helium light weight makes it useful in lighter-than -air blimps and balloons Neon and argon used in “neon lights” for advertising.

Properties of Metalloids share unusual characteristics form ionic and covalent bonds with other elements have metallic and nonmetallic properties

Properties of Metalloids – some can conduct electricity better than most nonmetals – not as well as some metals, giving them the name semiconductor. – except for aluminum the metalloids are the elements in the periodic table that are located along the stair-step line

The Boron Group Boron, – metalloid, first element in Group 13 – borax laundry products to soften water. – boric acid, a mild antiseptic.

The Carbon Group 14 – carbon family, has four electrons in its outer energy level – where much of the similarity ends.

The Carbon Group Carbon – nonmetal Silicon and germanium – metalloid Tin and lead – metals.

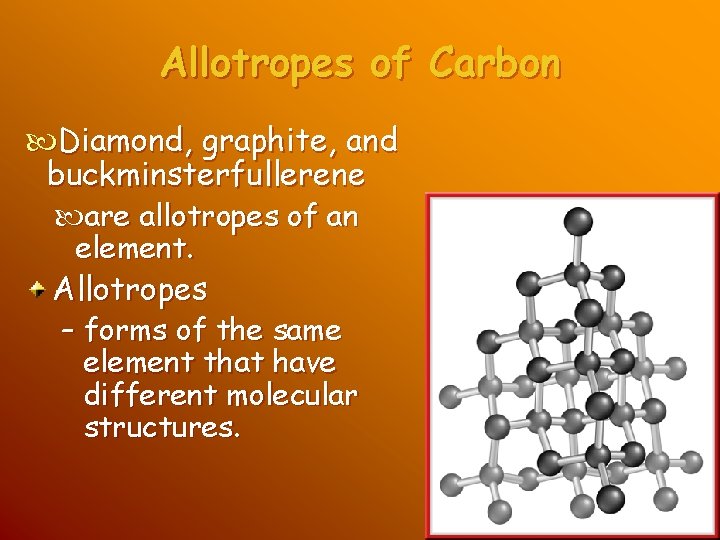
Allotropes of Carbon Diamond, graphite, and buckminsterfullerene are allotropes of an element. Allotropes – forms of the same element that have different molecular structures.

Allotropes of Carbon Diamond, each carbon atom is bonded to four other carbon atoms at the vertices, or corner points, of a tetrahedron – many tetrahedrons join together to form a giant molecule in which the atoms are held tightly in a strong crystalline structure.

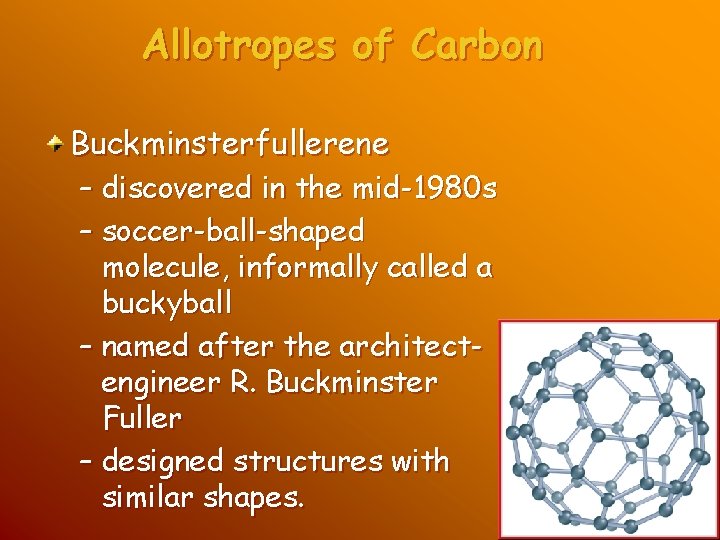
Allotropes of Carbon Buckminsterfullerene – discovered in the mid-1980 s – soccer-ball-shaped molecule, informally called a buckyball – named after the architectengineer R. Buckminster Fuller – designed structures with similar shapes.

Allotropes of Carbon Graphite – allotrope of carbon that is soft and can be used as a lubricant Diamonds – allotrope of carbon that is hard and is often used in jewelry.

The Nitrogen Group Nitrogen family – Group 15. – five electrons in its outer energy level – tend to share electrons – form covalent compounds with other elements.

The Nitrogen Group Nitrogen – fourth most abundant element in your body. – each breath is about 80 percent gaseous nitrogen in the form of diatomic molecules, N 2.

Uses of the Nitrogen Group Phosphorus – nonmetal that has three allotropes. Antimony – metalloid Bismuth – metal – both elements are used with other metals to lower their melting points.

The Oxygen Group 16 oxygen group. Oxygen nonmetal, exists in the air as diatomic molecules, O 2. during electrical storms, some oxygen molecules, O 2, change into ozone molecules, O 3.

The Oxygen Group sulfur – second element in the oxygen group Sulfur – nonmetal that exists in several allotropic forms. – exists as different-shaped crystals and as a noncrystalline solid.

The Oxygen Group Tellurium and polonium – metalloids Selenium – nonmetal – one of several that you need in trace amounts in your diet. – toxic if too much of it gets into your system. – most common of these three.

Synthetic Elements Synthetics – smashing existing elements with particles accelerated in a heavy ion accelerator, scientists have been successful in creating elements not typically found on Earth. – more than 92 protons – except for technetium-43 and promethium-61

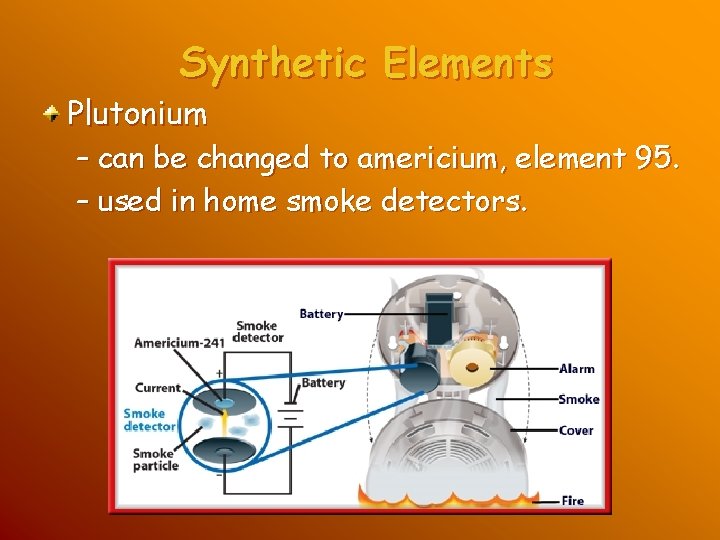
Synthetic Elements Plutonium – can be changed to americium, element 95. – used in home smoke detectors.

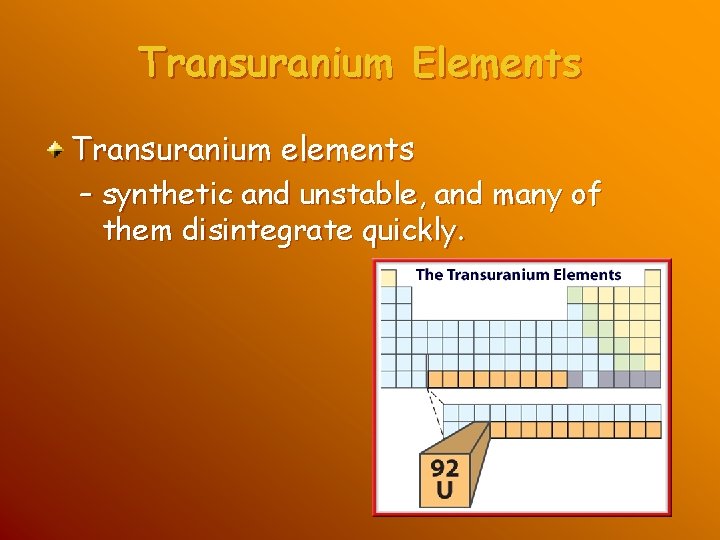
Transuranium Elements Transuranium elements – elements having more than 92 protons, the atomic number of uranium – do not belong exclusively to the metal, nonmetal, or metalloid group.

Transuranium Elements Transuranium elements – synthetic and unstable, and many of them disintegrate quickly.

Why make elements? Synthesized elements – by studying how they form and disintegrate, you can gain an understanding of the forces holding the nucleus together. – Radioactive elements can be useful. – technetium’s radioactivity makes it ideal for many medical applications. http: //www. youtube. com/watch? v=Jzqd. Hkp. Xuy 4&fea ture=plcp