Metallic Bonds and Properties of Metals Metals Metals

Metallic Bonds and Properties of Metals
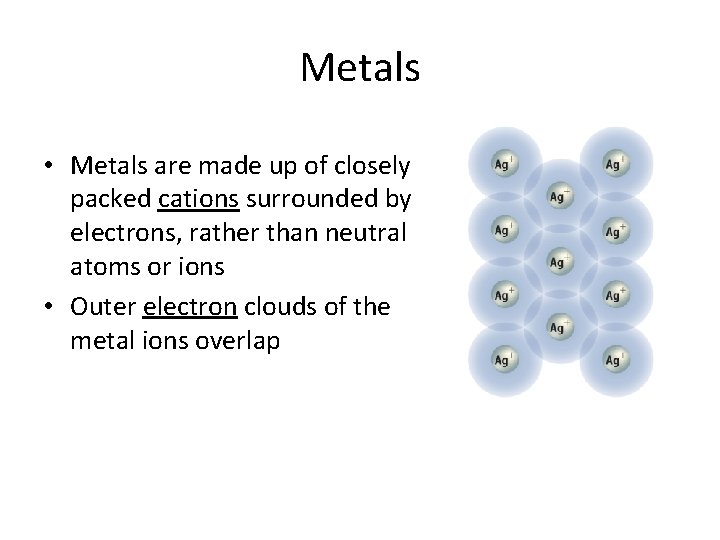
Metals • Metals are made up of closely packed cations surrounded by electrons, rather than neutral atoms or ions • Outer electron clouds of the metal ions overlap

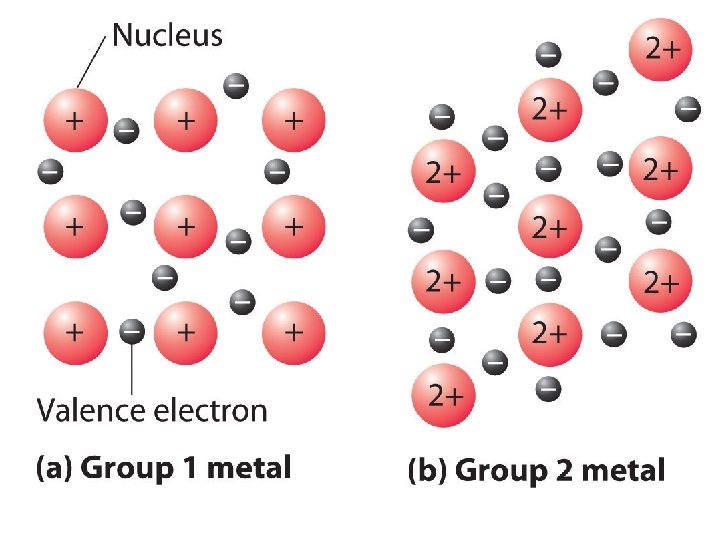
• Electron sea model – metals in a solid contribute their valence electrons to form a “sea of electrons” – Valence electrons are not held by any specific atom and can move easily from one atom to the next • Delocalized electrons –are free to move in the “sea of electrons”
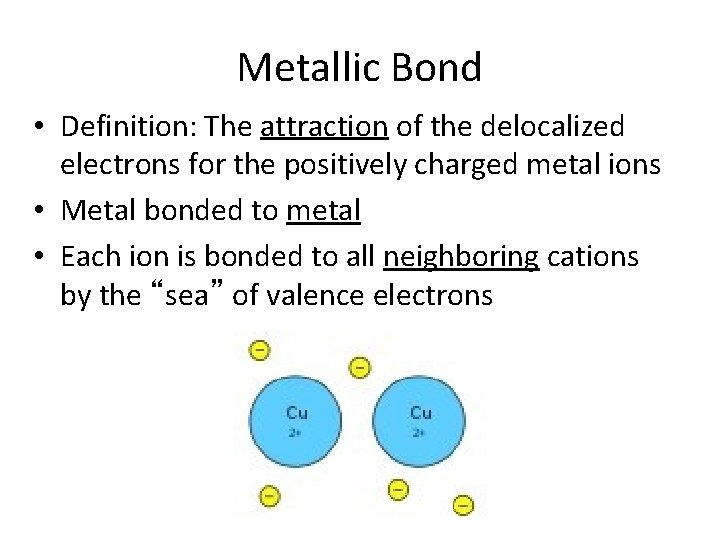
Metallic Bond • Definition: The attraction of the delocalized electrons for the positively charged metal ions • Metal bonded to metal • Each ion is bonded to all neighboring cations by the “sea” of valence electrons

Properties of Metals • In general, metals have moderately high melting and boiling points • Good conductors of heat and electricity • Malleable and Ductile – Mobile electrons can easily be pushed or pulled past each other
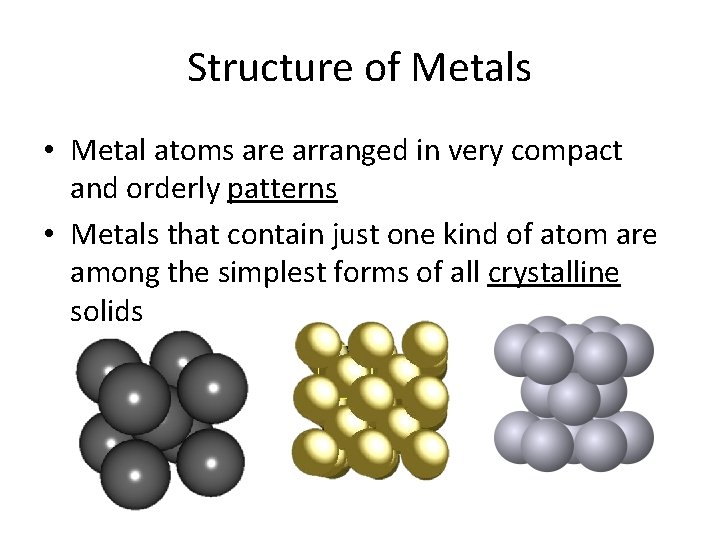
Structure of Metals • Metal atoms are arranged in very compact and orderly patterns • Metals that contain just one kind of atom are among the simplest forms of all crystalline solids

Metal Alloys • Alloy is a mixture of two or more elements (at least one must be a metal) • Alloy has metallic properties often superior to those of their component elements -Bronze: copper + tin -Steel: iron + carbon + other metals -Sterling silver: silver + copper
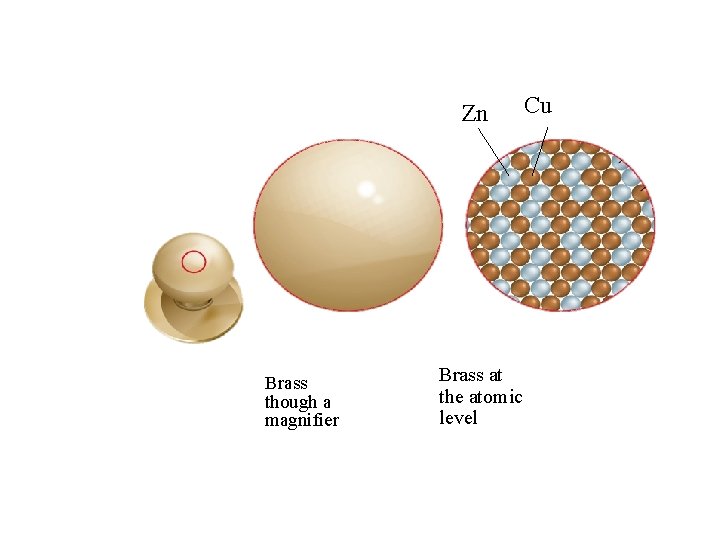
Zn Brass though a magnifier Brass at the atomic level Cu

2 common types of Alloys • Substitutional: one metal about the size of the other replaces it examples: Brass, pewter, 10 -carat gold • Interstitial: one metal smaller than the other fits between it example: Carbon steel
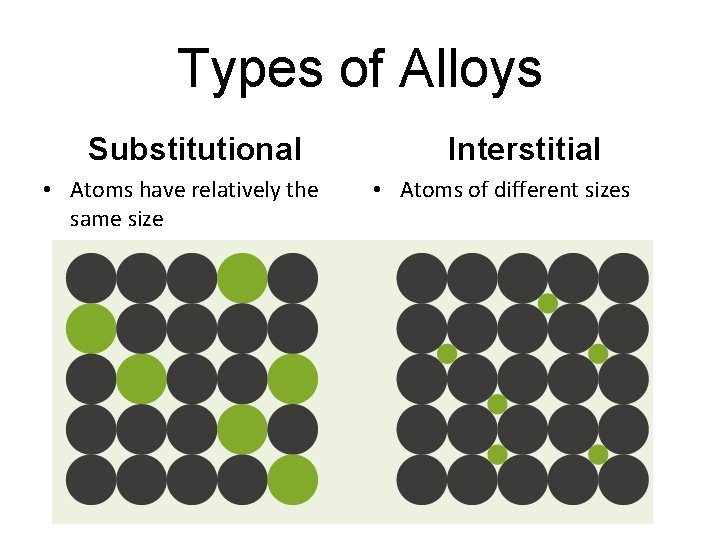
Types of Alloys Substitutional • Atoms have relatively the same size Interstitial • Atoms of different sizes
- Slides: 11