Nonmetals Section 20 2 and 20 3 Nonmetals





- Slides: 9

Nonmetals Section 20. 2 and 20. 3

Nonmetals n Nonmetals- gases or brittle solids at room temperature. – not malleable or ductile. – do not conduct heat or electricity well – not shiny n Nonmetals don’t conduct electricity because- electrons are strongly attracted to the nucleus

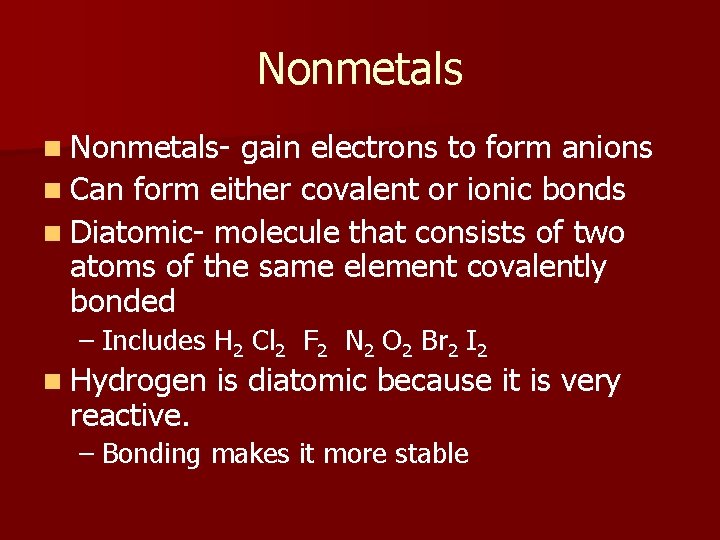
Nonmetals n Nonmetals- gain electrons to form anions n Can form either covalent or ionic bonds n Diatomic- molecule that consists of two atoms of the same element covalently bonded – Includes H 2 Cl 2 F 2 N 2 O 2 Br 2 I 2 n Hydrogen reactive. is diatomic because it is very – Bonding makes it more stable

Nonmetals n Halogens- nonmetals from group 17. – very reactive. – 7 valence electrons – form reactive diatomic molecules with distinctive colors n Sublimation- changing directly from a solid to a vapor n Noble gases- stable due to full outer energy levels. – don’t make compounds naturally

Metalloid & Allotropes n Metalloid- can form ionic or covalent bonds. – properties of both metals and nonmetals n Allotrope- element different forms of the same – have different molecular structures n Semiconductor- element that conducts electric current under certain conditionslike Silicon

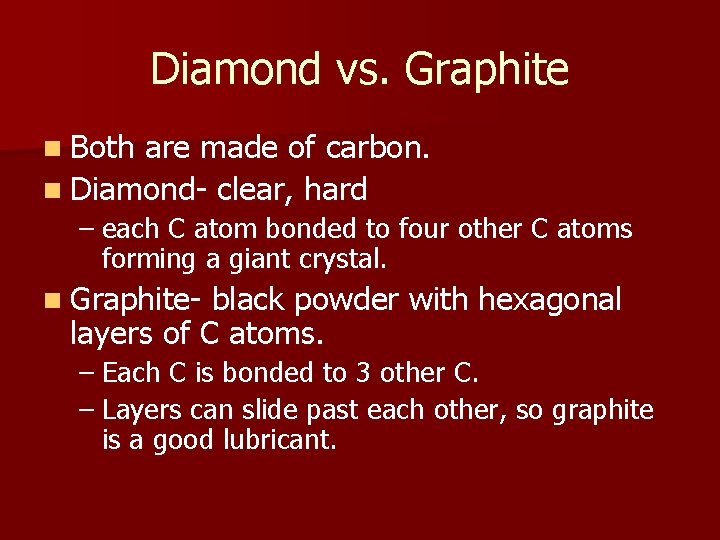
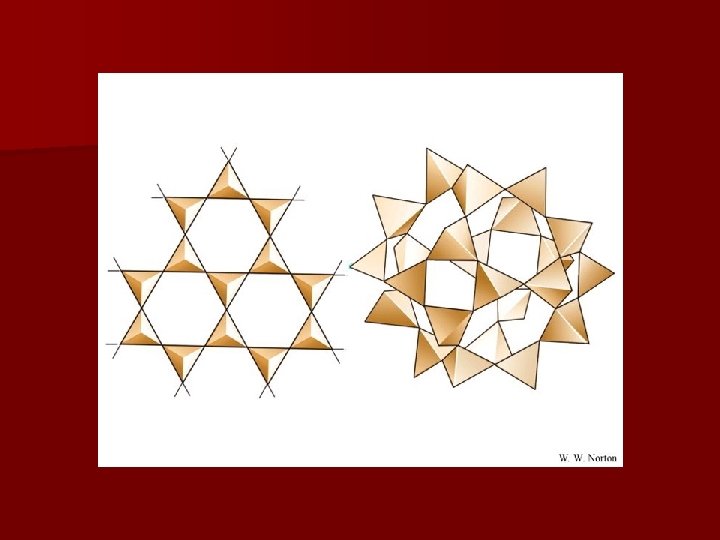
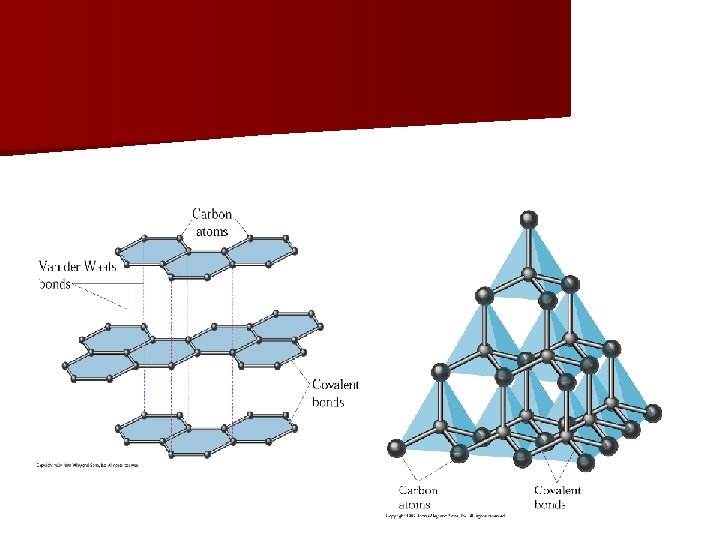
Diamond vs. Graphite n Both are made of carbon. n Diamond- clear, hard – each C atom bonded to four other C atoms forming a giant crystal. n Graphite- black powder with hexagonal layers of C atoms. – Each C is bonded to 3 other C. – Layers can slide past each other, so graphite is a good lubricant.



Synthetic Elements n Transuranium elements - elements that have an atomic number greater than 92 (uranium). – Synthetic (man made) and unstable n Scientists make elements to gain an understanding of the forces holding the nucleus together – To develop new technologies