Ionic Compound a metal reacts with a nonmetal


- Slides: 13


Ionic Compound – a metal reacts with a nonmetal • Ionic bonds form when an atom that loses electrons easily reacts with an atom that has a high affinity for electrons. • The charged ions are held together by their mutual attraction (Coulombic attraction). • Ionic bonds form because the ion pair has lower energy than the separated ions. • All bonds form in order to reach a lower energy level.

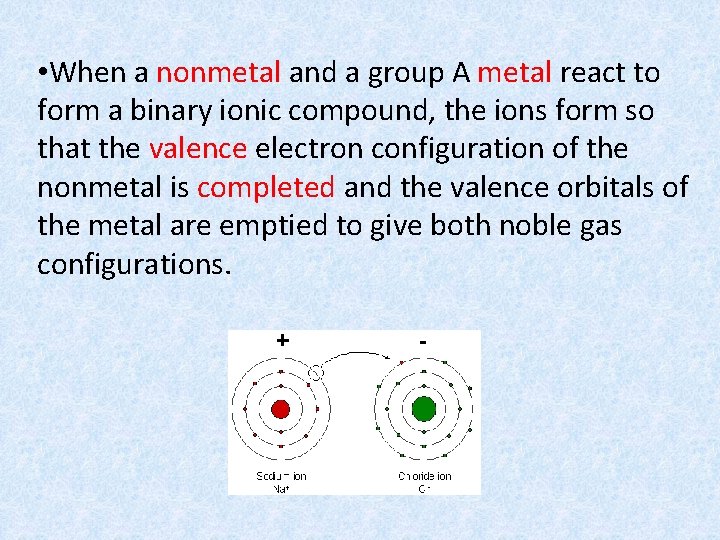
• When a nonmetal and a group A metal react to form a binary ionic compound, the ions form so that the valence electron configuration of the nonmetal is completed and the valence orbitals of the metal are emptied to give both noble gas configurations.

Ions form to get noble gas configurations: -exceptions in Group A metals Sn 2+ and Sn 4+ Pb 2+ and Pb 4+ Bi 3+ and Bi 5+ Tl+ and Tl 3+ Metals with d electrons will lose their highest numerical energy level electrons before losing their inner d electrons. •

Size of Ions: • Positive ions (cations) are smaller than their parent atoms since they are losing electrons. (More protons than electrons = greater nuclear pull) • Negative ions (anions) are larger than their parent atoms since they are gaining electrons. (Fewer protons than electrons = lower nuclear pull) Think: Monster Ants and miniature cats

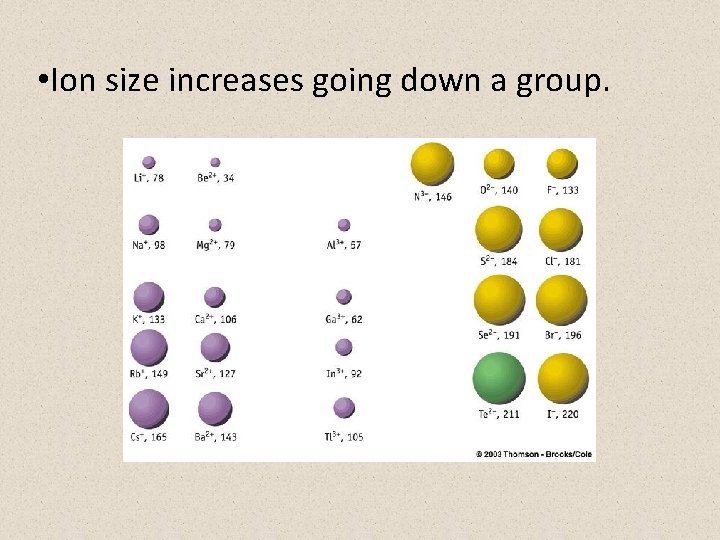
• Ion size increases going down a group.

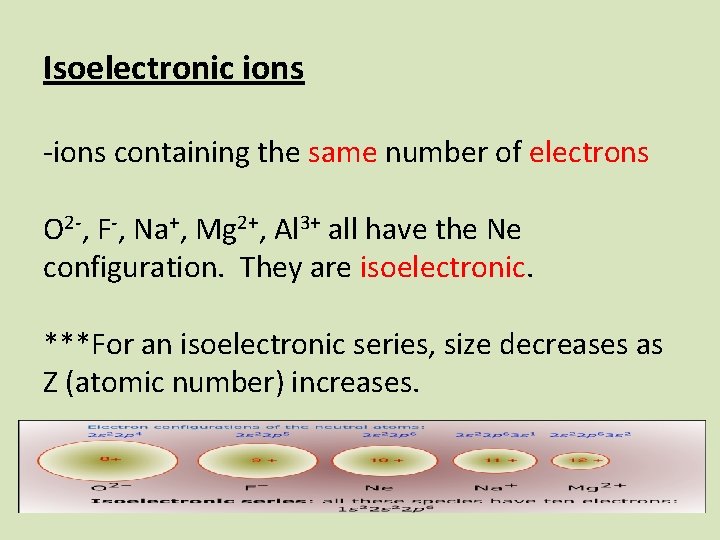
Isoelectronic ions -ions containing the same number of electrons O 2 -, F-, Na+, Mg 2+, Al 3+ all have the Ne configuration. They are isoelectronic. ***For an isoelectronic series, size decreases as Z (atomic number) increases.

Lattice Energy • Lattice energy – the change in energy that takes place when separated gaseous ions are packed together to form an ionic solid: M+(g) + X- (g) → MX(s) • Often defined as the energy released when an ionic solid forms from its ions. • By convention, lattice energy has a negative sign.

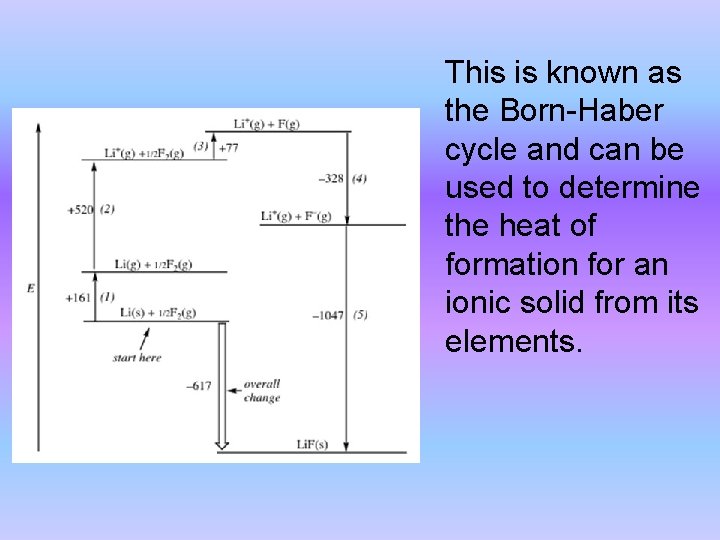
• Recall that energy is a state function. • To illustrate the energy changes involved in the formation of an ionic solid the process can be broken into steps. • The sum of the steps gives the overall reaction. • Consider the formation of solid lithium fluoride from its elements: Li(s) + ½ F 2(g) → Li. F(s) Step 1: Sublimation of solid lithium Li(s) → Li(g) ∆Hsub = 161 kj/mol

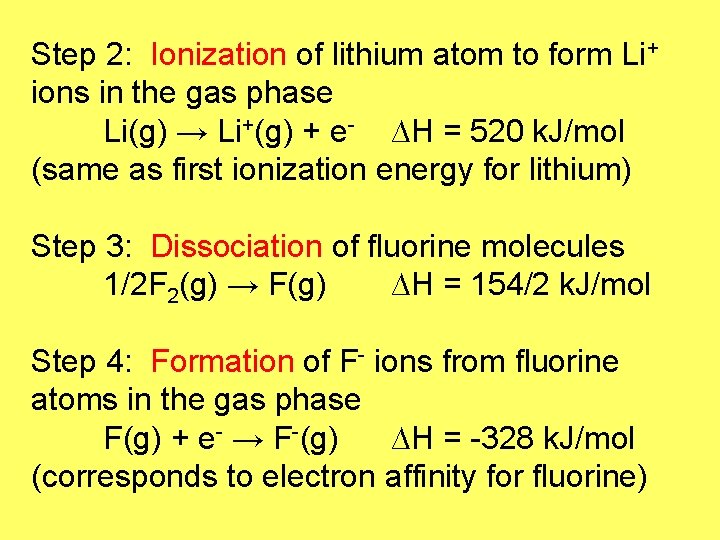
Step 2: Ionization of lithium atom to form Li+ ions in the gas phase Li(g) → Li+(g) + e- ∆H = 520 k. J/mol (same as first ionization energy for lithium) Step 3: Dissociation of fluorine molecules 1/2 F 2(g) → F(g) ∆H = 154/2 k. J/mol Step 4: Formation of F- ions from fluorine atoms in the gas phase F(g) + e- → F-(g) ∆H = -328 k. J/mol (corresponds to electron affinity for fluorine)

Step 5: Formation of solid lithium fluoride from the gaseous Li+ and F- ions Li+ (g) + F- (g) → Li. F(s) ∆H = -1047 k. J/mol (lattice energy for Li. F) The sum of these five processes yields the desired overall reaction, Overall: Li(s) + 1/2 F 2(g) → Li. F(s) the sum of the individual energy changes gives the overall energy change: -617 k. J/mol. This is the enthalpy of formation value for Li. F formed from its elements.

This is known as the Born-Haber cycle and can be used to determine the heat of formation for an ionic solid from its elements.

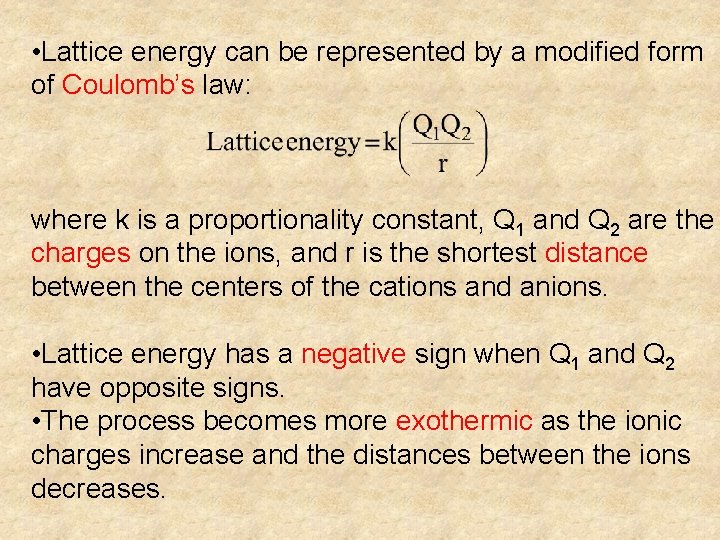
• Lattice energy can be represented by a modified form of Coulomb’s law: where k is a proportionality constant, Q 1 and Q 2 are the charges on the ions, and r is the shortest distance between the centers of the cations and anions. • Lattice energy has a negative sign when Q 1 and Q 2 have opposite signs. • The process becomes more exothermic as the ionic charges increase and the distances between the ions decreases.