Chapter 5 Section 1 History of the Periodic




- Slides: 8

Chapter 5 Section 1 History of the Periodic Table Lesson Starter Share what you have learned previously about the periodic table.

Chapter 5 Section 1 History of the Periodic Table Objectives • Explain the roles of Mendeleev and Moseley in the development of the periodic table. • Describe the modern periodic table. • Explain how the periodic law can be used to predict the physical and chemical properties of elements. • Describe how the elements belonging to a group of the periodic table are interrelated in terms of atomic number.

Chapter 5 Section 1 History of the Periodic Table Mendeleev and Chemical Periodicity • Mendeleev noticed that when the elements were arranged in order of increasing atomic mass, certain similarities in their chemical properties appeared at regular intervals. • Repeating patterns are referred to as periodic. • Mendeleev created a table in which elements with similar properties were grouped together—a periodic table of the elements.

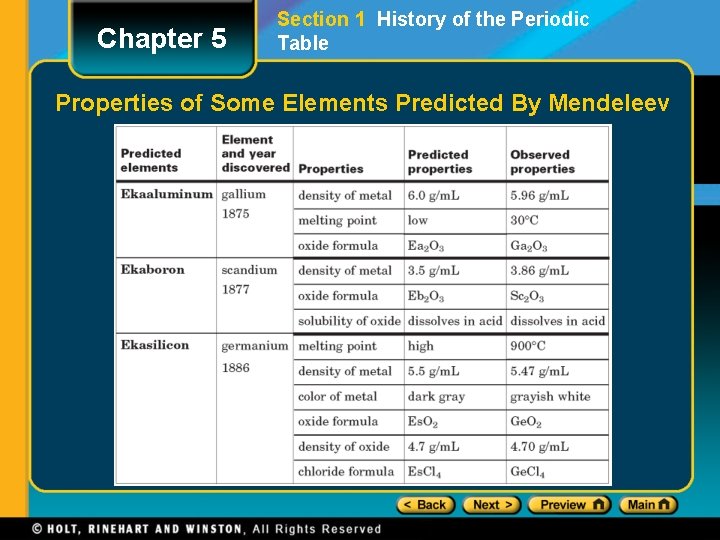
Chapter 5 Section 1 History of the Periodic Table Mendeleev and Chemical Periodicity, continued • After Mendeleev placed all the known elements in his periodic table, several empty spaces were left. • In 1871 Mendeleev predicted the existence and properties of elements that would fill three of the spaces. • By 1886, all three of these elements had been discovered.

Chapter 5 Section 1 History of the Periodic Table Properties of Some Elements Predicted By Mendeleev

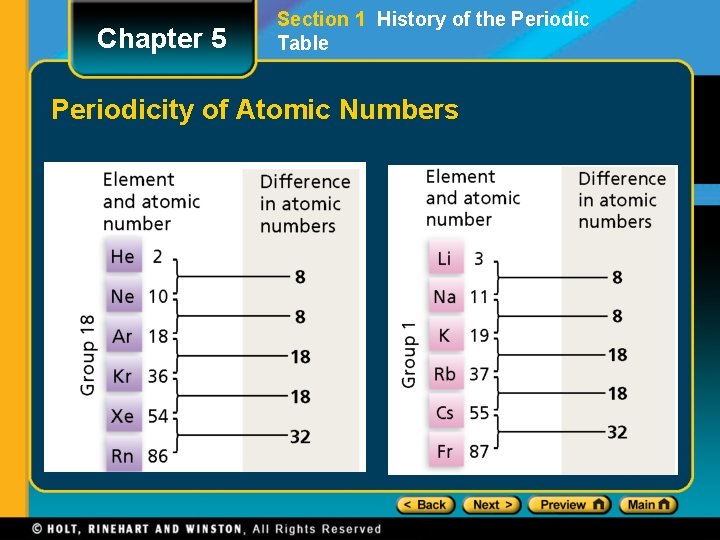
Chapter 5 Section 1 History of the Periodic Table Moseley and the Periodic Law • In 1911, the English scientist Henry Moseley discovered that the elements fit into patterns better when they were arranged according to atomic number, rather than atomic weight. • The Periodic Law states that the physical and chemical properties of the elements are periodic functions of their atomic numbers.

Chapter 5 Section 1 History of the Periodic Table Periodicity of Atomic Numbers

Chapter 5 Section 1 History of the Periodic Table The Modern Periodic Table • The Periodic Table is an arrangement of the elements in order of their atomic numbers so that elements with similar properties fall in the same column, or group.
 Chapter 5 section 1 history of the periodic table
Chapter 5 section 1 history of the periodic table 6 the periodic table
6 the periodic table Chapter 6 periodic table
Chapter 6 periodic table The periodic table and periodic law chapter 6
The periodic table and periodic law chapter 6 Trends in the periodic table
Trends in the periodic table Periodic family names
Periodic family names Chapter 17 section 3 luther leads the reformation
Chapter 17 section 3 luther leads the reformation Chapter 30 section 2 world history
Chapter 30 section 2 world history What was the counter-reformation?
What was the counter-reformation?