Periodic Table Elements Elements are pure substances made
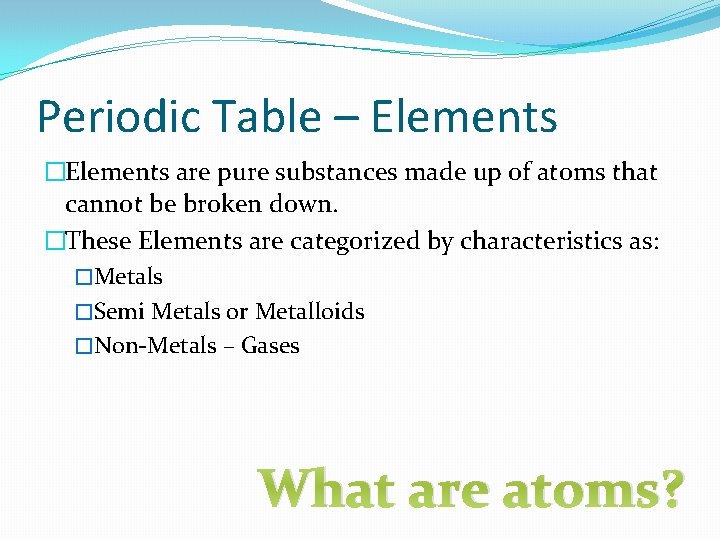
Periodic Table – Elements �Elements are pure substances made up of atoms that cannot be broken down. �These Elements are categorized by characteristics as: �Metals �Semi Metals or Metalloids �Non-Metals – Gases What are atoms?
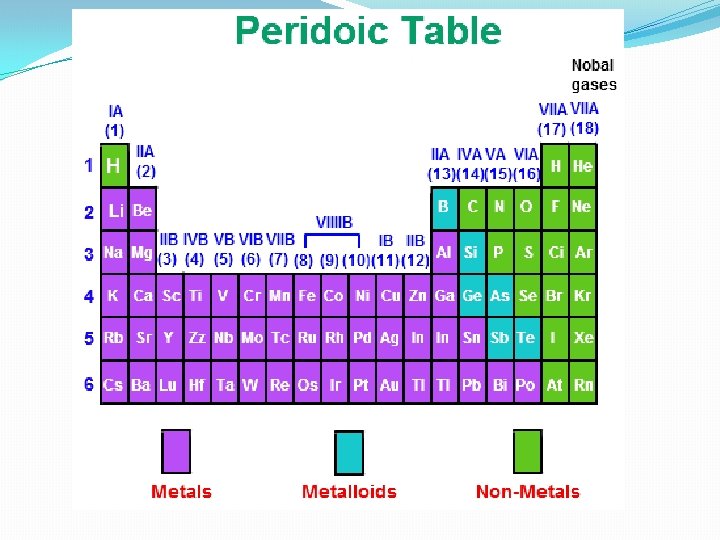
Characteristics of Metals �Metals have few electrons in their outer energy level �Metals are solid at room temperature except Mercury which is a liquid

Characteristics of Metals cont. . . �Metals are good conductors of heat and electricity �Metals are malleable which means they can be flattened into thin sheets �Metals are ductile which means they can be drawn into thin wires �Metals are shiny �https: //www. youtube. com/watch? v=c 382 zi. Upbbc �https: //www. youtube. com/watch? v=k. MR 5 Kb. NZ 7 b 8
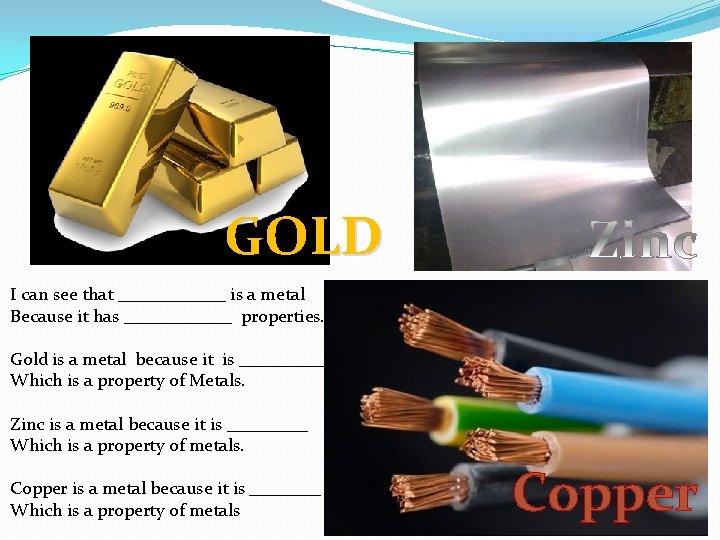
GOLD I can see that ______ is a metal Because it has ______ properties. Gold is a metal because it is _____ Which is a property of Metals. Zinc is a metal because it is _____ Which is a property of metals. Copper is a metal because it is ____ Which is a property of metals Copper

Characteristics of nonmetals �Nonmetals have almost a complete set of electrons in their outer energy level �Nonmetals are mostly gases at room temperature �Nonmetals are not good conductors of heat & electricity �Nonmetals are not malleable �Nonmetals are not ductile – they are brittle and will break or shatter when hit with a hammer �Nonmetals are not shiny �Nonmetals do not have the characteristics of metals
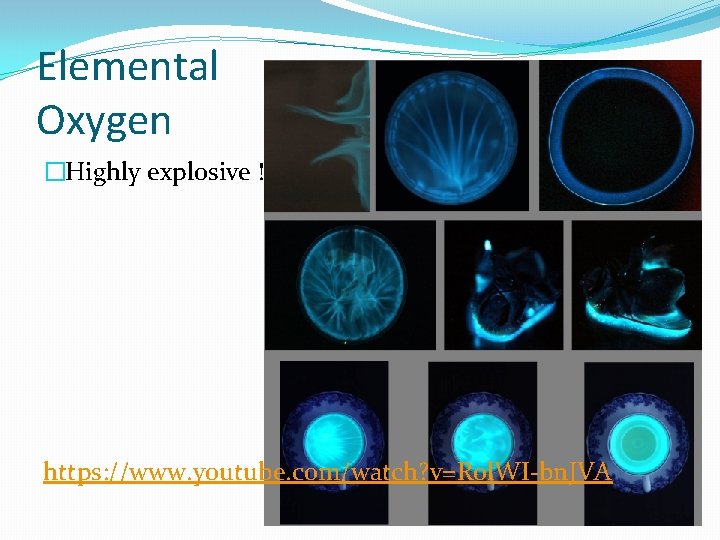
Elemental Oxygen �Highly explosive ! https: //www. youtube. com/watch? v=R 0 l. WI-bn. JVA
Characteristics of Semi Metals �Metalloids have a ½ complete set of electrons in their outer energy level �Metalloids have some properties of metals and some properties of nonmetals.
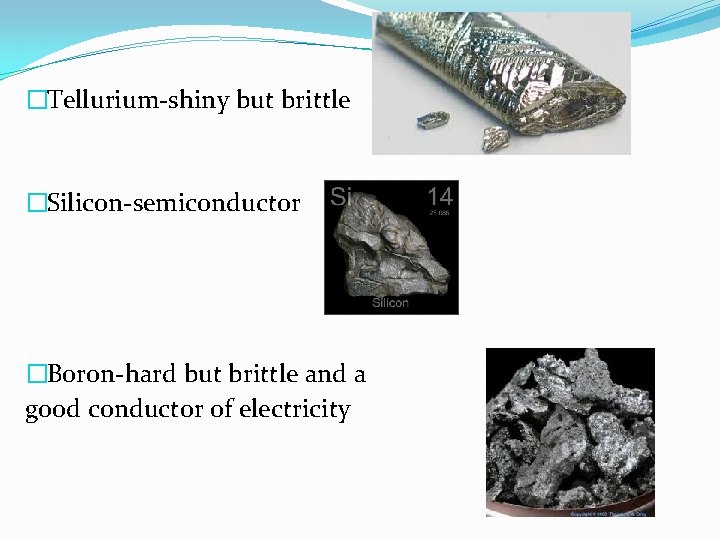
�Tellurium-shiny but brittle �Silicon-semiconductor �Boron-hard but brittle and a good conductor of electricity

Nobel gases �Noble Gases have a complete set of electrons in their outer energy level – all but Helium have 8. Helium has 2. �Noble Gases are gases at room temperature �Noble Gases are nonmetals �Noble Gases are colorless �Noble Gases are odorless �Noble Gases are unreactive �Noble Gases are found in very small amounts on Earth


Is this element Metal, Non-metal or Metalloid (Semimetal) ? Aluminum Tellurium Physical Properties: �Conductor �Shiny Luster �Malleable �Non-Magnetic �Not Ductile �Solid at room temperature Physical Properties: �Semiconductor �Shiny Luster �Brittle �Non Magnetic �Not Ductile �Solid at Room temperature

Is this element Metal, Non-metal or Metalloid (Semimetal) ? Copper Germanium Physical Properties: �Conductor �Orange Luster �Malleable �Not Magnetic �Ductile �Solid at room Temperature �Physical Properties: �Semiconductor �Shiny Luster �Brittle �Not Magnetic �Not Ductile �Solid at Room temperature

Is this element Metal, Non-metal or Metalloid (Semimetal) ? Sulfer Chlorine �No conduction �Yellow Color �Brittle �Non Magnetic �Not Ductile �Solid @ room temperature �No Conduction �Yellow Green Color �No Brittle / Malleable �Not Magnetic �Not Ductile �Gas at room Temp
Molecules and Compounds Monday October 5, 2015 How are compounds formed?
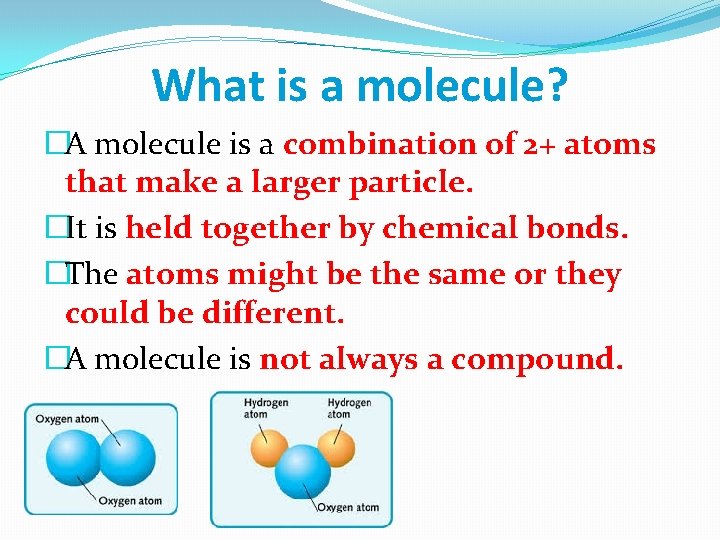
What is a molecule? �A molecule is a combination of 2+ atoms that make a larger particle. �It is held together by chemical bonds. �The atoms might be the same or they could be different. �A molecule is not always a compound.
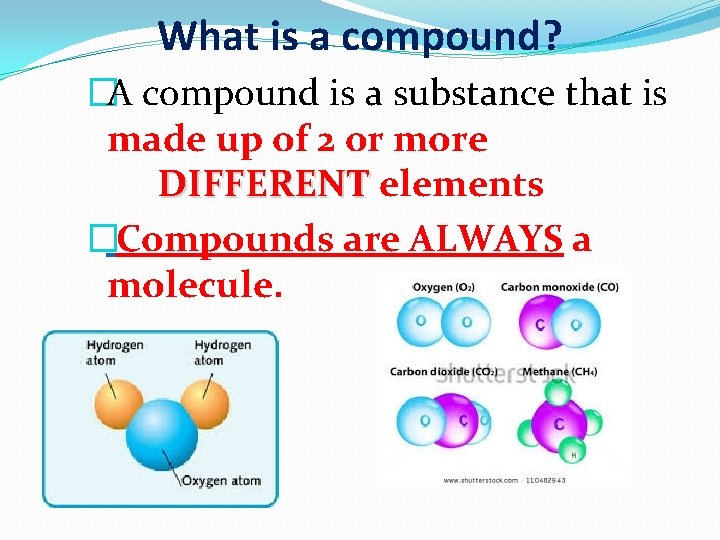
What is a compound? �A compound is a substance that is made up of 2 or more DIFFERENT elements �Compounds are ALWAYS a molecule.

Properties of the compound are different than the properties of the elements that make them up. Compound H 2 0 Na. Cl Element property Common Compound Property H= gas O= gas liquid Na=dangerous metal Cl=dangerous nonmetal Na. Cl=table salt (eat it!)

�For a compound to be formed a chemical reaction must take place. This is when 2 elements or molecules are rearranged through a chemical process to form something NEW and DIFFERENT!
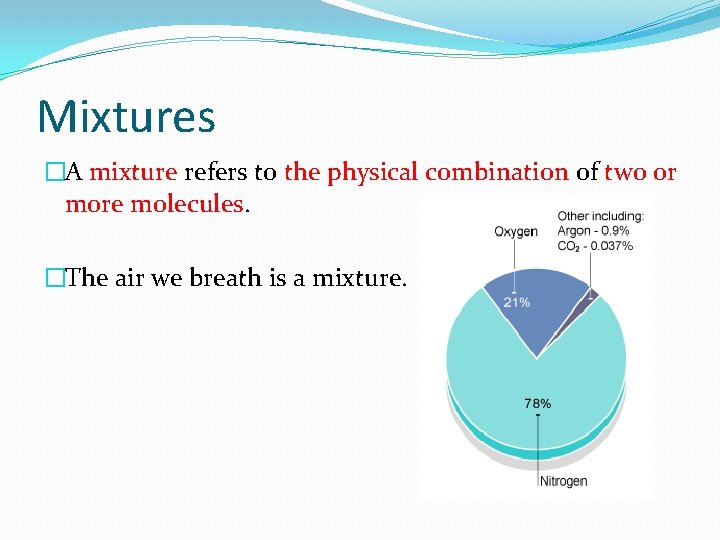
Mixtures �A mixture refers to the physical combination of two or more molecules. �The air we breath is a mixture.
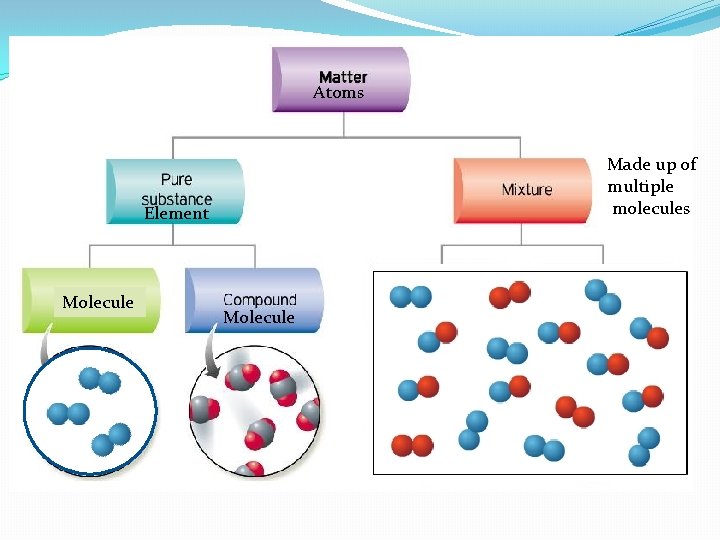
Atoms Made up of multiple molecules Element Molecule
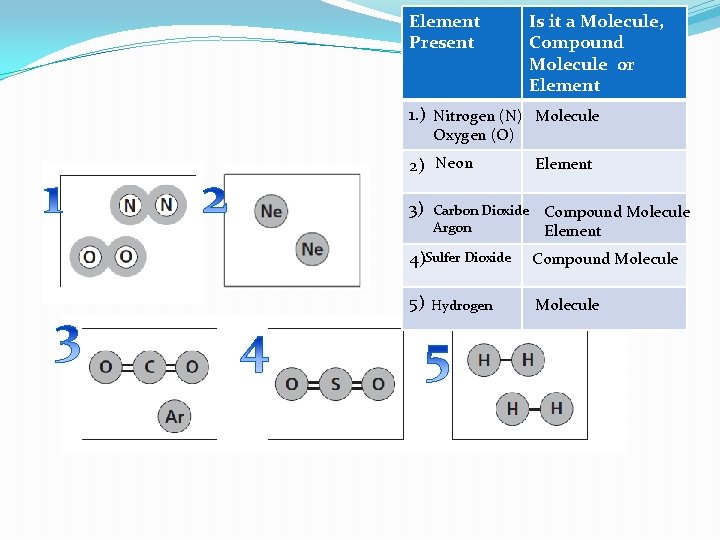
Element Present Is it a Molecule, Compound Molecule 0 r Element 1. ) Nitrogen (N) Molecule Oxygen (O) 2) Neon 3) Carbon Dioxide Argon Element Compound Molecule Element 4)Sulfer Dioxide Compound Molecule 5) Molecule Hydrogen
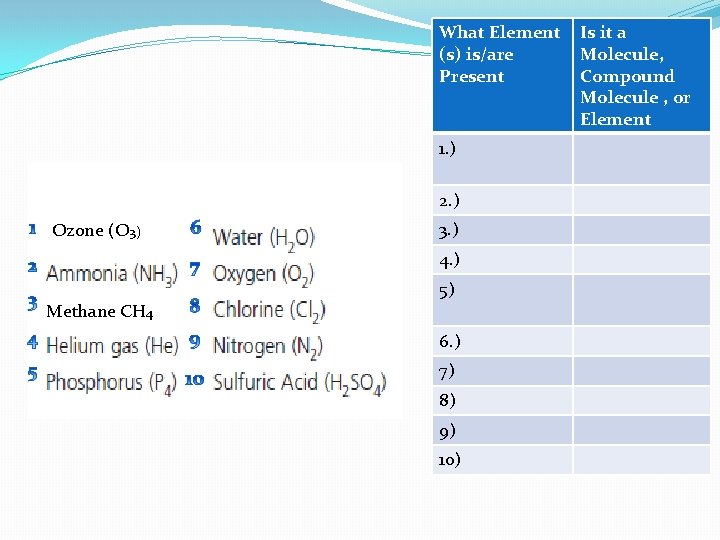
What Element (s) is/are Present 1. ) 2. ) Ozone (O 3) 3. ) 4. ) 5) Methane CH 4 6. ) 7) 8) 9) 10) Is it a Molecule, Compound Molecule , or Element
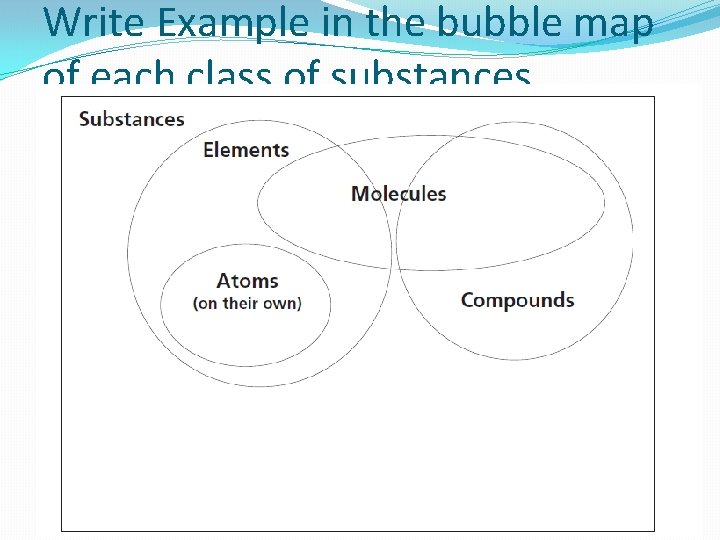
Write Example in the bubble map of each class of substances.
- Slides: 24