QHesss Law Prem Sattsangi Copyright 2007 2 Hesss




- Slides: 11

Q-Hess’s Law Prem Sattsangi Copyright 2007

#2 -Hess’s Law Study • Please have your pencil and paper and BLB text book as you attempt these problem. • Write your response. • Press enter to check the correct answer.

#3 -Definitions BLB-Page 170 Please Define: SYSTEM: Portion of the Universe under study. SURROUNDINGS: Rest of the Universe.

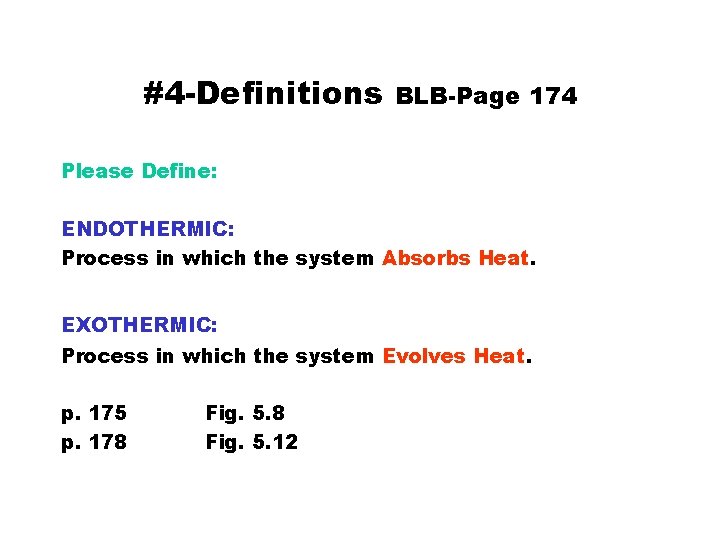
#4 -Definitions BLB-Page 174 Please Define: ENDOTHERMIC: Process in which the system Absorbs Heat. EXOTHERMIC: Process in which the system Evolves Heat. p. 175 p. 178 Fig. 5. 12

#5 -Definitions BLB-Page 183 Please Define: HEAT CAPACITY: Amount of Heat required to raise the temperature of an object by 1 o. C. SPECIFIC HEAT : HEAT CAPACITY per gram of a substance. OR Amount of Heat required to raise the temperature of 1 g of a substance by 1 o. C.

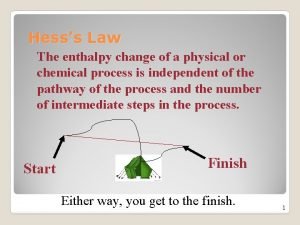
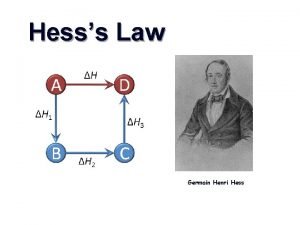
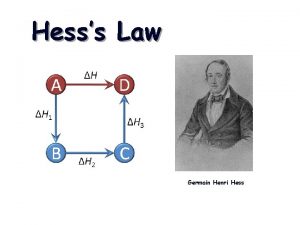
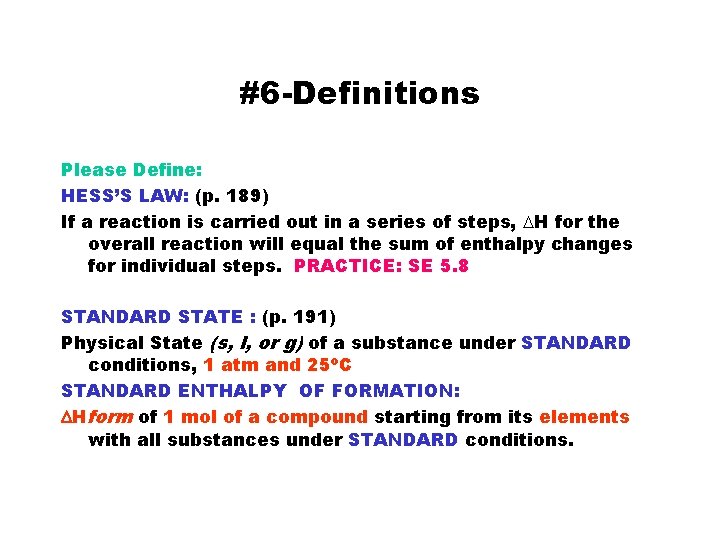
#6 -Definitions Please Define: HESS’S LAW: (p. 189) If a reaction is carried out in a series of steps, DH for the overall reaction will equal the sum of enthalpy changes for individual steps. PRACTICE: SE 5. 8 STANDARD STATE : (p. 191) Physical State (s, l, or g) of a substance under STANDARD conditions, 1 atm and 25 o. C STANDARD ENTHALPY OF FORMATION: DHform of 1 mol of a compound starting from its elements with all substances under STANDARD conditions.

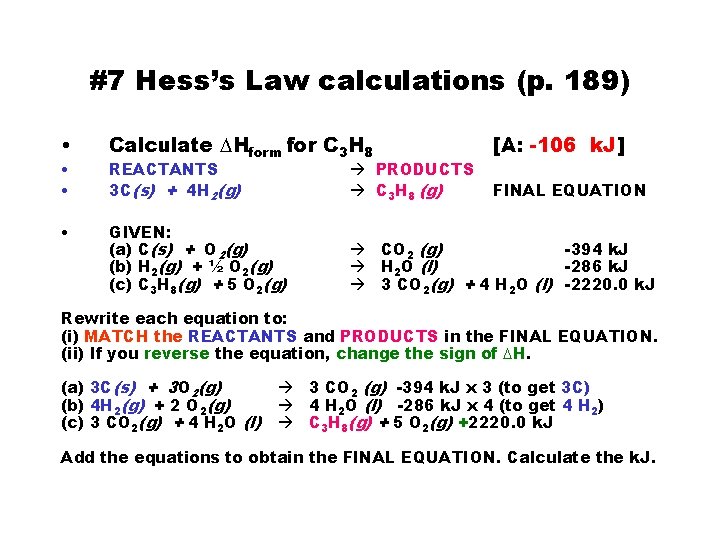
#7 Hess’s Law calculations (p. 189) • • Calculate DHform for C 3 H 8 [A: -106 k. J] REACTANTS 3 C(s) + 4 H 2(g) PRODUCTS C 3 H 8 (g) GIVEN: (a) C(s) + O 2(g) (b) H 2(g) + ½ O 2(g) (c) C 3 H 8(g) + 5 O 2(g) CO 2 (g) -394 k. J H 2 O (l) -286 k. J 3 CO 2(g) + 4 H 2 O (l) -2220. 0 k. J FINAL EQUATION Rewrite each equation to: (i) MATCH the REACTANTS and PRODUCTS in the FINAL EQUATION. (ii) If you reverse the equation, change the sign of DH. (a) 3 C(s) + 3 O 2(g) (b) 4 H 2(g) + 2 O 2(g) (c) 3 CO 2(g) + 4 H 2 O (l) 3 CO 2 (g) -394 k. J x 3 (to get 3 C) 4 H 2 O (l) -286 k. J x 4 (to get 4 H 2) C 3 H 8(g) + 5 O 2(g) +2220. 0 k. J Add the equations to obtain the FINAL EQUATION. Calculate the k. J.

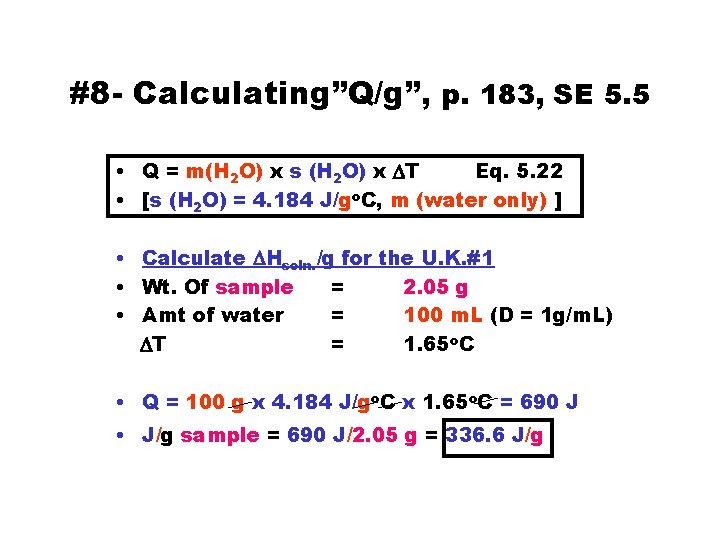
#8 - Calculating”Q/g”, p. 183, SE 5. 5 • Q = m(H 2 O) x s (H 2 O) x DT Eq. 5. 22 • [s (H 2 O) = 4. 184 J/go. C, m (water only) ] • Calculate DHsoln. /g for the U. K. #1 • Wt. Of sample = 2. 05 g • Amt of water = 100 m. L (D = 1 g/m. L) DT = 1. 65 o. C • Q = 100 g x 4. 184 J/go. C x 1. 65 o. C = 690 J • J/g sample = 690 J/2. 05 g = 336. 6 J/g

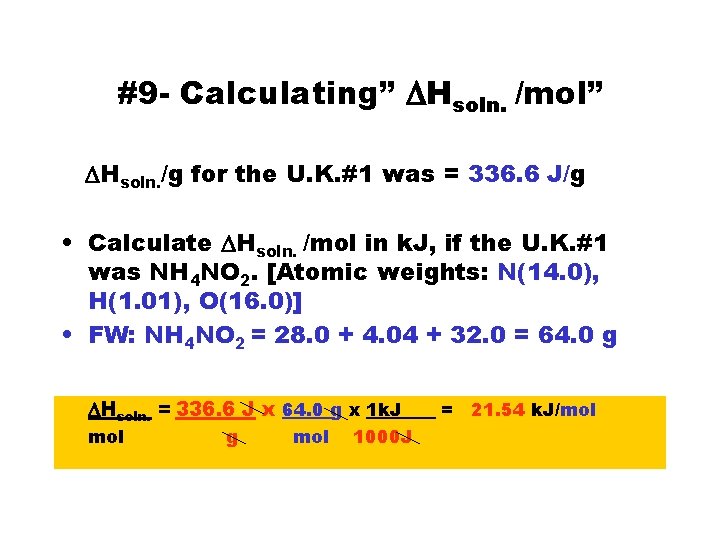
#9 - Calculating” DHsoln. /mol” DHsoln. /g for the U. K. #1 was = 336. 6 J/g • Calculate DHsoln. /mol in k. J, if the U. K. #1 was NH 4 NO 2. [Atomic weights: N(14. 0), H(1. 01), O(16. 0)] • FW: NH 4 NO 2 = 28. 0 + 4. 04 + 32. 0 = 64. 0 g DHsoln. = 336. 6 J x 64. 0 g x 1 k. J___ = 21. 54 k. J/mol g mol 1000 J

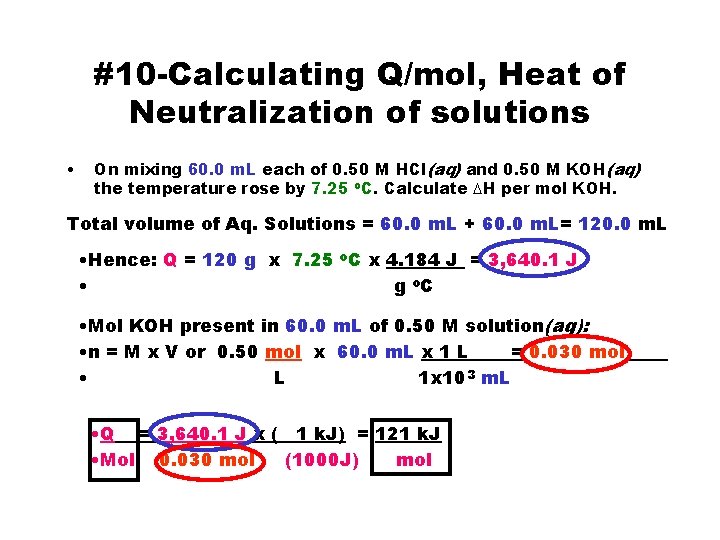
#10 -Calculating Q/mol, Heat of Neutralization of solutions • On mixing 60. 0 m. L each of 0. 50 M HCl(aq) and 0. 50 M KOH(aq) the temperature rose by 7. 25 o. C. Calculate DH per mol KOH. Total volume of Aq. Solutions = 60. 0 m. L + 60. 0 m. L= 120. 0 m. L • Hence: Q = 120 g x 7. 25 o. C x 4. 184 J = 3, 640. 1 J • g o. C • Mol KOH present in 60. 0 m. L of 0. 50 M solution(aq): • n = M x V or 0. 50 mol x 60. 0 m. L x 1 L = 0. 030 mol • L 1 x 10 3 m. L • Q = 3, 640. 1 J x ( 1 k. J) = 121 k. J • Mol 0. 030 mol (1000 J) mol

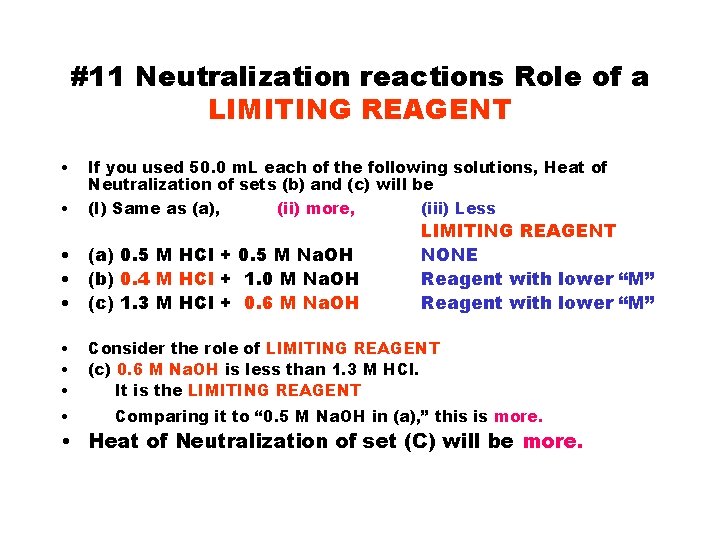
#11 Neutralization reactions Role of a LIMITING REAGENT • • If you used 50. 0 m. L each of the following solutions, Heat of Neutralization of sets (b) and (c) will be (I) Same as (a), (ii) more, (iii) Less • (a) 0. 5 M HCl + 0. 5 M Na. OH • (b) 0. 4 M HCl + 1. 0 M Na. OH • (c) 1. 3 M HCl + 0. 6 M Na. OH • • LIMITING REAGENT NONE Reagent with lower “M” Consider the role of LIMITING REAGENT (c) 0. 6 M Na. OH is less than 1. 3 M HCl. It is the LIMITING REAGENT Comparing it to “ 0. 5 M Na. OH in (a), ” this is more. • Heat of Neutralization of set (C) will be more.