Thermodynamics 1 2 1 Enthalpy 2 1 Enthalpy







- Slides: 15

Thermodynamics 1. 2. 1 Enthalpy

2. 1 Enthalpy ¥ Enthalpy is the heat content of a system, or the amount of energy within a substance, both kinetic and potential. ¥ Every substance possesses both potential energy, and kinetic energy from to the constant motion of the particles. This total amount of energy is enthalpy.

Enthalpy ¥ Symbol for enthalpy: H ¥ Unit for enthalpy: the joule, J

Heat of Reaction ¥ It isn’t possible to directly measure the heat content of a substance- chemists can only measure how much enthalpy changes ¥ Therefore, we will generally refer to the change in enthalpy, or ΔH. This is also know as the heat of the reaction.

Exothermic Reactions ¥ ¥ ¥ During exothermic reactions, more energy is released during bond formation than is required to break bonds. An exothermic reaction is written like this: Cu(s) + Cl 2(g) → Cu. Cl 2(g) + 220. 1 k. J There is a net release of 220. 1 k. J of energy, this appears on the product side.

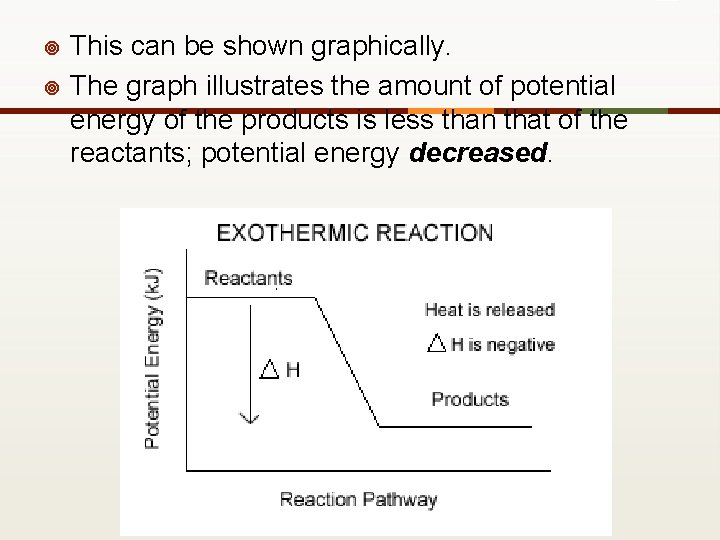
Exothermic reactions ¥ Exothermic reactions release energy because the reactants have more potential energy than the products. ¥ The "excess" energy is released to the surroundings.

¥ This can be shown graphically. The graph illustrates the amount of potential energy of the products is less than that of the reactants; potential energy decreased. _ ¥

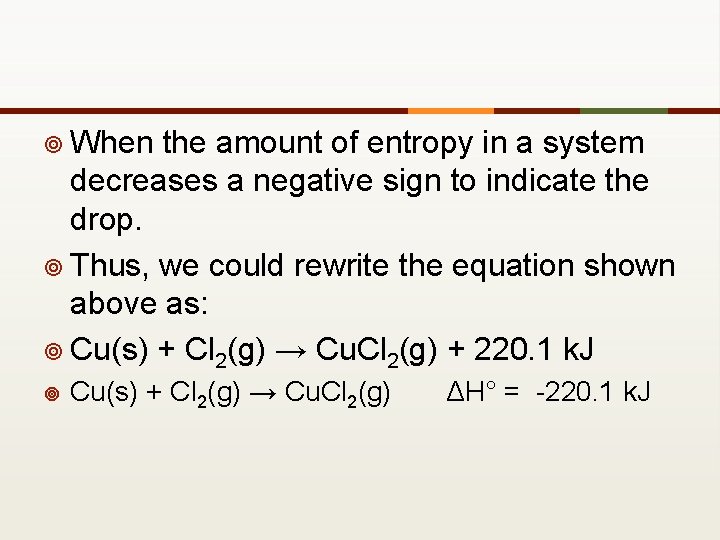
¥ When the amount of entropy in a system decreases a negative sign to indicate the drop. ¥ Thus, we could rewrite the equation shown above as: ¥ Cu(s) + Cl 2(g) → Cu. Cl 2(g) + 220. 1 k. J ¥ Cu(s) + Cl 2(g) → Cu. Cl 2(g) ΔH° = -220. 1 k. J

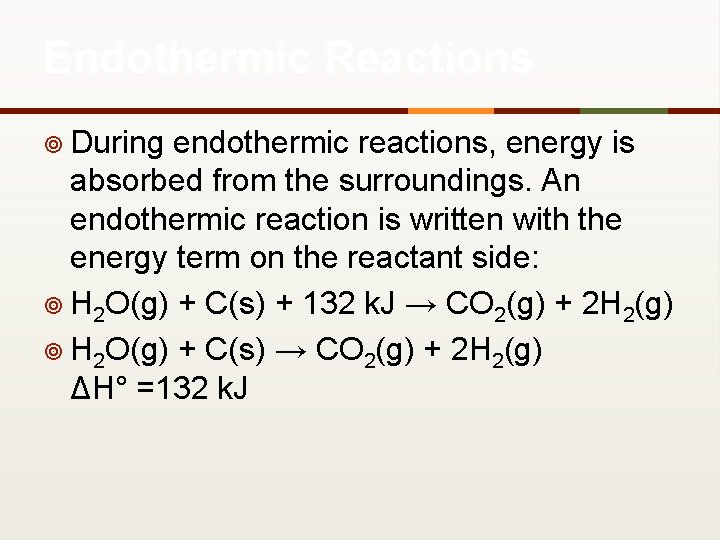
Endothermic Reactions ¥ During endothermic reactions, energy is absorbed from the surroundings. An endothermic reaction is written with the energy term on the reactant side: ¥ H 2 O(g) + C(s) + 132 k. J → CO 2(g) + 2 H 2(g) ¥ H 2 O(g) + C(s) → CO 2(g) + 2 H 2(g) ΔH° =132 k. J

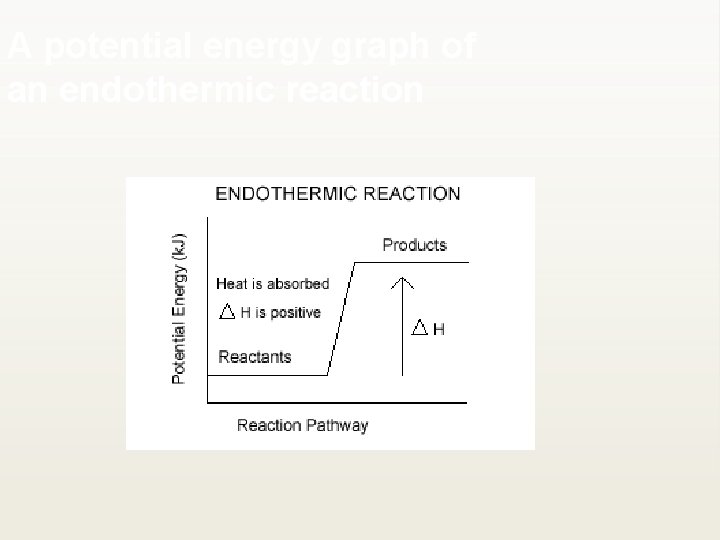
Endothermic reactions ¥ An input of energy is required ¥ The potential energy of the reactants is lower than the potential energy of the products; energy must be added to the reaction for it to occur.

A potential energy graph of an endothermic reaction

¥ We can remove the energy term from the equation and write it as a positive value, indicating that enthalpy increased: ¥ H 2 O(g) + C(s) → CO 2(g) + 2 H 2(g) ΔH° = +132 k. J

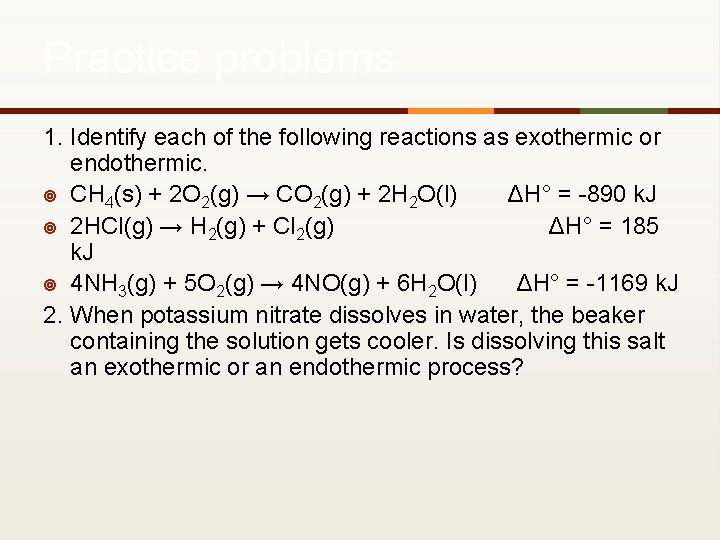
Practice problems 1. Identify each of the following reactions as exothermic or endothermic. ¥ CH 4(s) + 2 O 2(g) → CO 2(g) + 2 H 2 O(l) ΔH° = -890 k. J ¥ 2 HCl(g) → H 2(g) + Cl 2(g) ΔH° = 185 k. J ¥ 4 NH 3(g) + 5 O 2(g) → 4 NO(g) + 6 H 2 O(l) ΔH° = -1169 k. J 2. When potassium nitrate dissolves in water, the beaker containing the solution gets cooler. Is dissolving this salt an exothermic or an endothermic process?

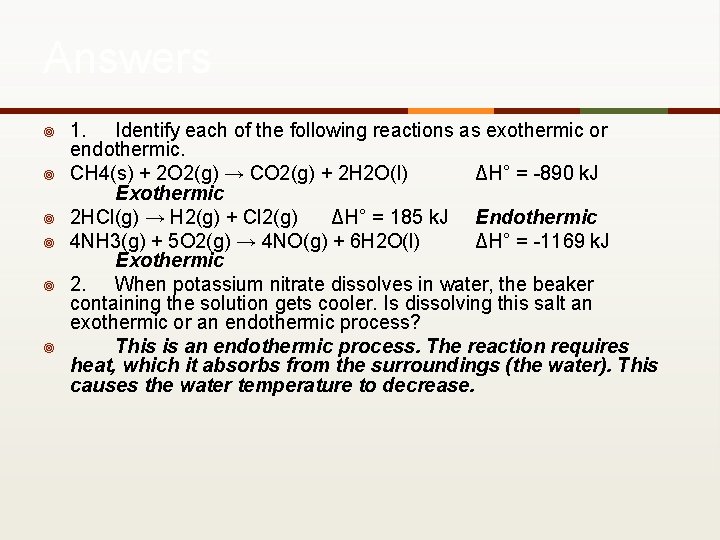
Answers ¥ ¥ ¥ 1. Identify each of the following reactions as exothermic or endothermic. CH 4(s) + 2 O 2(g) → CO 2(g) + 2 H 2 O(l) ΔH° = -890 k. J Exothermic 2 HCl(g) → H 2(g) + Cl 2(g) ΔH° = 185 k. J Endothermic 4 NH 3(g) + 5 O 2(g) → 4 NO(g) + 6 H 2 O(l) ΔH° = -1169 k. J Exothermic 2. When potassium nitrate dissolves in water, the beaker containing the solution gets cooler. Is dissolving this salt an exothermic or an endothermic process? This is an endothermic process. The reaction requires heat, which it absorbs from the surroundings (the water). This causes the water temperature to decrease.

Assignment ¥ Assignment 1. 1. 1