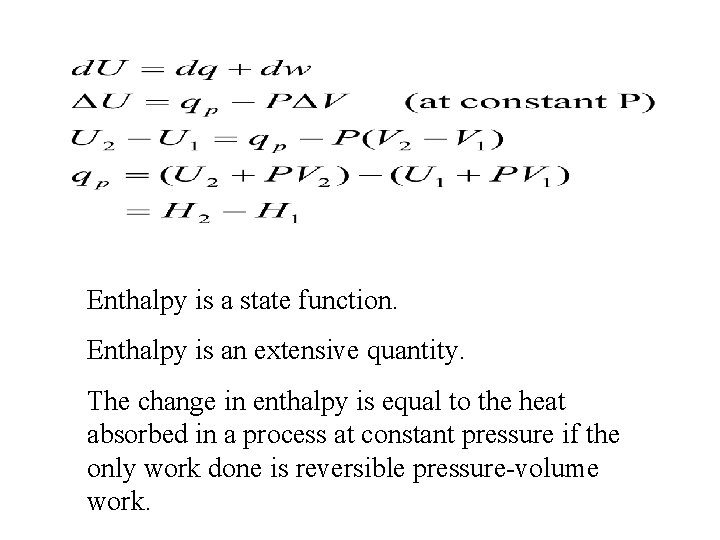
Enthalpy is a state function Enthalpy is an



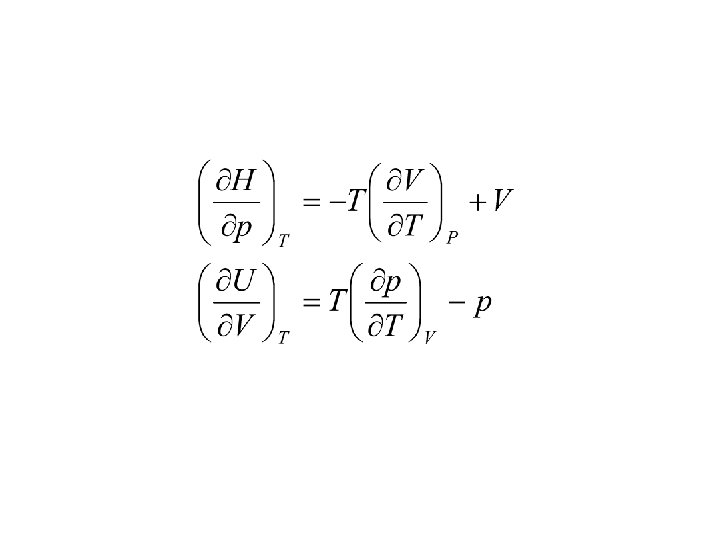





- Slides: 20

Enthalpy is a state function. Enthalpy is an extensive quantity. The change in enthalpy is equal to the heat absorbed in a process at constant pressure if the only work done is reversible pressure-volume work.

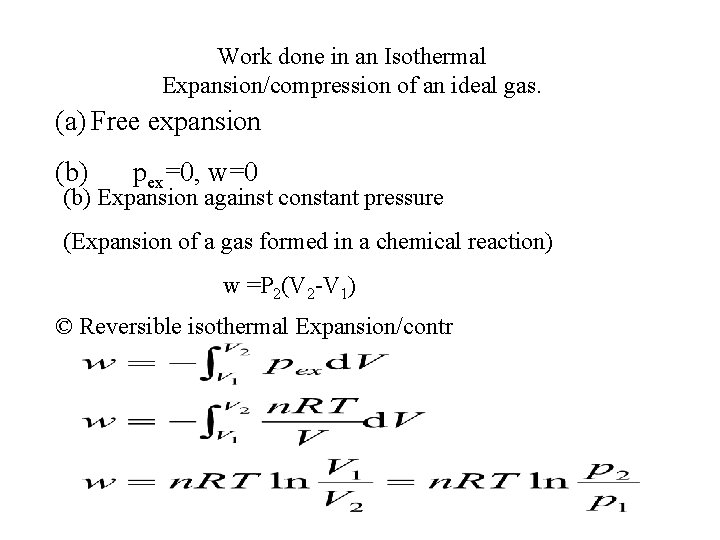
Work done in an Isothermal Expansion/compression of an ideal gas. (a) Free expansion (b) pex=0, w=0 (b) Expansion against constant pressure (Expansion of a gas formed in a chemical reaction) w =P 2(V 2 -V 1) © Reversible isothermal Expansion/contr

Internal Energy Change in an Isothermal Expansion/compression of an ideal Gas

Thermodynamics changes in an adiabatic process of an ideal gas.


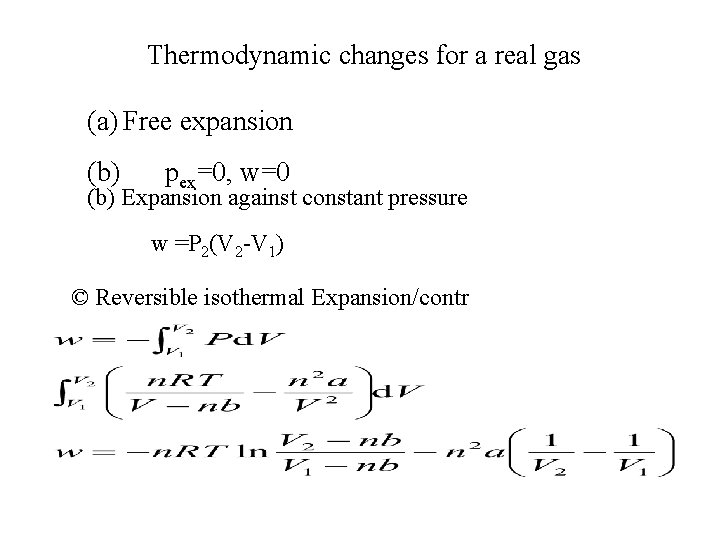
Thermodynamic changes for a real gas (a) Free expansion (b) pex=0, w=0 (b) Expansion against constant pressure w =P 2(V 2 -V 1) © Reversible isothermal Expansion/contr

Internal energy change in an isothermal process for a real gas.

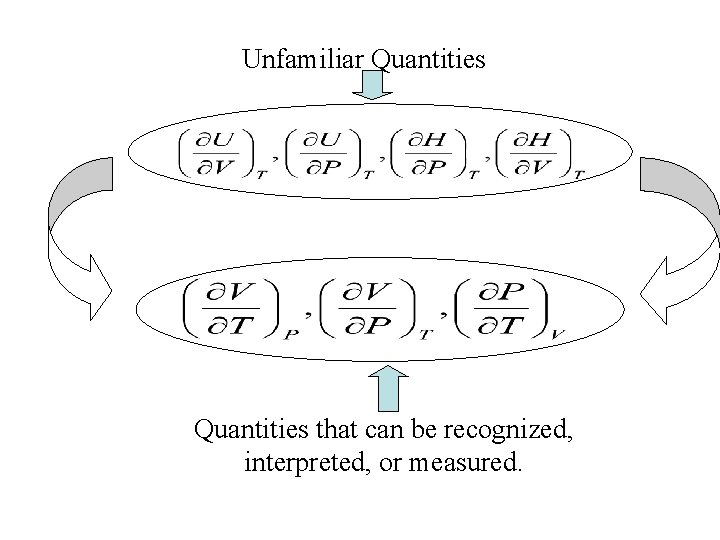
Unfamiliar Quantities that can be recognized, interpreted, or measured.

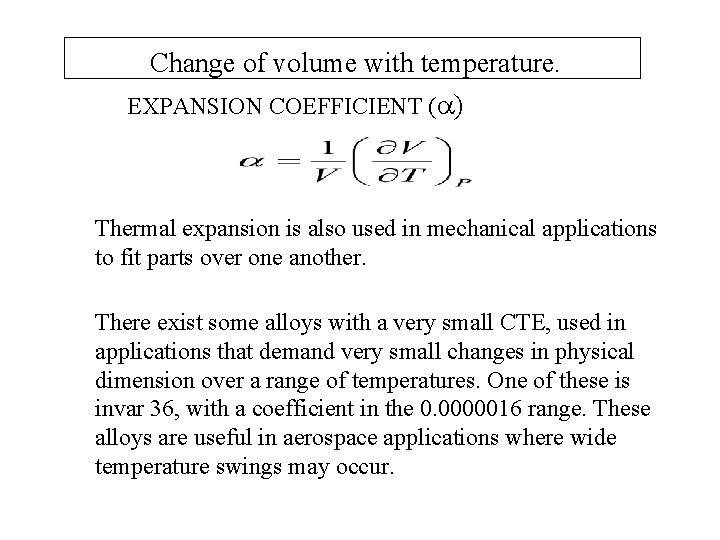
Change of volume with temperature. EXPANSION COEFFICIENT ( ) Thermal expansion is also used in mechanical applications to fit parts over one another. There exist some alloys with a very small CTE, used in applications that demand very small changes in physical dimension over a range of temperatures. One of these is invar 36, with a coefficient in the 0. 0000016 range. These alloys are useful in aerospace applications where wide temperature swings may occur.

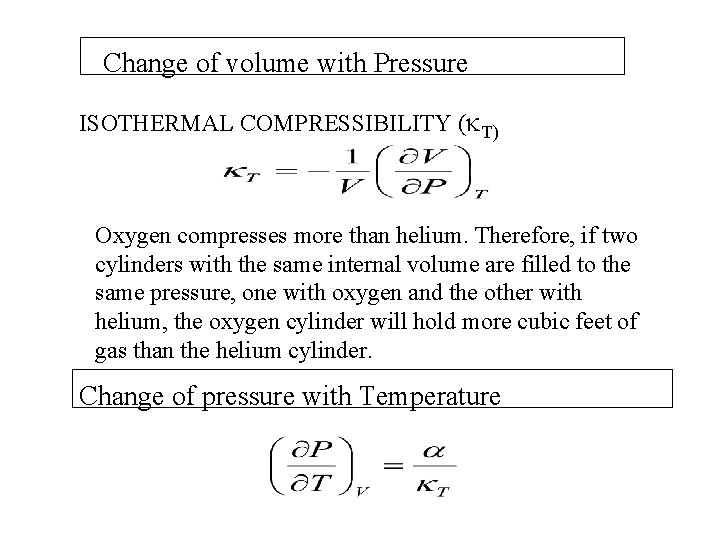
Change of volume with Pressure ISOTHERMAL COMPRESSIBILITY ( T) Oxygen compresses more than helium. Therefore, if two cylinders with the same internal volume are filled to the same pressure, one with oxygen and the other with helium, the oxygen cylinder will hold more cubic feet of gas than the helium cylinder. Change of pressure with Temperature

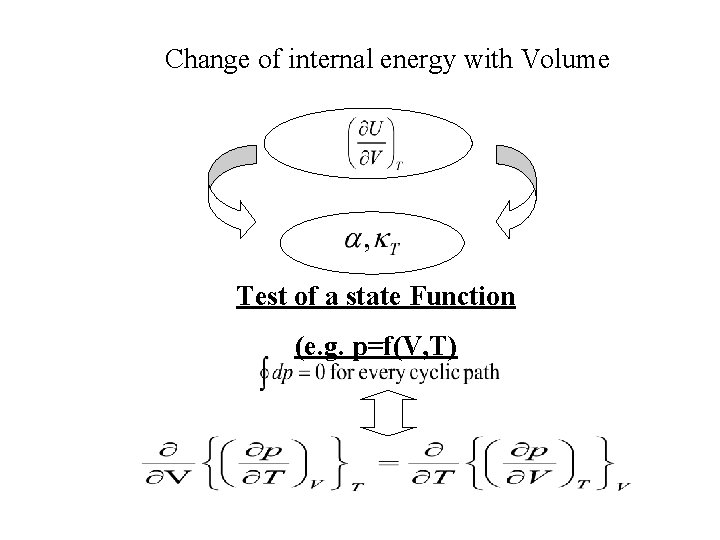
Change of internal energy with Volume Test of a state Function (e. g. p=f(V, T)
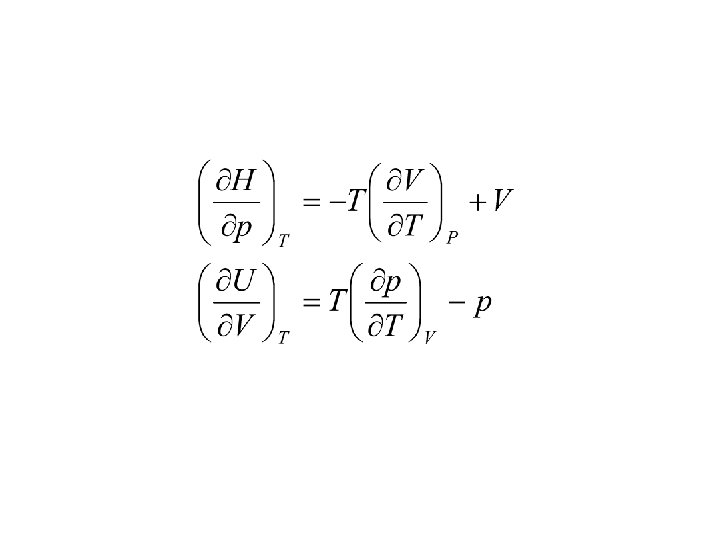

Internal energy change in an isothermal process for a real gas.

Internal energy change in an adiabatic process for a real gas.

Enthalpy changes in a thermodynamic Processes.

Changes in Internal energy with temperature at constant pressure

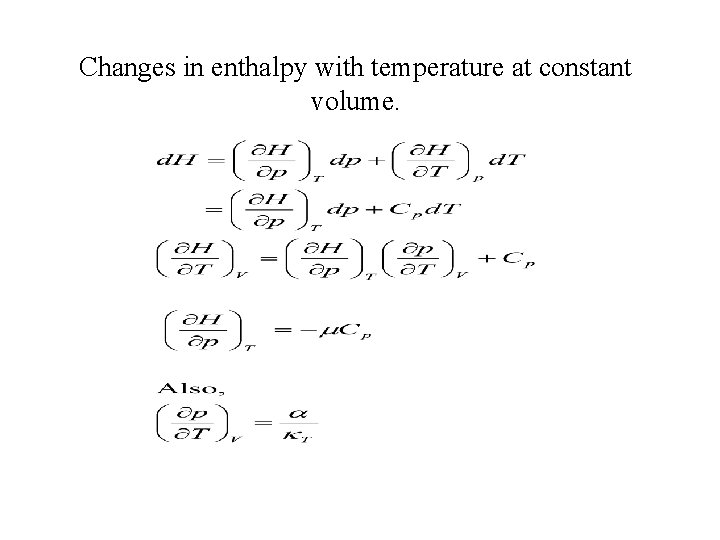
Changes in enthalpy with temperature at constant volume.


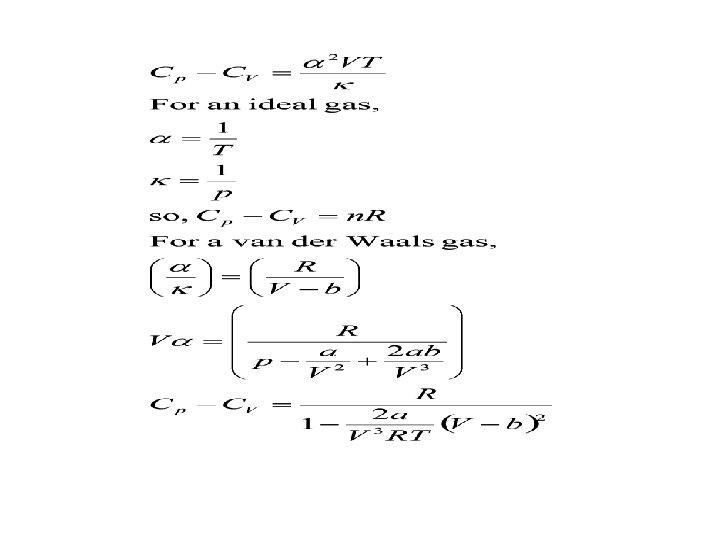
Relation between Cp and CV
