Chemistry 200 Focus 4 Thermodynamics The First Law

























- Slides: 81

Chemistry 200 Focus 4 Thermodynamics: The First Law

Thermodynamics, is the study of Work and Heat are Energy Work Energy Heat

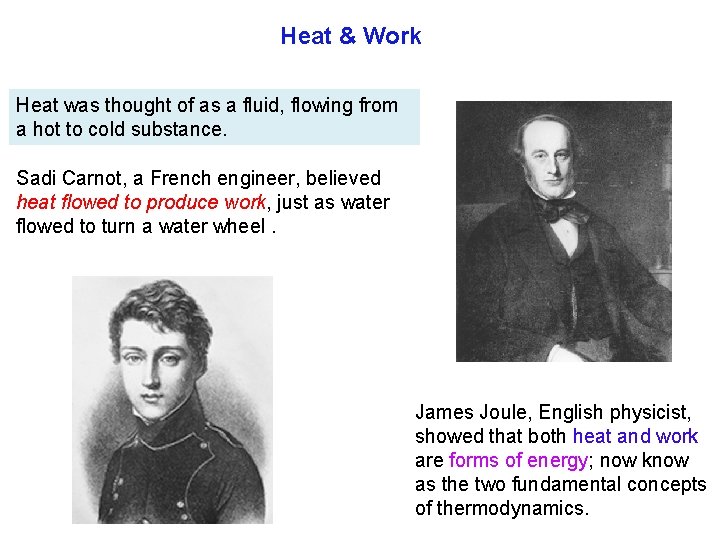
Heat & Work Heat was thought of as a fluid, flowing from a hot to cold substance. Sadi Carnot, a French engineer, believed heat flowed to produce work, just as water flowed to turn a water wheel. James Joule, English physicist, showed that both heat and work are forms of energy; now know as the two fundamental concepts of thermodynamics.

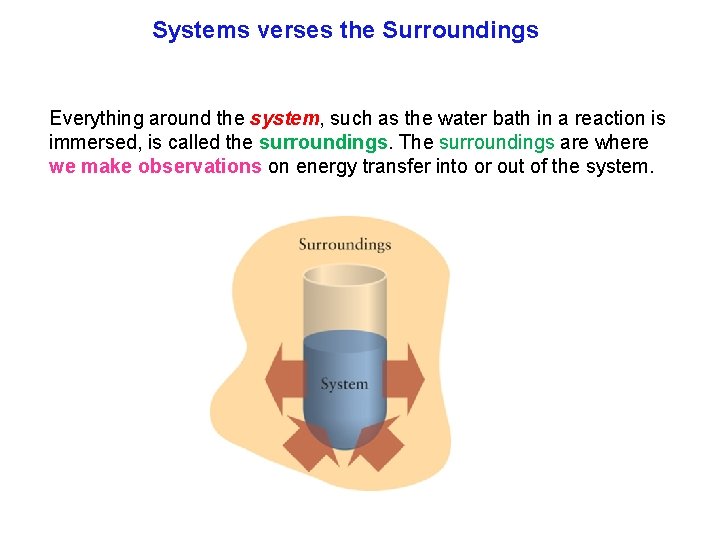
Systems verses the Surroundings Thermodynamics studies how energy is transformed from one form into another and transferred. Electricity generated in a power station is used a great distance away. The System (transformed) The Surroundings (transferred) To keep track of changes in energy, we always separate these two components.

Systems verses the Surroundings Everything around the system, such as the water bath in a reaction is immersed, is called the surroundings. The surroundings are where we make observations on energy transfer into or out of the system.

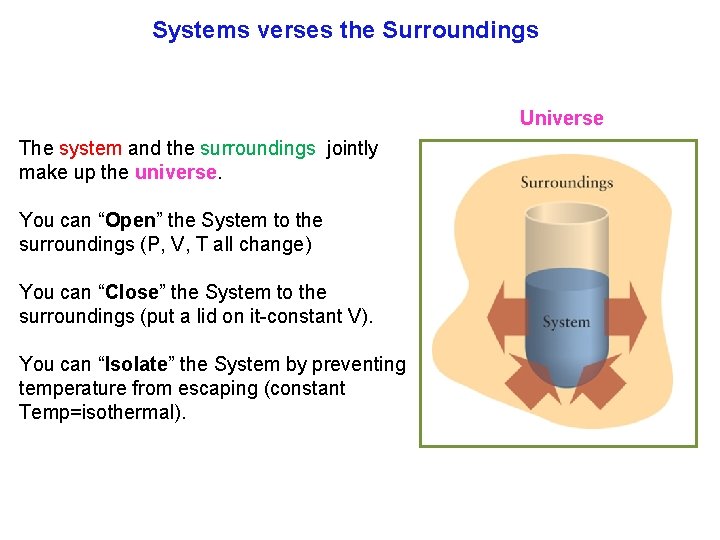
Systems verses the Surroundings Universe The system and the surroundings jointly make up the universe. You can “Open” the System to the surroundings (P, V, T all change) You can “Close” the System to the surroundings (put a lid on it constant V). You can “Isolate” the System by preventing temperature from escaping (constant Temp=isothermal).

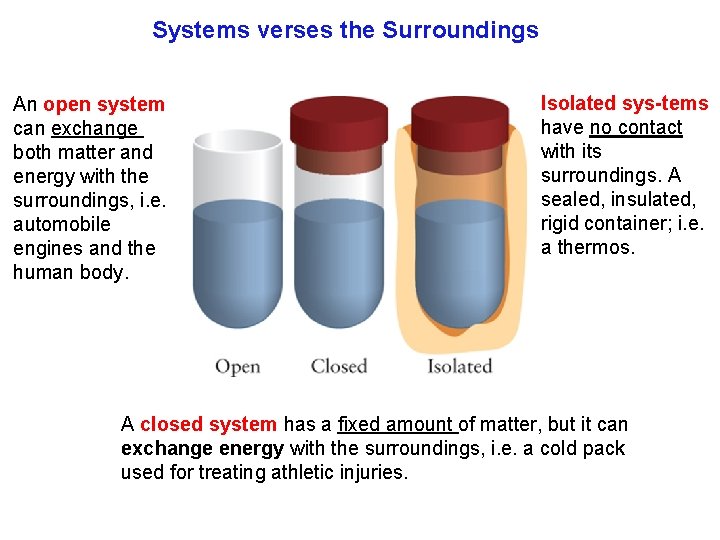
Systems verses the Surroundings An open system can exchange both matter and energy with the surroundings, i. e. automobile engines and the human body. Isolated sys tems have no contact with its surroundings. A sealed, insulated, rigid container; i. e. a thermos. A closed system has a fixed amount of matter, but it can exchange energy with the surroundings, i. e. a cold pack used for treating athletic injuries.

Work & Energy Work is motion against an oppos ing force. Work = opposing force × distance moved The SI unit for work (energy), is joule, J, where 1 J = 1 kg·m 2·s 2

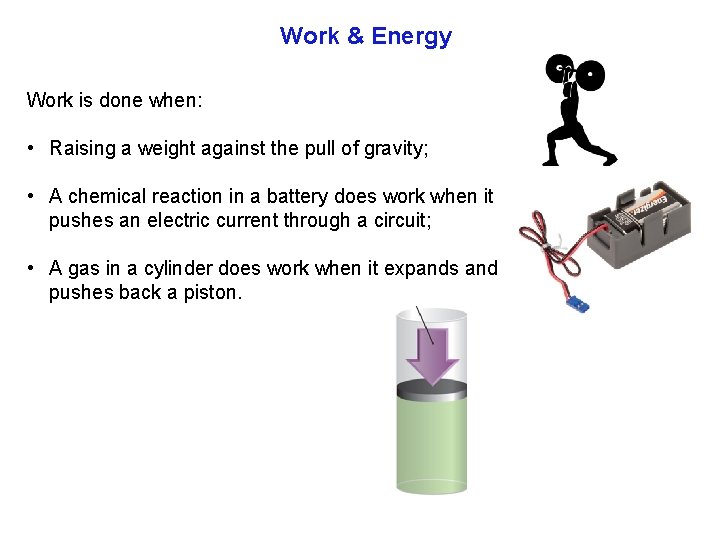
Work & Energy Work is done when: • Raising a weight against the pull of gravity; • A chemical reaction in a battery does work when it pushes an electric current through a circuit; • A gas in a cylinder does work when it expands and pushes back a piston.

Internal energy, U In thermodynamics, the capacity of a system to do work is called its internal energy, U. Internal energy (U): sum of the kinetic and potential energies. Absolute internal energy is not measurable because it includes the energies of all the atoms, their electrons, and the components of their nuclei. The best we can do is to measure changes in internal energy or ΔX: ΔX = Xfinal Xinitial

Internal energy, U If a system does 15 J of work, it used up some of its store of energy, so we say its internal energy has fallen by 15 J and write ΔX = 15 J (when your body burns food). A negative value of ΔX, or ΔU 15 J, means X has decreased. If we do work on the system, lets say 15 J of work, we say its internal energy has increased by 15 J and write ΔX = +15 J (winding a spring increases U of the system, or we eat a sandwich). A positive value of ΔX, or ΔU + 15 J, means X has increased.

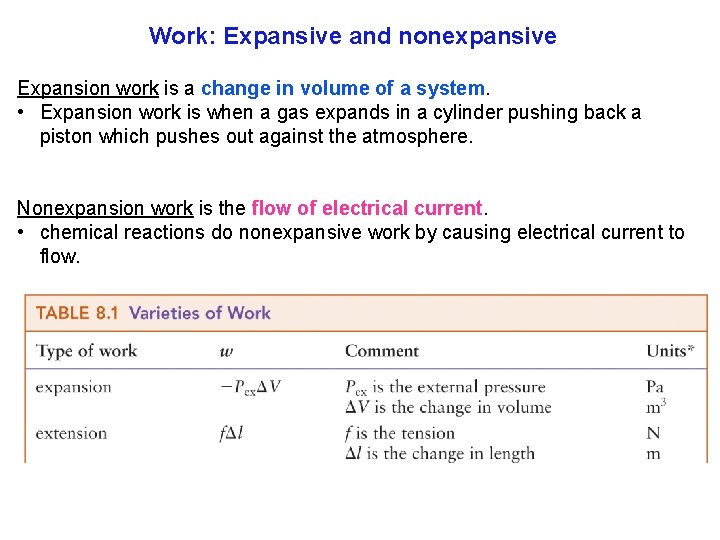
Work: Expansive and nonexpansive Expansion work is a change in volume of a system. • Expansion work is when a gas expands in a cylinder pushing back a piston which pushes out against the atmosphere. Nonexpansion work is the flow of electrical current. • chemical reactions do nonexpansive work by causing electrical current to flow. 12

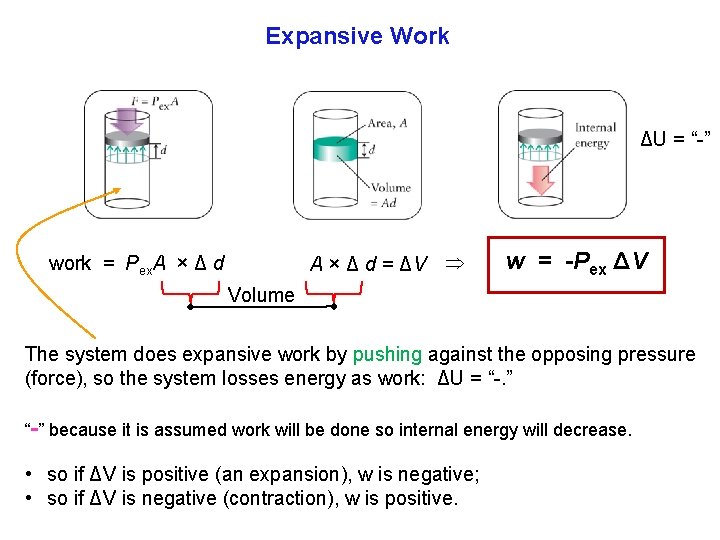
Expansive Work We now introduce two forms of Expansive Work (W = PΔV): 1. Expansion at constant pressure; 2. Expansion at constant temperature with changing Pexternal. Work Energy Heat

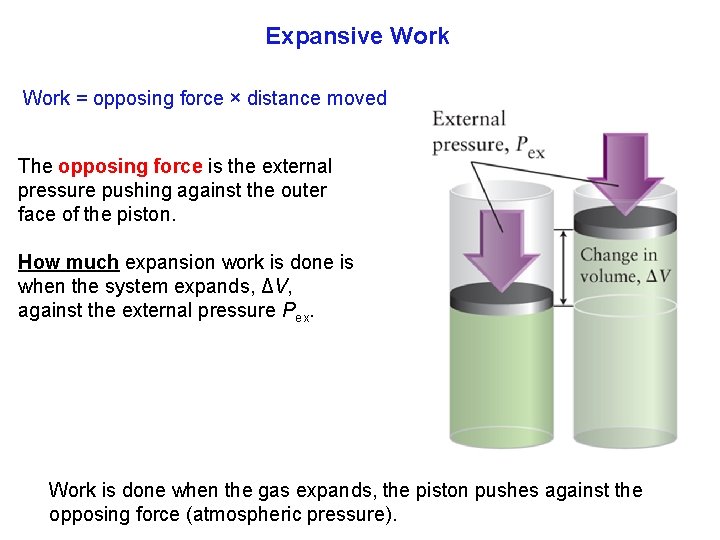
Expansive Work = opposing force × distance moved The opposing force is the external pressure pushing against the outer face of the piston. How much expansion work is done is when the system expands, ΔV, against the external pressure Pex. Work is done when the gas expands, the piston pushes against the opposing force (atmospheric pressure).

Expansive Work ΔU = “ ” A × Δ d = ΔV work = Pex. A × Δ d w = Pex ΔV Volume The system does expansive work by pushing against the opposing pressure (force), so the system losses energy as work: ΔU = “. ” “ ” because it is assumed work will be done so internal energy will decrease. • so if ΔV is positive (an expansion), w is negative; • so if ΔV is negative (contraction), w is positive.

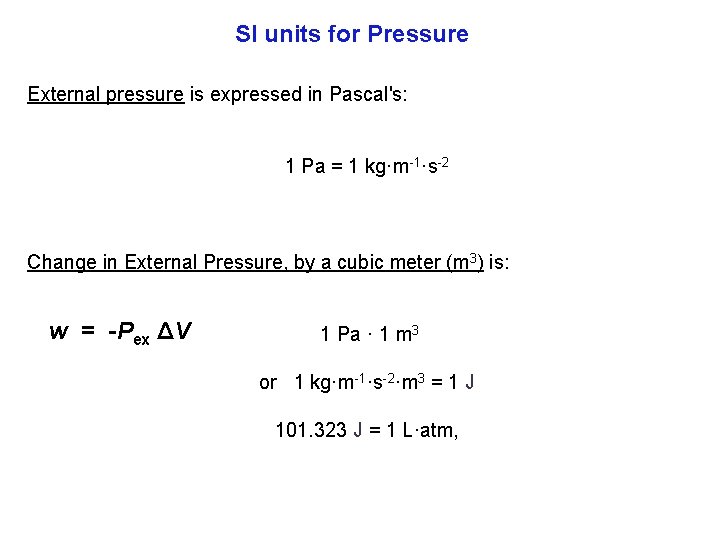
SI units for Pressure External pressure is expressed in Pascal's: 1 Pa = 1 kg·m 1·s 2 Change in External Pressure, by a cubic meter (m 3) is: w = Pex ΔV 1 Pa · 1 m 3 or 1 kg·m 1·s 2·m 3 = 1 J 101. 323 J = 1 L·atm,

Work: Free Expansion If the external pressure is zero (a vacuum), than w = 0 No expansion work is done in a vacuum because there is no opposing force, there is nothing to push against. Expansion against zero pressure is called free expansion.

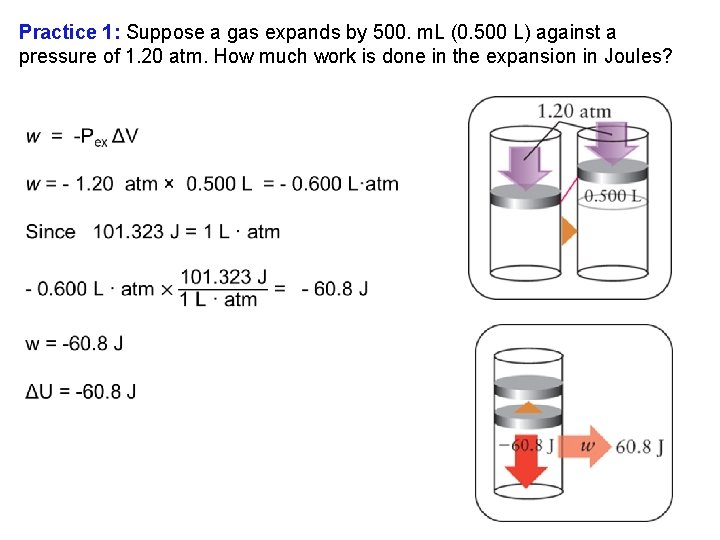
Practice 1: Suppose a gas expands by 500. m. L (0. 500 L) against a pressure of 1. 20 atm. How much work is done in the expansion in Joules?

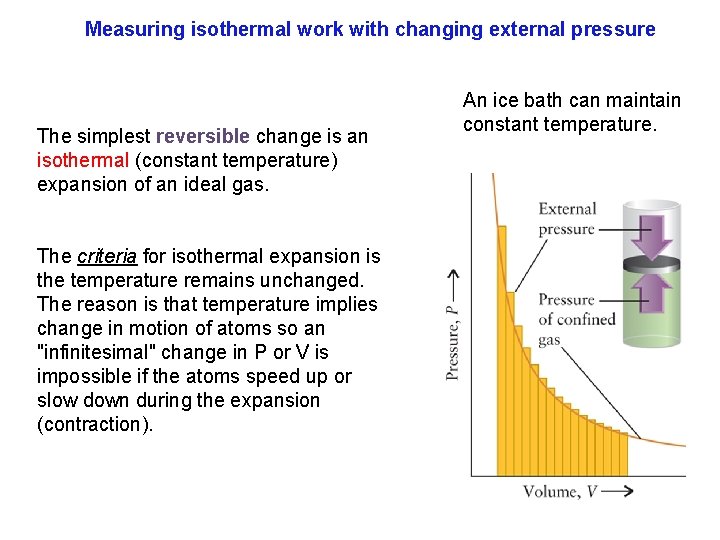
Changing external pressure Work is a measure of P and V changes. Measuring work where V changes in either direction as a "reversible" expansion must be done in "infinitesimal" changes. Of course this means that pressure increases or decreases infinitesimally as well. These reversible processes are of the greatest importance in thermodynamics, and we shall encounter them many times.

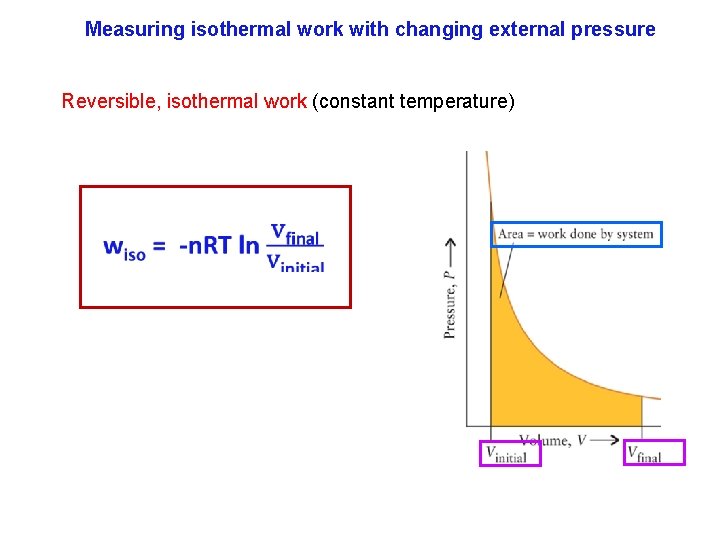
Measuring isothermal work with changing external pressure The simplest reversible change is an isothermal (constant temperature) expansion of an ideal gas. The criteria for isothermal expansion is the temperature remains unchanged. The reason is that temperature implies change in motion of atoms so an "infinitesimal" change in P or V is impossible if the atoms speed up or slow down during the expansion (contraction). An ice bath can maintain constant temperature.

Measuring isothermal work with changing external pressure Reversible, isothermal work (constant temperature)

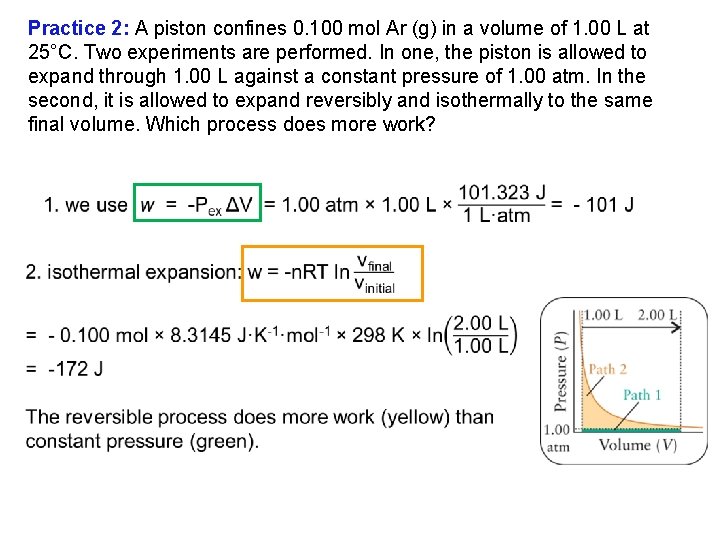
Practice 2: A piston confines 0. 100 mol Ar (g) in a volume of 1. 00 L at 25°C. Two experiments are performed. 1. The piston is allowed to expand through 1. 00 L against a constant pressure of 1. 00 atm. The piston is allowed to expand reversibly and isothermally to the same final volume. 2. Which process does more work?

Practice 2: A piston confines 0. 100 mol Ar (g) in a volume of 1. 00 L at 25°C. Two experiments are performed. In one, the piston is allowed to expand through 1. 00 L against a constant pressure of 1. 00 atm. In the second, it is allowed to expand reversibly and isothermally to the same final volume. Which process does more work?

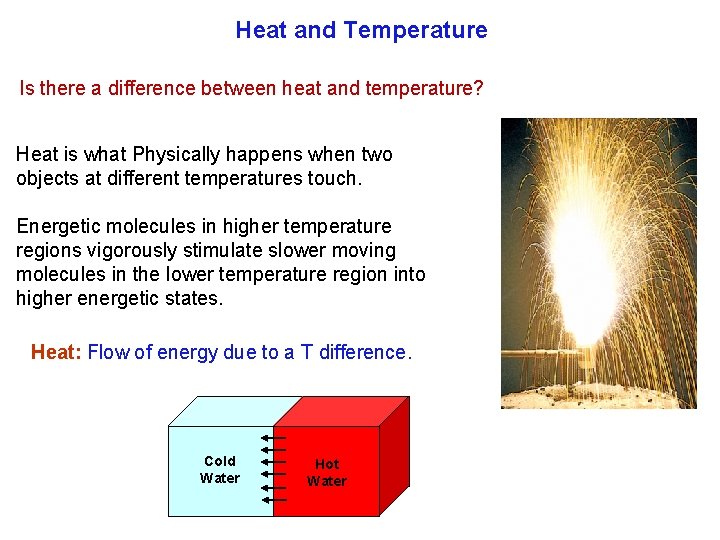
Heat and Temperature Is there a difference between heat and temperature? Heat is what Physically happens when two objects at different temperatures touch. Energetic molecules in higher temperature regions vigorously stimulate slower moving molecules in the lower temperature region into higher energetic states. Heat: Flow of energy due to a T difference. Cold Water Hot Water

Heat and Temperature This results in a decrease for higher energy molecules (they become cooler) and an increase the lower energetic molecules (they become warmer). Temperature is measured with a thermometer, and it must come into contact with an object. Fluid inside thermometer, often mercury or alcohol, reacts to changes in the energetic molecules it makes contact with.

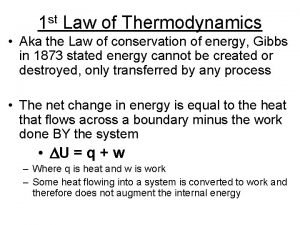
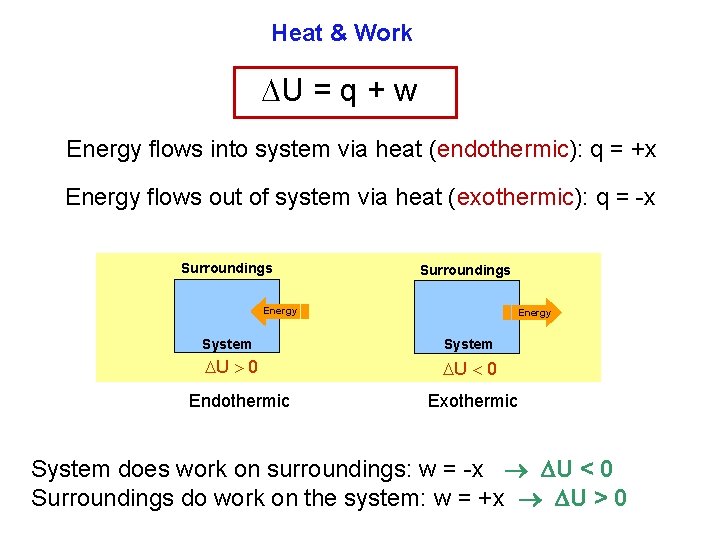
Heat & Work U = q + w Energy flows into system via heat (endothermic): q = +x Energy flows out of system via heat (exothermic): q = x Surroundings Energy System U 0 Endothermic Exothermic System does work on surroundings: w = x U < 0 Surroundings do work on the system: w = +x U > 0

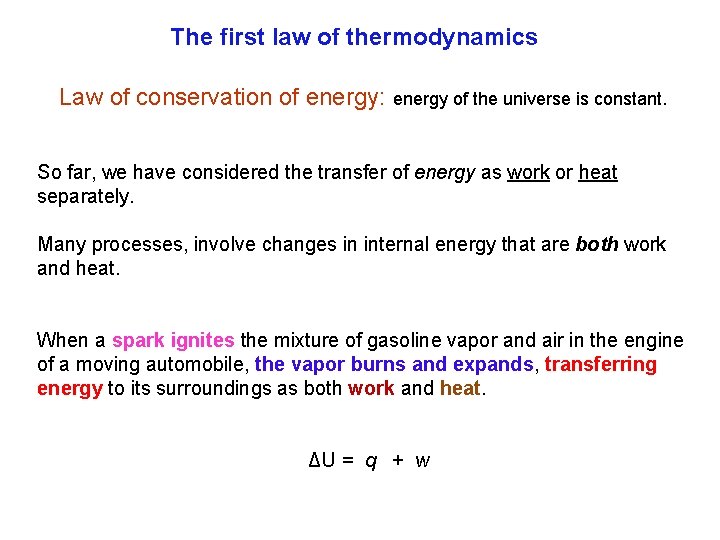
The first law of thermodynamics Law of conservation of energy: energy of the universe is constant. So far, we have considered the transfer of energy as work or heat separately. Many processes, involve changes in internal energy that are both work and heat. When a spark ignites the mixture of gasoline vapor and air in the engine of a moving automobile, the vapor burns and expands, transferring energy to its surroundings as both work and heat. ΔU = q + w

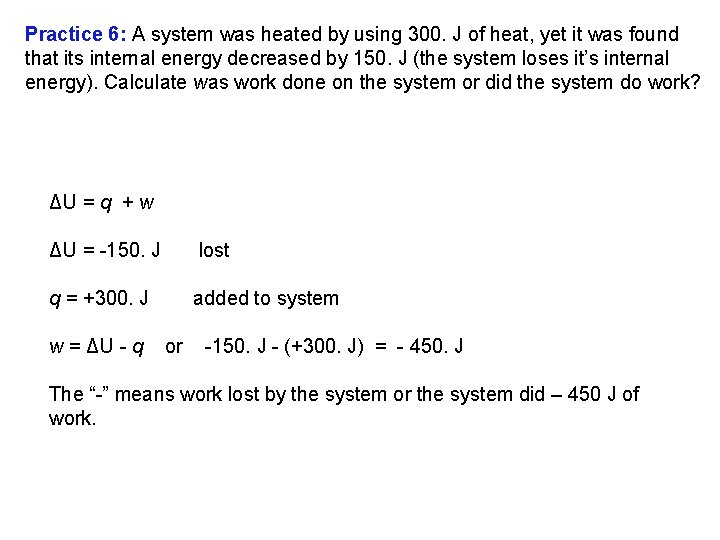
Practice 6: A system was heated by using 300. J of heat, yet it was found that its internal energy decreased by 150. J (the system loses it’s internal energy). Calculate was work done on the system or did the system do work? ΔU = q + w ΔU = 150. J lost q = +300. J added to system w = ΔU q or 150. J (+300. J) = 450. J The “ ” means work lost by the system or the system did – 450 J of work.

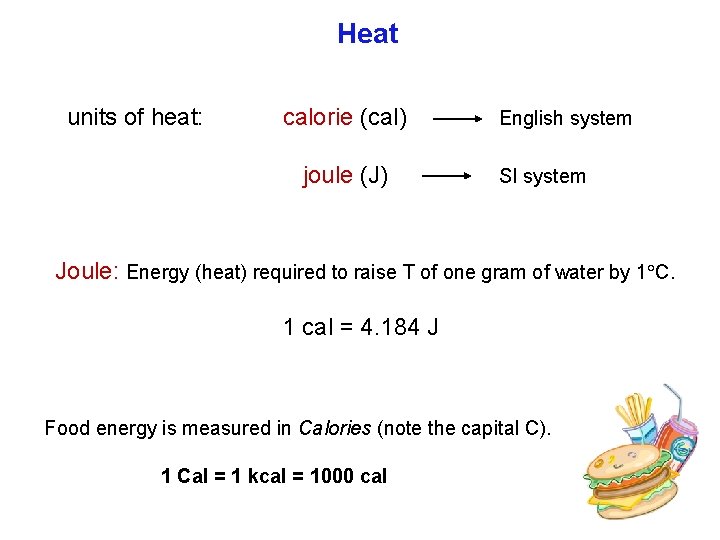
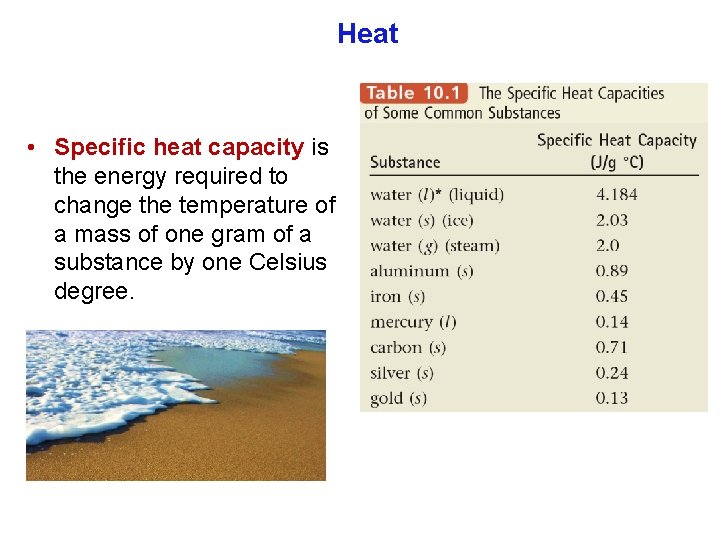
Heat units of heat: calorie (cal) joule (J) English system SI system Joule: Energy (heat) required to raise T of one gram of water by 1 C. 1 cal = 4. 184 J Food energy is measured in Calories (note the capital C). 1 Cal = 1 kcal = 1000 cal

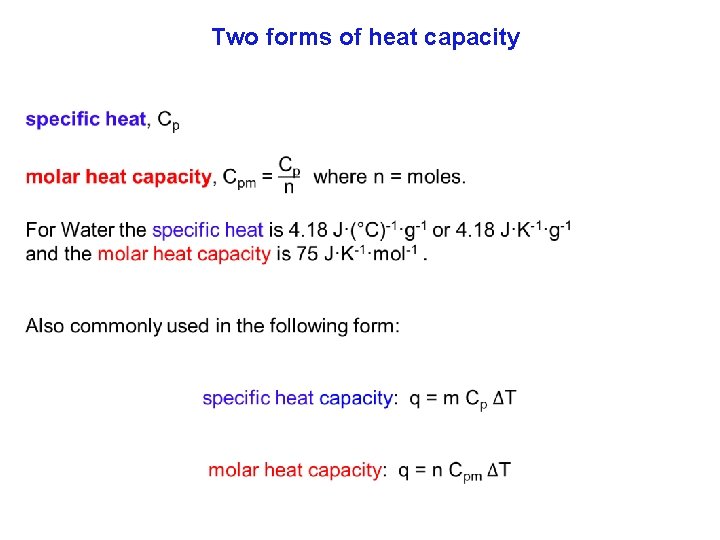
Heat Amount of heat = specific heat capacity × mass × change in temperature q = Cp × m × (Tfinal – Tinitial) Cp = Specific heat capacity (cal/g °C or J/g °C) Tfinal = final temperature (ºC) m = mass (g) Tinitial = initial temperature(ºC)

Heat • Specific heat capacity is the energy required to change the temperature of a mass of one gram of a substance by one Celsius degree.

Two forms of heat capacity

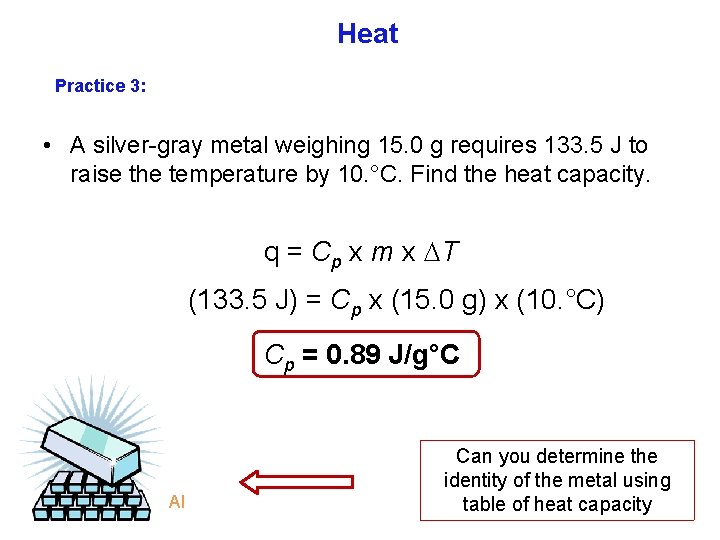
Heat Practice 3: • A silver gray metal weighing 15. 0 g requires 133. 5 J to raise the temperature by 10. °C. Find the heat capacity. q = Cp x m x T (133. 5 J) = Cp x (15. 0 g) x (10. °C) Cp = 0. 89 J/g°C Al Can you determine the identity of the metal using table of heat capacity

Practice 4: Calculating the heat needed to bring about a rise in temperature Calculate the heat necessary to increase the temperature of 2. 00 mol H 2 O(l) by 20. °C from room temperature. (Cpm = 75 J. K 1. mol 1)

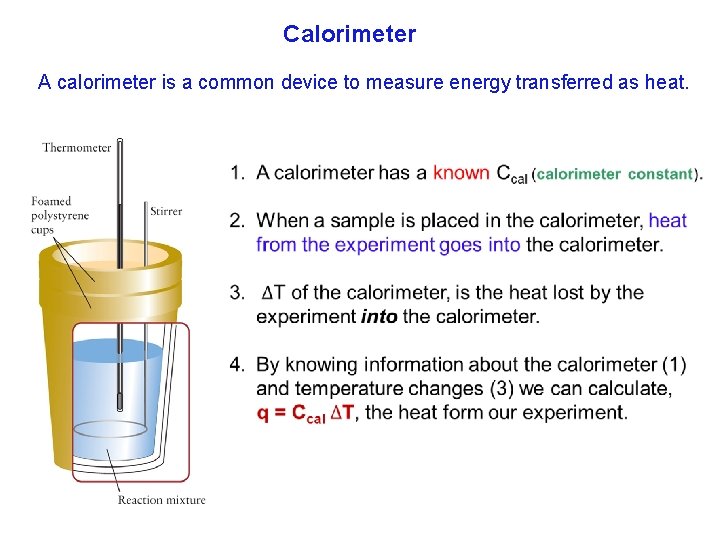
Calorimeter A calorimeter is a common device to measure energy transferred as heat.

Calorimeter Heat from the Experiment = Heat of the Calorimeter. The sample generates heat. Then, that heat is absorbed the calorimeter (qcal = qexp). Calorimeters are calibrated with a known specific heat, usually water. Calibration means finding the calorimeter constant Ccal. A more sophisticated version made of metal, called a bomb calorimeter. 36

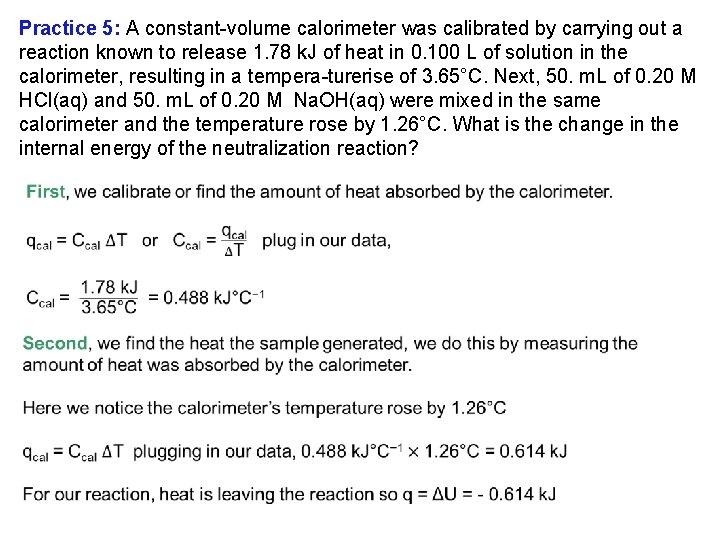
Practice 5: A constant volume calorimeter was calibrated by carrying out a reaction known to release 1. 78 k. J of heat in 0. 100 L of solution in the calorimeter, resulting in a tempera turerise of 3. 65°C. Next, 50. m. L of 0. 20 M HCl(aq) and 50. m. L of 0. 20 M Na. OH(aq) were mixed in the same calorimeter and the temperature rose by 1. 26°C. What is the change in the internal energy of the neutralization reaction?

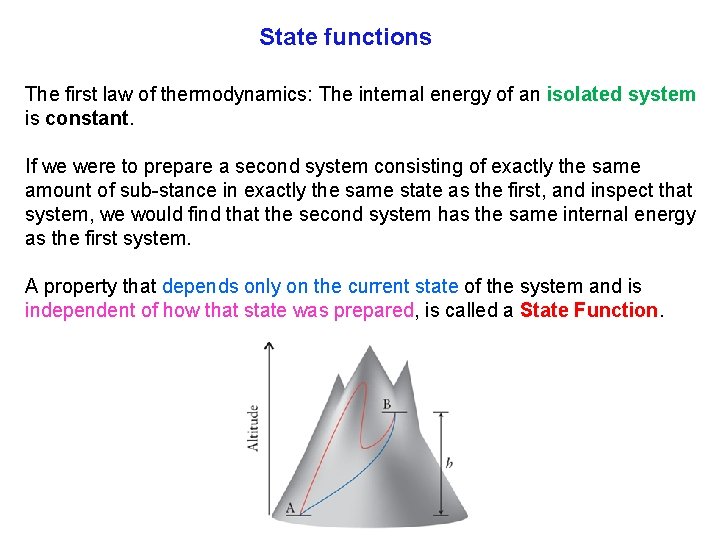
State functions The first law of thermodynamics: The internal energy of an isolated system is constant. If we were to prepare a second system consisting of exactly the same amount of sub stance in exactly the same state as the first, and inspect that system, we would find that the second system has the same internal energy as the first system. A property that depends only on the current state of the system and is independent of how that state was prepared, is called a State Function.

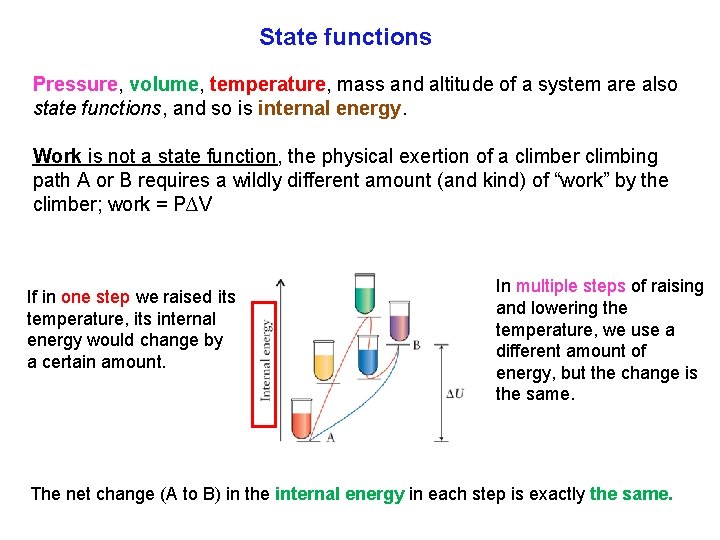
State functions Pressure, volume, temperature, mass and altitude of a system are also state functions, and so is internal energy. Work is not a state function, the physical exertion of a climber climbing path A or B requires a wildly different amount (and kind) of “work” by the climber; work = P∆V If in one step we raised its temperature, its internal energy would change by a certain amount. In multiple steps of raising and lowering the temperature, we use a different amount of energy, but the change is the same. The net change (A to B) in the internal energy in each step is exactly the same.

State functions In the next example internal energy, ΔU = q + w, is calculated by two different paths, both over the same range. 1. One path calculates internal energy only reversibly, 2. The other path is a combination of both reversible and irreversible. The biggest difference between the two is for a reversible process the temperature remains constant (isothermal). Also remember that all isothermal expansions are the most efficient (though not common).

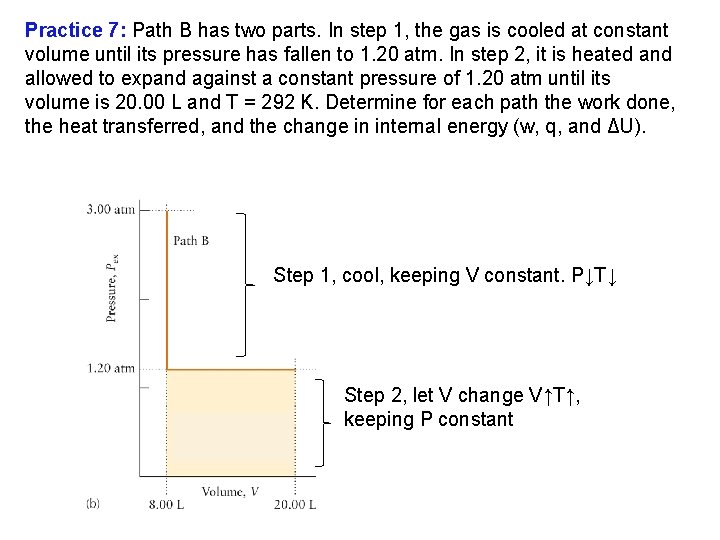
Practice 7: Suppose that 1. 00 mol of ideal gas molecules at 292 K at 3. 00 atm expands from 8. 00 L to 20. 00 L and has a final pressure of 1. 20 atm. Find the amount of work by two different paths. (a) Path A is an isothermal, reversible expansion. (b) Path B has two parts. In step 1, the gas is cooled at constant volume until its pressure has fallen to 1. 20 atm. In step 2, it is heated and allowed to expand against a constant pressure of 1. 20 atm until its volume is 20. 00 L and T = 292 K. Determine for each path the work done, the heat transferred, and the change in internal energy (w, q, and ΔU).

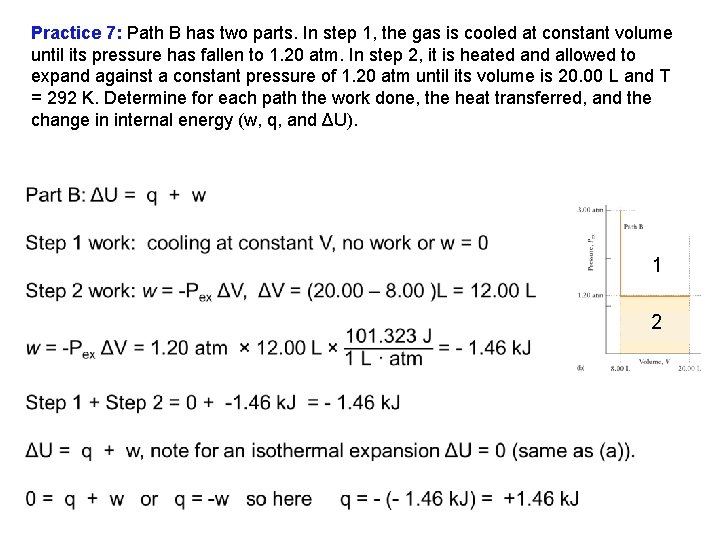
Practice 7: Path B has two parts. In step 1, the gas is cooled at constant volume until its pressure has fallen to 1. 20 atm. In step 2, it is heated and allowed to expand against a constant pressure of 1. 20 atm until its volume is 20. 00 L and T = 292 K. Determine for each path the work done, the heat transferred, and the change in internal energy (w, q, and ΔU). Step 1, cool, keeping V constant. P↓T↓ Step 2, let V change V↑T↑, keeping P constant

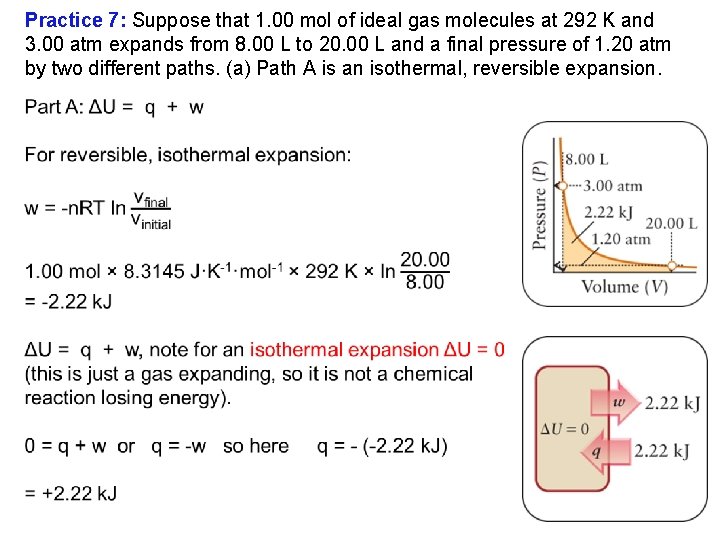
Practice 7: Suppose that 1. 00 mol of ideal gas molecules at 292 K and 3. 00 atm expands from 8. 00 L to 20. 00 L and a final pressure of 1. 20 atm by two different paths. (a) Path A is an isothermal, reversible expansion.

Practice 7: Path B has two parts. In step 1, the gas is cooled at constant volume until its pressure has fallen to 1. 20 atm. In step 2, it is heated and allowed to expand against a constant pressure of 1. 20 atm until its volume is 20. 00 L and T = 292 K. Determine for each path the work done, the heat transferred, and the change in internal energy (w, q, and ΔU). 1 2

Practice 7: Suppose that 1. 00 mol of ideal gas molecules at 292 K and 3. 00 atm expands from 8. 00 L to 20. 00 L and a final pressure of 1. 20 atm by two different paths. (a) Path A is an isothermal, reversible expansion. (b) Path B has two parts. In step 1, the gas is cooled at constant volume until its pressure has fallen to 1. 20 atm. In step 2, it is heated and allowed to expand against a constant pressure of 1. 20 atm until its volume is 20. 00 L and T = 292 K. Determine for each path the work done, the heat transferred, and the change in internal energy (w, q, and ΔU). Nonreversible does less work, +1. 46 k. J. Isothermal, reversible does more work, +2. 22 k. J. ΔU = 0 for both

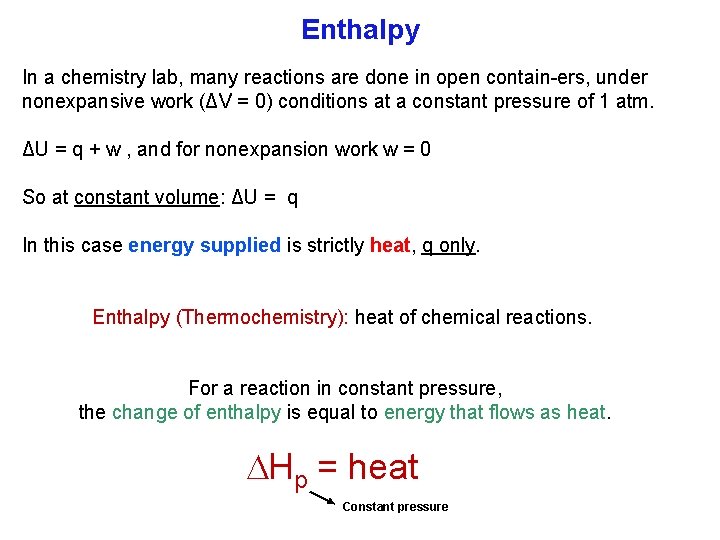
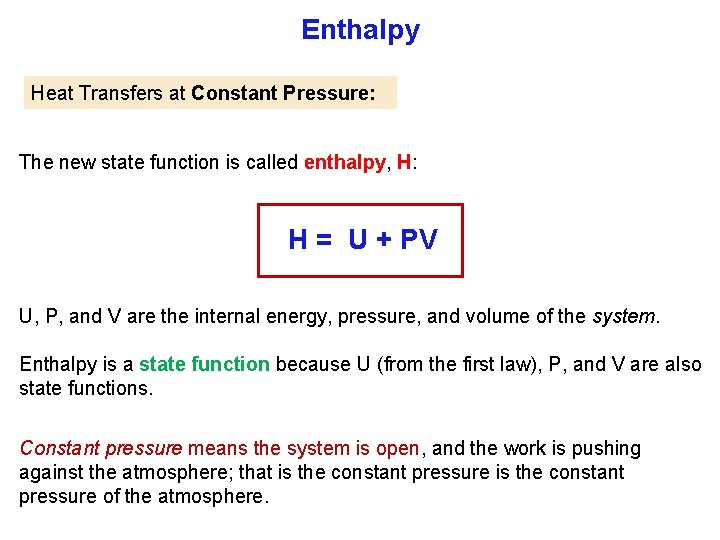
Enthalpy In a chemistry lab, many reactions are done in open contain ers, under nonexpansive work (ΔV = 0) conditions at a constant pressure of 1 atm. ΔU = q + w , and for nonexpansion work w = 0 So at constant volume: ΔU = q In this case energy supplied is strictly heat, q only. Enthalpy (Thermochemistry): heat of chemical reactions. For a reaction in constant pressure, the change of enthalpy is equal to energy that flows as heat. Hp = heat Constant pressure

Enthalpy Heat Transfers at Constant Pressure: The new state function is called enthalpy, H: H = U + PV U, P, and V are the internal energy, pressure, and volume of the system. Enthalpy is a state function because U (from the first law), P, and V are also state functions. Constant pressure means the system is open, and the work is pushing against the atmosphere; that is the constant pressure of the atmosphere.

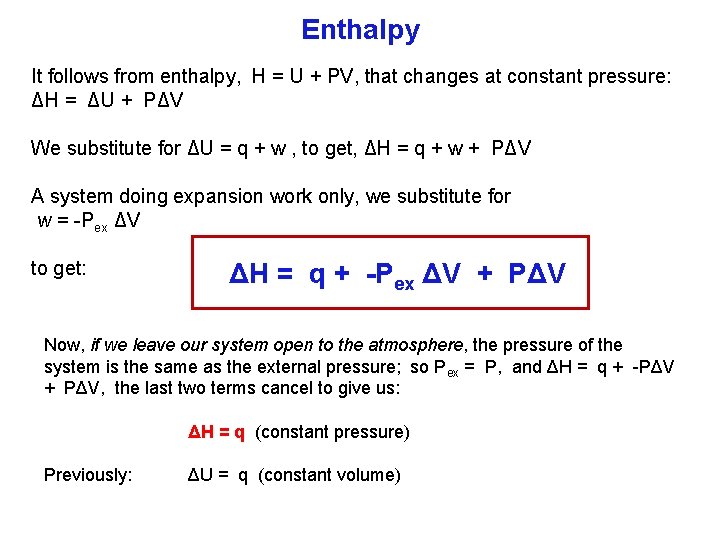
Enthalpy It follows from enthalpy, H = U + PV, that changes at constant pressure: ΔH = ΔU + PΔV We substitute for ΔU = q + w , to get, ΔH = q + w + PΔV A system doing expansion work only, we substitute for w = Pex ΔV to get: ΔH = q + Pex ΔV + PΔV Now, if we leave our system open to the atmosphere, the pressure of the system is the same as the external pressure; so Pex = P, and ΔH = q + PΔV, the last two terms cancel to give us: ΔH = q (constant pressure) Previously: ΔU = q (constant volume)

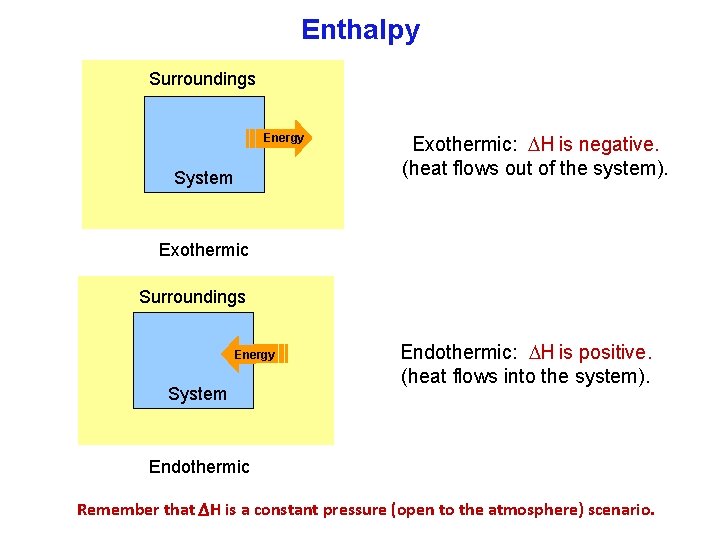
Enthalpy Surroundings Energy System Exothermic: H is negative. (heat flows out of the system). Exothermic Surroundings Energy System Endothermic: H is positive. (heat flows into the system). Endothermic Remember that ΔH is a constant pressure (open to the atmosphere) scenario.

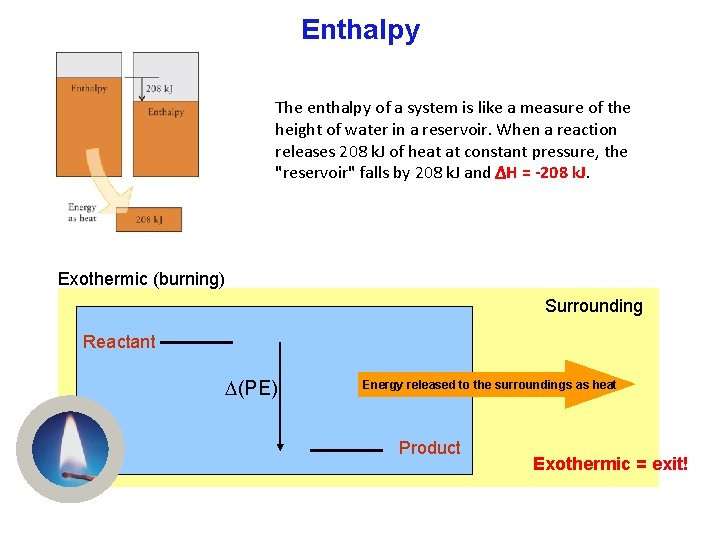
Enthalpy The enthalpy of a system is like a measure of the height of water in a reservoir. When a reaction releases 208 k. J of heat at constant pressure, the "reservoir" falls by 208 k. J and ΔH = -208 k. J. Exothermic (burning) Surrounding Reactant (PE) Energy released to the surroundings as heat Product Exothermic = exit!

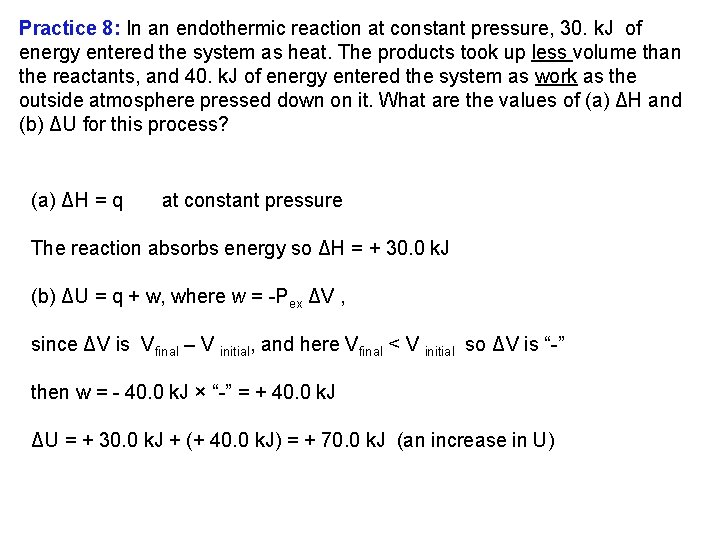
Practice 8: In an endothermic reaction at constant pressure, 30. k. J of energy entered the system as heat. The products took up less volume than the reactants, and 40. k. J of energy entered the system as work as the outside atmosphere pressed down on it. What are the values of (a) ΔH and (b) ΔU for this process? (a) ΔH = q at constant pressure The reaction absorbs energy so ΔH = + 30. 0 k. J (b) ΔU = q + w, where w = Pex ΔV , since ΔV is Vfinal – V initial, and here Vfinal < V initial so ΔV is “ ” then w = 40. 0 k. J × “ ” = + 40. 0 k. J ΔU = + 30. 0 k. J + (+ 40. 0 k. J) = + 70. 0 k. J (an increase in U)

The Enthalpy of Physical Change Phase changes approximate how far apart molecules are. Vaporization, requires adding energy to break and separate intermolecular attractions, an endothermic process. Freezing, is exothermic because energy is given off to allow new intermolecular attractions to form between molecules. Phase changes usually take place at constant pressure, the heat transfer is due to changes in enthalpy.

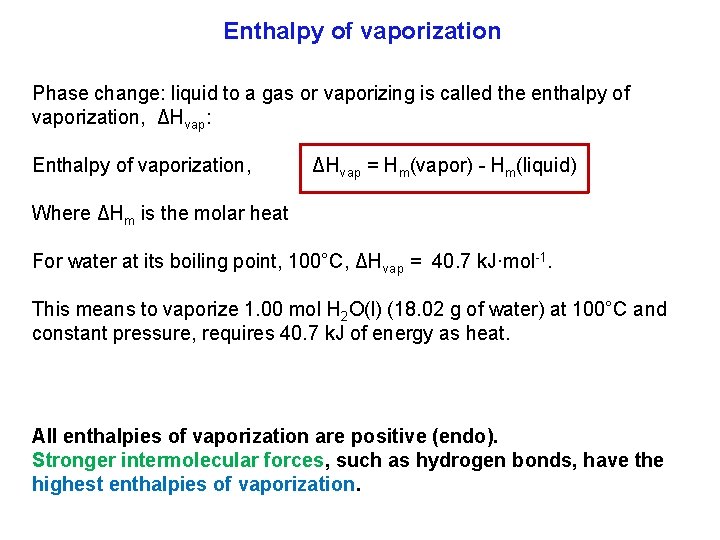
Enthalpy of vaporization Phase change: liquid to a gas or vaporizing is called the enthalpy of vaporization, ΔHvap: Enthalpy of vaporization, ΔHvap = Hm(vapor) Hm(liquid) Where ΔHm is the molar heat For water at its boiling point, 100°C, ΔHvap = 40. 7 k. J·mol 1. This means to vaporize 1. 00 mol H 2 O(l) (18. 02 g of water) at 100°C and constant pressure, requires 40. 7 k. J of energy as heat. All enthalpies of vaporization are positive (endo). Stronger intermolecular forces, such as hydrogen bonds, have the highest enthalpies of vaporization.

Practice 9: Heating a sample of ethanol, C 2 H 5 OH, of mass 23 g to its boiling point, it was found that 22 k. J was required to vaporize all the ethanol. What is the enthalpy of vaporization of ethanol at its boiling point?

Enthalpy of fusion Phase change: solid to a liquid or melting (fusion) is called the enthalpy of fusion, ΔHfus = Hm(liquid) Hm(solid) The enthalpy of fusion of water at 0. 0 °C is 6. 0 k. J·mole 1: to melt 1. 0 mol H 2 O(s) (18 g of ice) at 0. 0 °C, we have to sup ply 6. 0 k. J of heat. Vaporizing water takes much more energy (more than 40 k. J) because to vaporize a gas, its molecules are separated completely, and KE increases dramatically. In melting, the molecules stay close together, and so the forces of attraction and repulsion are nearly as strong as in the solid.

Enthalpy of freezing Freezing is the change from liquid to solid. Enthalpy is a state property: ΔHreverse process = ΔHforward process Since the enthalpy of fusion of water at 0. 0°C is 6. 0 k. J·mole 1 the enthalpy of freezing for water is at 0. 0°C is 6. 0 k. J·mole 1 Enthalpy of condensation Condensation is the change from gas to liquid. So this process is the reverse of ΔHvap. For water at boiling, 100°C, ΔHvap = 40. 7 k. J·mol 1 , therefore ΔHcondensation = 40. 7 k. J·mol 1

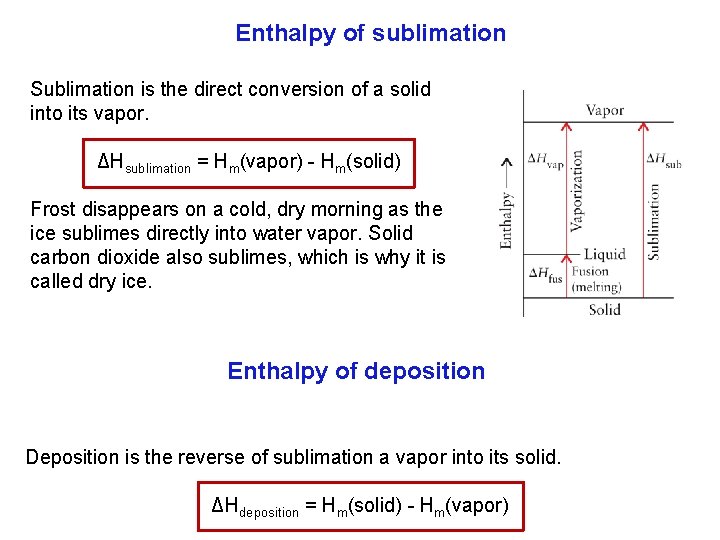
Enthalpy of sublimation Sublimation is the direct conversion of a solid into its vapor. ΔHsublimation = Hm(vapor) Hm(solid) Frost disappears on a cold, dry morning as the ice sublimes directly into water vapor. Solid carbon dioxide also sublimes, which is why it is called dry ice. Enthalpy of deposition Deposition is the reverse of sublimation a vapor into its solid. ΔHdeposition = Hm(solid) Hm(vapor)

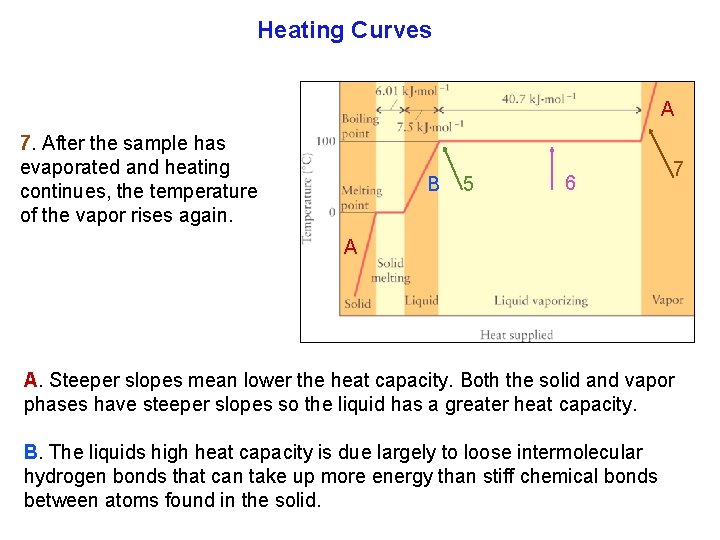
Heating Curves A heating curve shows the variation in the temperature of a heated sample. As a sample of ice is heated the temperature rises steadily. 2 1 1. In a solid the molecules are still locked together, as they oscillate vigorously around their mean positions. 2. As the temperature rises reaching the melting point, the molecules have enough energy to move past one another, overcoming the attractive forces between molecules.

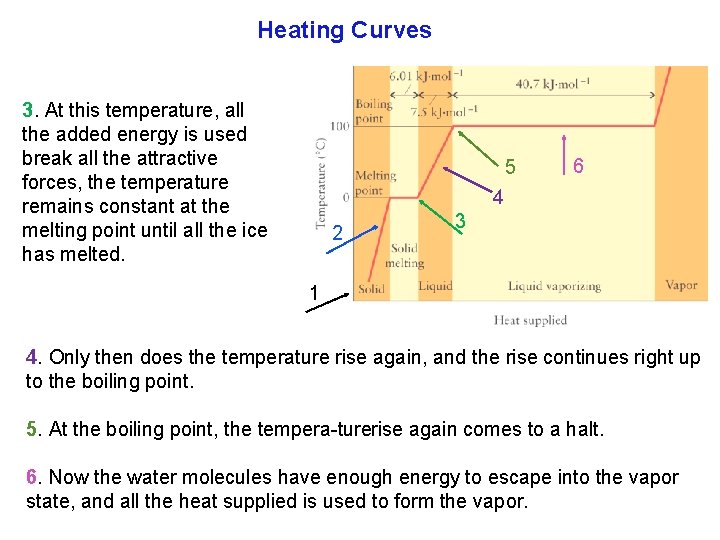
Heating Curves 3. At this temperature, all the added energy is used break all the attractive forces, the temperature remains constant at the melting point until all the ice has melted. 5 6 4 2 3 1 4. Only then does the temperature rise again, and the rise continues right up to the boiling point. 5. At the boiling point, the tempera turerise again comes to a halt. 6. Now the water molecules have enough energy to escape into the vapor state, and all the heat supplied is used to form the vapor.

Heating Curves A 7. After the sample has evaporated and heating continues, the temperature of the vapor rises again. B 5 6 7 A A. Steeper slopes mean lower the heat capacity. Both the solid and vapor phases have steeper slopes so the liquid has a greater heat capacity. B. The liquids high heat capacity is due largely to loose intermolecular hydrogen bonds that can take up more energy than stiff chemical bonds between atoms found in the solid.

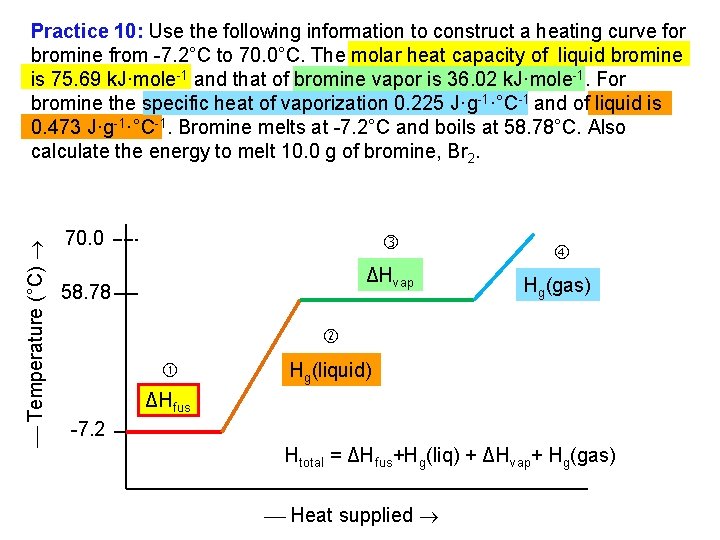
Temperature (°C) Practice 10: Use the following information to construct a heating curve for bromine from 7. 2°C to 70. 0°C. The molar heat capacity of liquid bromine is 75. 69 k. J·mole 1 and that of bromine vapor is 36. 02 k. J·mole 1. For bromine the specific heat of vaporization 0. 225 J·g 1·°C 1 and of liquid is 0. 473 J·g 1·°C 1. Bromine melts at 7. 2°C and boils at 58. 78°C. Also calculate the energy to melt 10. 0 g of bromine, Br 2. 70. 0 ΔHvap 58. 78 Hg(gas) Hg(liquid) ΔHfus 7. 2 Htotal = ΔHfus+Hg(liq) + ΔHvap+ Hg(gas) Heat supplied


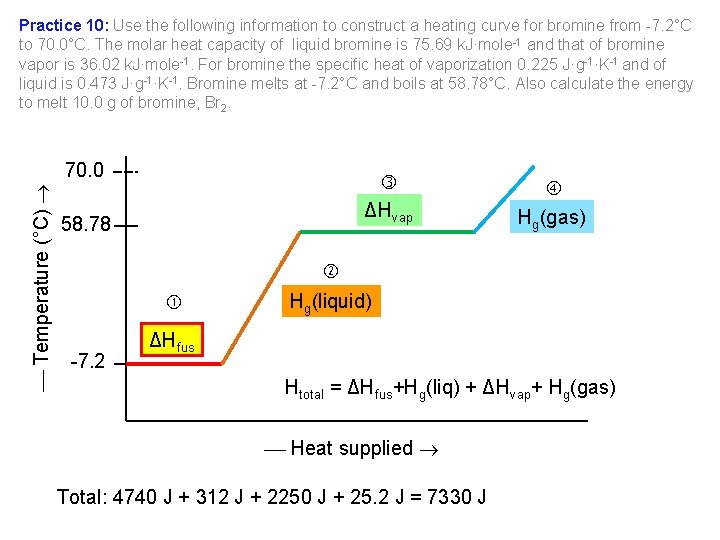
Practice 10: Use the following information to construct a heating curve for bromine from 7. 2°C to 70. 0°C. The molar heat capacity of liquid bromine is 75. 69 k. J·mole 1 and that of bromine vapor is 36. 02 k. J·mole 1. For bromine the specific heat of vaporization 0. 225 J·g 1·K 1 and of liquid is 0. 473 J·g 1·K 1. Bromine melts at 7. 2°C and boils at 58. 78°C. Also calculate the energy to melt 10. 0 g of bromine, Br 2. Temperature (°C) 70. 0 ΔHvap 58. 78 Hg(gas) 7. 2 Hg(liquid) ΔHfus Htotal = ΔHfus+Hg(liq) + ΔHvap+ Hg(gas) Heat supplied Total: 4740 J + 312 J + 2250 J + 25. 2 J = 7330 J

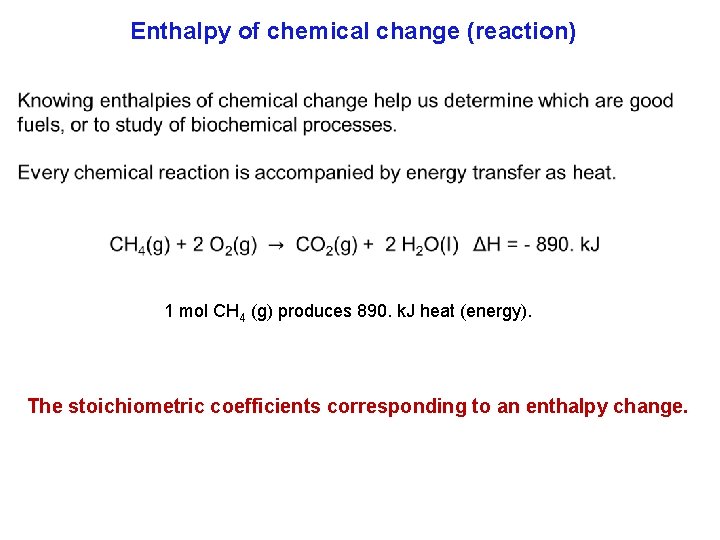
Enthalpy of chemical change (reaction) 1 mol CH 4 (g) produces 890. k. J heat (energy). The stoichiometric coefficients corresponding to an enthalpy change.

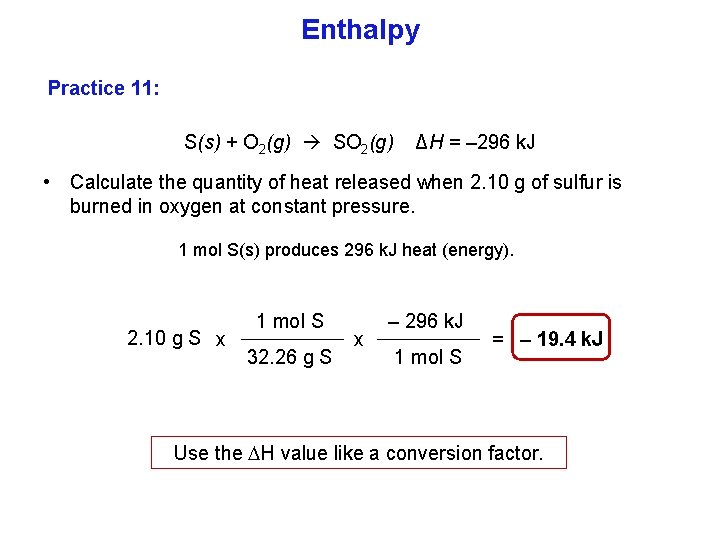
Enthalpy Practice 11: S(s) + O 2(g) SO 2(g) ΔH = – 296 k. J • Calculate the quantity of heat released when 2. 10 g of sulfur is burned in oxygen at constant pressure. 1 mol S(s) produces 296 k. J heat (energy). 2. 10 g S x 1 mol S 32. 26 g S x – 296 k. J 1 mol S = – 19. 4 k. J Use the H value like a conversion factor.

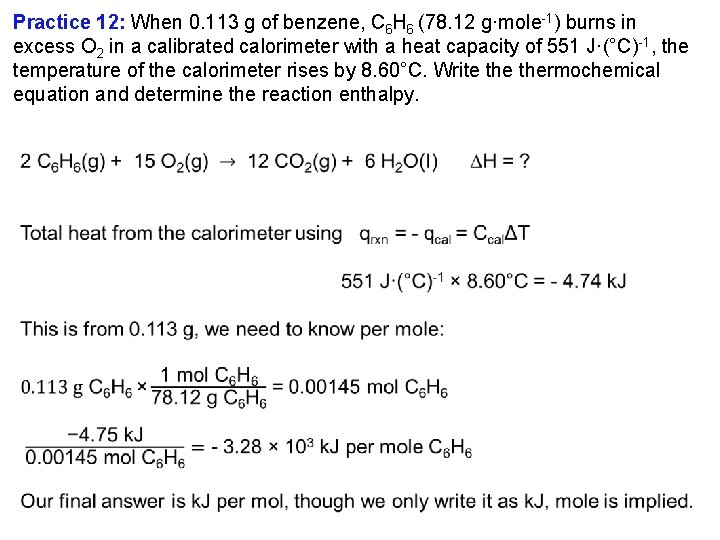
Practice 12: When 0. 113 g of benzene, C 6 H 6 (78. 12 g·mole 1) burns in excess O 2 in a calibrated calorimeter with a heat capacity of 551 J·(°C) 1, the temperature of the calorimeter rises by 8. 60°C. Write thermochemical equation and determine the reaction enthalpy.

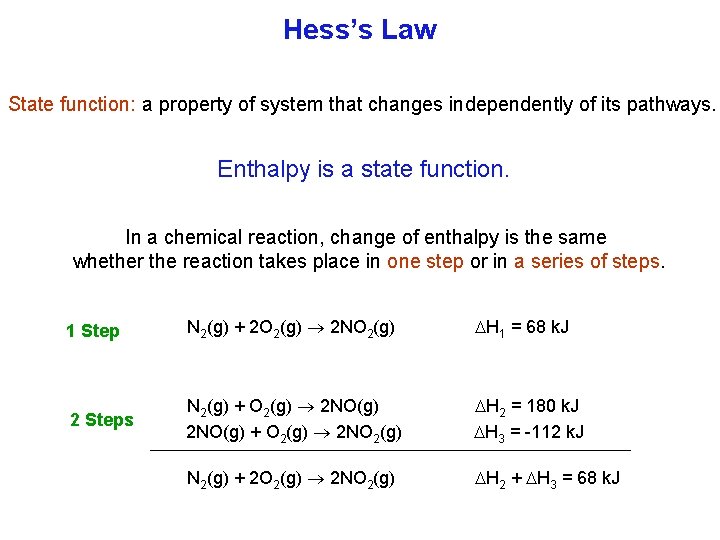
Hess’s Law State function: a property of system that changes independently of its pathways. Enthalpy is a state function. In a chemical reaction, change of enthalpy is the same whether the reaction takes place in one step or in a series of steps. 1 Step N 2(g) + 2 O 2(g) 2 NO 2(g) H 1 = 68 k. J 2 Steps N 2(g) + O 2(g) 2 NO(g) + O 2(g) 2 NO 2(g) H 2 = 180 k. J H 3 = 112 k. J N 2(g) + 2 O 2(g) 2 NO 2(g) H 2 + H 3 = 68 k. J

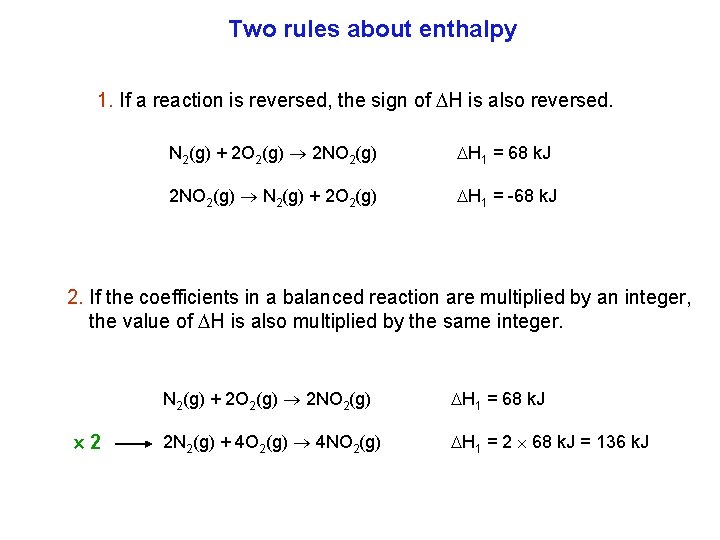
Two rules about enthalpy 1. If a reaction is reversed, the sign of H is also reversed. N 2(g) + 2 O 2(g) 2 NO 2(g) H 1 = 68 k. J 2 NO 2(g) N 2(g) + 2 O 2(g) H 1 = 68 k. J 2. If the coefficients in a balanced reaction are multiplied by an integer, the value of H is also multiplied by the same integer. 2 N 2(g) + 2 O 2(g) 2 NO 2(g) H 1 = 68 k. J 2 N 2(g) + 4 O 2(g) 4 NO 2(g) H 1 = 2 68 k. J = 136 k. J

Combining Reaction Enthalpies: Hess's Law Hess's law is a way to find unknown enthalpies. • Step 1: Select one of the reactants in the overall reaction and write down a chemical equation in which it also appears as a reactant; • Step 2: Select one of the products in the overall reaction and write down a chemical equation in which it also appears as a product; • Step 3: Cancel unwanted species in the sum; • Step 4: Once the sequence is complete, combine the standard reaction enthalpies; Note: Reversing the equation, changes the sign of enthalpy; Note: Mul tiplying by a factor and so multiply the reaction enthalpyby the same factor.



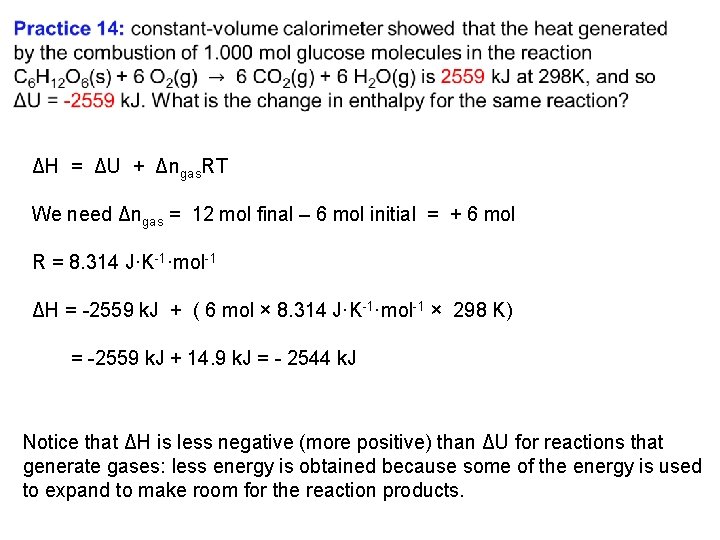
The Relation Between ΔH and ΔU Previous: ΔH = ΔU + PΔV For reactions in which no gas is generated or consumed, little expansion work is done the difference between ΔH and ΔU is negligible; so we can set ΔH = ΔU. However, if a gas is formed in the reaction and expansion work is done the difference can be significant We can use the ideal gas law to relate the values of ΔH and ΔU for gases that behave ideally. The relation between ΔH and ΔU, is H = U + PV, or H = U + n. RT ΔH = ΔU + Δngas. RT

ΔH = ΔU + Δngas. RT We need Δngas = 12 mol final – 6 mol initial = + 6 mol R = 8. 314 J·K 1·mol 1 ΔH = 2559 k. J + ( 6 mol × 8. 314 J·K 1·mol 1 × 298 K) = 2559 k. J + 14. 9 k. J = 2544 k. J Notice that ΔH is less negative (more positive) than ΔU for reactions that generate gases: less energy is obtained because some of the energy is used to expand to make room for the reaction products.

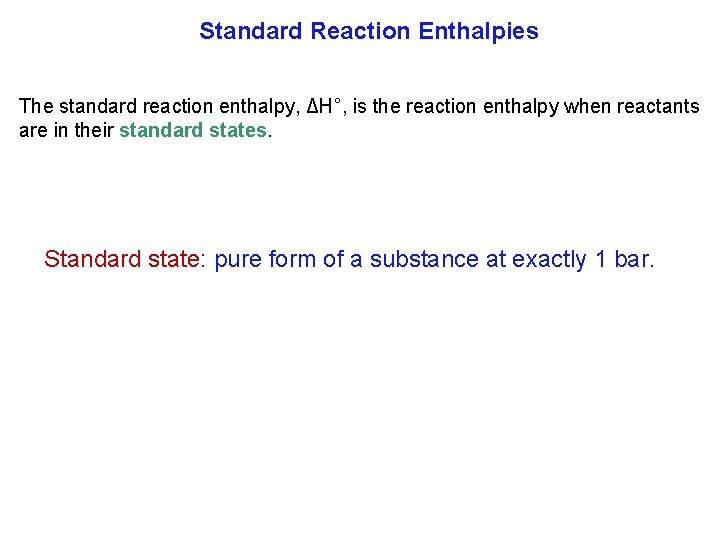
Standard Reaction Enthalpies The standard reaction enthalpy, ΔH°, is the reaction enthalpy when reactants are in their standard states. Standard state: pure form of a substance at exactly 1 bar.

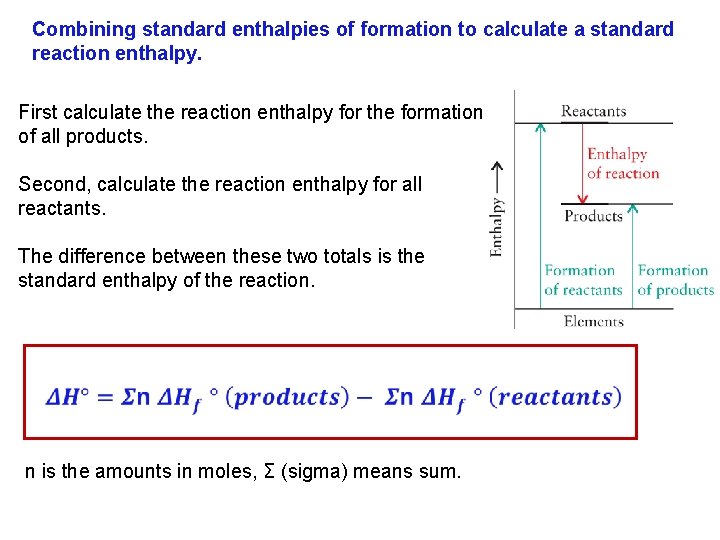
Combining standard enthalpies of formation to calculate a standard reaction enthalpy. First calculate the reaction enthalpy for the formation of all products. Second, calculate the reaction enthalpy for all reactants. The difference between these two totals is the standard enthalpy of the reaction. n is the amounts in moles, Σ (sigma) means sum.


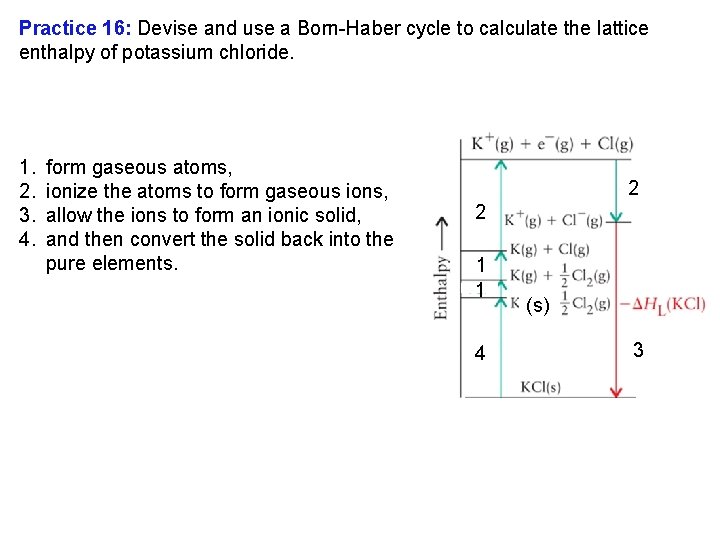
The Born Haber Cycle In focus 2 we calculated energy changes in an ionic system to find lattice energy. The difference in molar enthalpy of solid and gas is called the lattice enthalpy of the solid, ΔHL. The lattice enthalpy of a solid cannot be measured directly. Instead we measure it indirectly by using an application of Hess's law. The procedure uses a Born Haber cycle, a closed path of steps.

The Born Haber Cycle In a Born Haber cycle, we a) break apart the bulk elements into atoms, b) ionize the atoms, b a c) combine the gaseous ions to form the ionic solid, d d) then form the elements again from the ionic solid, The sum of a complete Born Haber cycle is zero, because the enthalpy of the system must be the same at the start and finish. Note: Lattice energies are “ ”, though when calculated with the Born Haber cycle the value will be positive. c

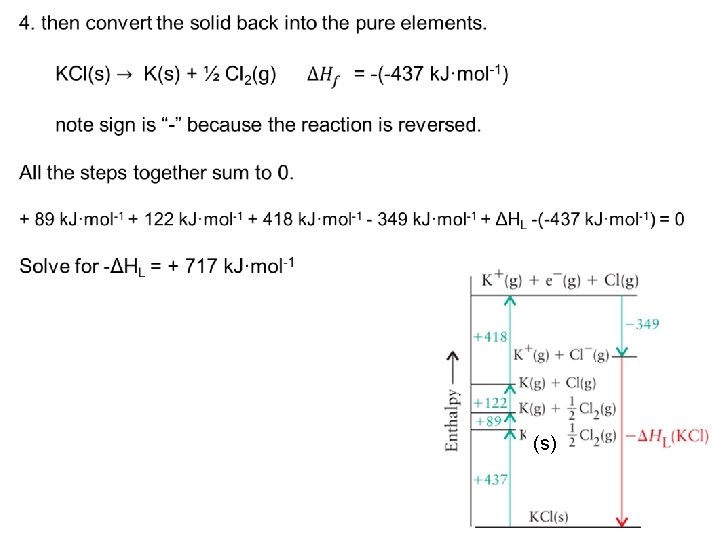
Practice 16: Devise and use a Born Haber cycle to calculate the lattice enthalpy of potassium chloride. 1. 2. 3. 4. form gaseous atoms, ionize the atoms to form gaseous ions, allow the ions to form an ionic solid, and then convert the solid back into the pure elements. 2 2 1 1 4 (s) 3


(s) 81
 200 + 200 + 300
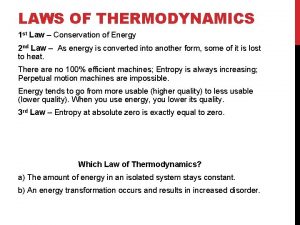
200 + 200 + 300 Newtons third law of thermodynamics
Newtons third law of thermodynamics Usuf thermodynamics
Usuf thermodynamics Isobaric process formula
Isobaric process formula 1th law of thermodynamics
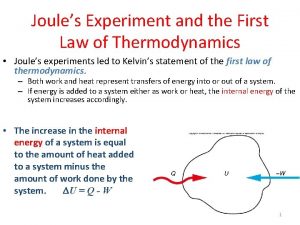
1th law of thermodynamics Joule's experiment in thermodynamics
Joule's experiment in thermodynamics Thermodynamics first law
Thermodynamics first law In a flow process the work transfer may be of which type
In a flow process the work transfer may be of which type First law of thermodynamics sign convention
First law of thermodynamics sign convention Enthalpy vs internal energy
Enthalpy vs internal energy Gibbs free energy
Gibbs free energy Thermodynamics of ideal gases
Thermodynamics of ideal gases Newton's first law and second law and third law
Newton's first law and second law and third law Si unit of newton's first law
Si unit of newton's first law Language
Language Porter's competitive strategies
Porter's competitive strategies Differentiation business level strategy
Differentiation business level strategy Actor focus vs object focus
Actor focus vs object focus Statistical thermodynamics in chemistry
Statistical thermodynamics in chemistry 11th chemistry thermodynamics lec 13
11th chemistry thermodynamics lec 13 Ap chemistry thermodynamics
Ap chemistry thermodynamics Thermodynamics ap chemistry
Thermodynamics ap chemistry 11th chemistry thermodynamics lec 10
11th chemistry thermodynamics lec 10 200+200+100+100
200+200+100+100 400+400+400+200
400+400+400+200 300 + 200 + 200
300 + 200 + 200 200+200+100
200+200+100 100 + 100 200
100 + 100 200 100 200 300
100 200 300 Dr erukhimova
Dr erukhimova Thermodynamic
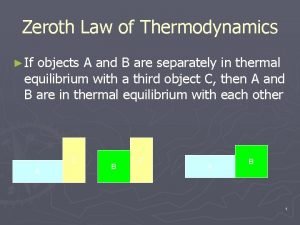
Thermodynamic Zeroth law of thermodynamics examples
Zeroth law of thermodynamics examples Kelvin planck statement
Kelvin planck statement Zeroth law of thermodynamics
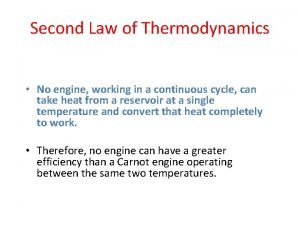
Zeroth law of thermodynamics What is second law of thermodynamics
What is second law of thermodynamics Law of thermodynamics
Law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Thermodynamics enthalpy of reaction and hess's law
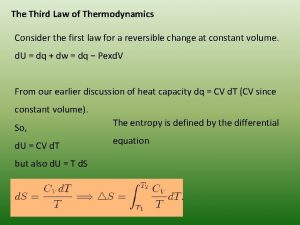
Thermodynamics enthalpy of reaction and hess's law Third law of thermodynamics is depend on
Third law of thermodynamics is depend on Dq=cdt
Dq=cdt 1st law of thermodynamics
1st law of thermodynamics 1st law of thermodynamics
1st law of thermodynamics Zeroth law of thermodynamics
Zeroth law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Zeroth law of thermodynamics
Zeroth law of thermodynamics 2nd law of thermodynamics
2nd law of thermodynamics Laws of thermodynamics
Laws of thermodynamics Frist law of thermodynamics
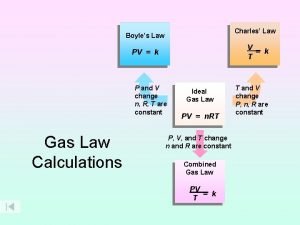
Frist law of thermodynamics V=k/p
V=k/p P=k/v
P=k/v Ib organic chemistry
Ib organic chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Frameset trong html5
Frameset trong html5 Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Tư thế worm breton là gì
Tư thế worm breton là gì Hát lên người ơi
Hát lên người ơi Môn thể thao bắt đầu bằng chữ f
Môn thể thao bắt đầu bằng chữ f Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế
Cái miệng nó xinh thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Giọng cùng tên là
Giọng cùng tên là Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Fecboak
Fecboak Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ