Chapter 19 Spontaneity entropy and free energy rev
































- Slides: 40

Chapter 19 Spontaneity, entropy and free energy (rev. 11/09/08)

Definition l Entropy (S) is disorder, or randomness, of a system

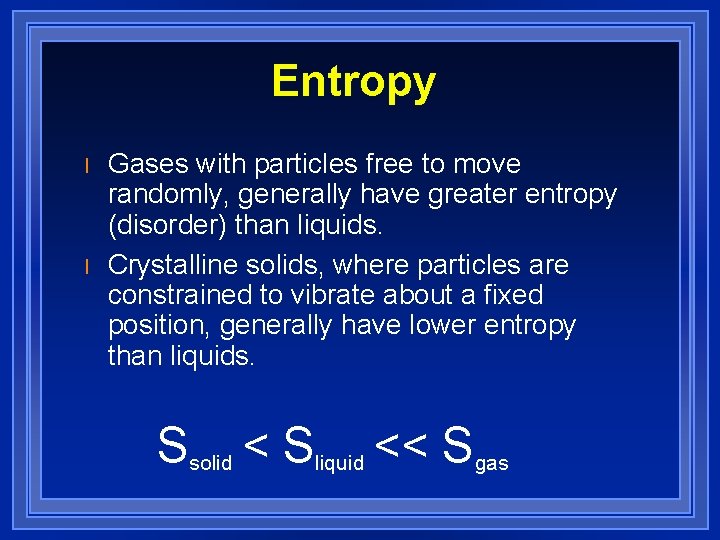
Entropy l l Gases with particles free to move randomly, generally have greater entropy (disorder) than liquids. Crystalline solids, where particles are constrained to vibrate about a fixed position, generally have lower entropy than liquids. Ssolid < Sliquid << Sgas

Entropy and Gases l l Within a sample of gas, the entropy increases with increased volume (and consequently decreases with increased pressure), as a greater volume allows the particles greater freedom of position and increased disorder. The entropy of a gas sample also increases with increased temperature

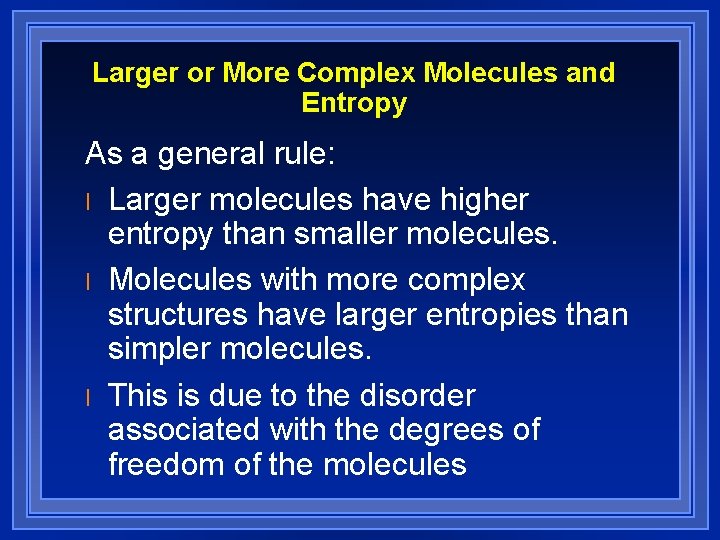
Larger or More Complex Molecules and Entropy As a general rule: l Larger molecules have higher entropy than smaller molecules. l Molecules with more complex structures have larger entropies than simpler molecules. l This is due to the disorder associated with the degrees of freedom of the molecules

Thermodynamics l 1 st Law- the energy of the universe is constant. l 2 nd Law - the entropy of the universe increases.

3 rd Law l l The entropy of a pure crystal at 0 K is 0. The pure crystal entropy gives us a starting point. All other entropy values must be >0. (liquids and gases are more random, therefore their values should be more positive)

l The entropy of an element or compound under any set of conditions is the entropy gained by converting the substance from 0 K to the defined conditions.

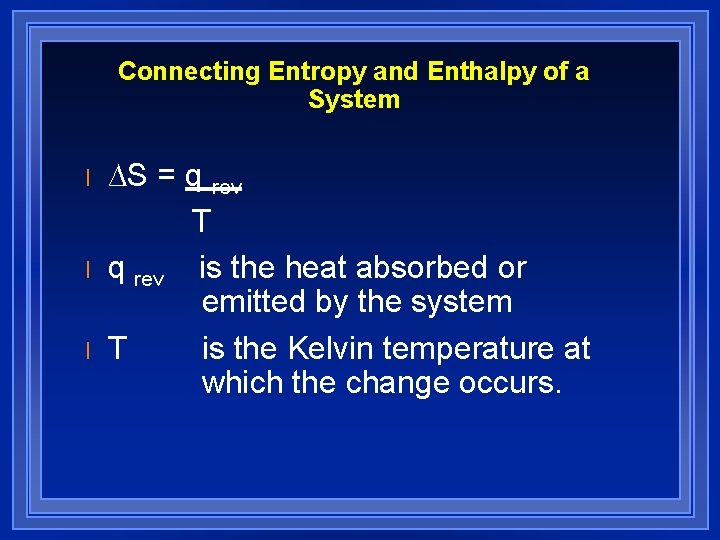
Connecting Entropy and Enthalpy of a System l l l ∆S = q rev T q rev is the heat absorbed or emitted by the system T is the Kelvin temperature at which the change occurs.

l l Because it is necessary to add heat to raise the temperature, all substances have positive entropy values at temperatures above 0 K. Based on the 3 rd law of thermodynamics, negative values of entropy cannot occur.

l Standard Entropy Sº (at 298 K and 1 atm) of substances are listed in the textbook Appendix. The standard entropy, Sº , of a substance, is the entropy gained by converting it from a perfect crystal at 0 K to standard state conditions. Units for entropy are: J/mole • K

l l l DSº = ∑ n. S º prod - ∑ m. S º reac Sº is a state function “n” and “m” are the number of moles from the balanced equation.

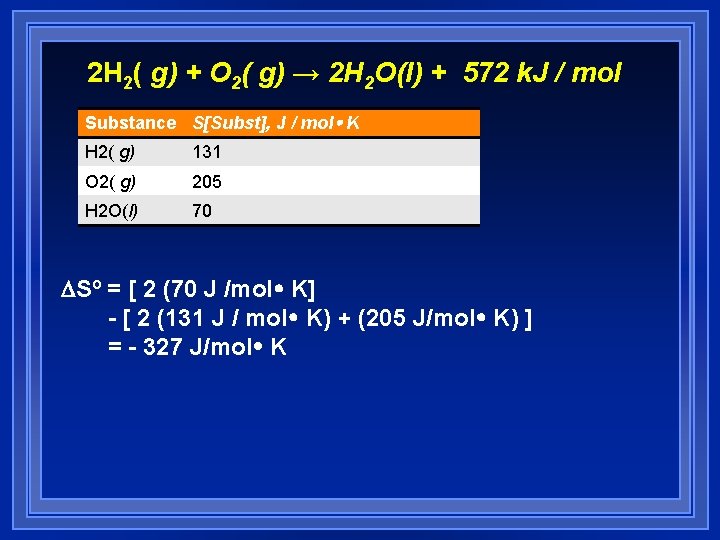
2 H 2( g) + O 2( g) → 2 H 2 O(l) + 572 k. J / mol Substance S[Subst], J / mol K H 2( g) 131 O 2( g) 205 H 2 O(l) 70 DSº = [ 2 (70 J /mol K] - [ 2 (131 J / mol K) + (205 J/mol K) ] = - 327 J/mol K

Intuitively Speaking l Intuitively, the balanced equation reflects the conversion of three moles of highly disordered gases to form two moles of less disordered liquid, it is expected that the change in entropy of the reaction would be negative.

l The second law of thermodynamics states that “in any spontaneously occurring process, the total entropy of the universe will increase. ” Thus, a chemical reaction or physical change will proceed spontaneously in the direction that increases the total entropy of the universe.

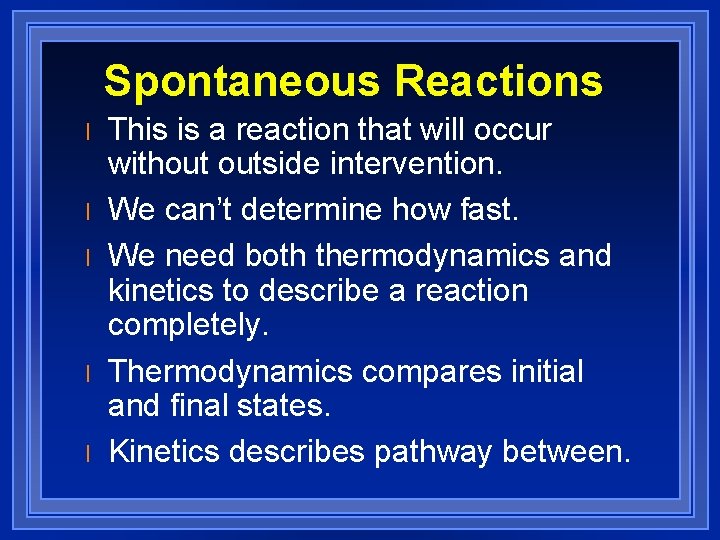
Spontaneous Reactions l l l This is a reaction that will occur without outside intervention. We can’t determine how fast. We need both thermodynamics and kinetics to describe a reaction completely. Thermodynamics compares initial and final states. Kinetics describes pathway between.

Entropy of Solutions l Solutions form because there are many more possible arrangements of dissolved pieces than if they stay separate.

2 nd Law l DSuniv = DSsys + DSsurr l If DSuniv is positive the process is spontaneous. l If DSuniv is negative the process is spontaneous in the opposite direction.

l l l For exothermic processes DSsurr is positive. For endothermic processes DSsurr is negative. Consider this process H 2 O(l)® H 2 O(g) DSsys is positive DSsurr is negative DSuniv depends on temperature.

Temperature and Spontaneity l l l Entropy changes in the surroundings are determined by the heat flow. An exothermic process is favored because by giving up heat the entropy of the surroundings increases. The magnitude of DSsurr depends on temperature DSsurr = -DH/T In a previous slide you saw DS = qrev /T the DS was for the system

DSsys DSsurr DSuniv + + + Yes No, Reverse + - ? At High temp. - + ? At Low temp. Spontaneous?

Free Energy And Work l l l Free energy is: energy free to do work. It is the maximum amount of work possible at a given temperature and pressure. It is never really achieved because some of the free energy is changed to heat during a reaction, so it can’t be used to do work.

Gibbs Free Energy l Gibbs free energy is a thermodynamic property that indicates the amount of energy available for the system to do useful work at constant T and P and GFE is used to predict whether a process will occur spontaneously at constant temperature and pressure. Gibbs free energy “G” is defined as G = H - TS where H, T and S are the enthalpy, temperature, and entropy.

Gibb's Free Energy l l l l G = H - TS The equation is never used this way. DG=DH-TDS at constant temperature Divide by -T - DG/T = - DH/T - DS - DG/T = DSsurr + DSsys - DG/T = DSuniv If DG is negative at constant T and P, the process is spontaneous.

Let’s Check l l l For the reaction H 2 O(s) ® H 2 O(l) DSº = 22. 1 J/K mol DHº =6030 J/mol Look at the equation DG=DH-TDS Spontaneity can be predicted from the sign of DH and DS. Predict the sign of DG.

Try the Calculation l For the reaction H 2 O(s) ® H 2 O(l) DSº = 22. 1 J/K mol DHº =6030 J/mol l Calculate DG at 10ºC and -10ºC l

Objectives l l l SWBAT Practice solving for ΔG using various methods. Practice solving for ΔG when the conditions are not standard state.

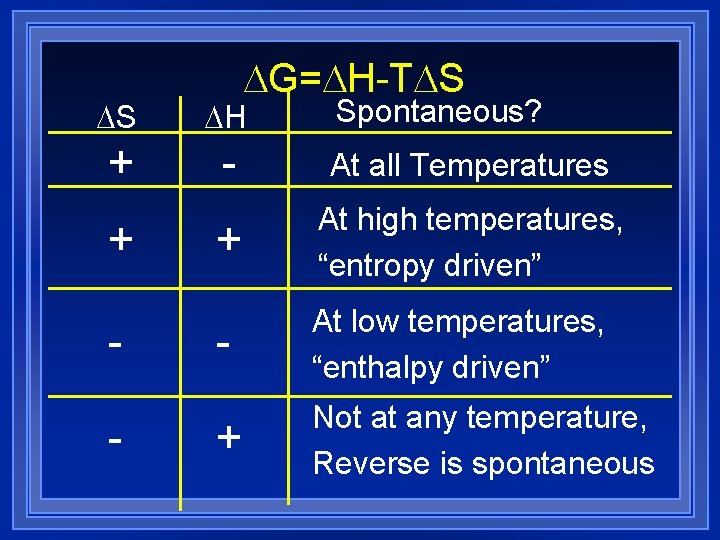
DG=DH-TDS Spontaneous? DS DH + - At all Temperatures + At high temperatures, “entropy driven” - At low temperatures, “enthalpy driven” + Not at any temperature, Reverse is spontaneous + -

Free Energy in Reactions l l l DGº = standard free energy change. Free energy change that will occur if reactants (in their standard state) turn into products (in their standard state). Can’t be measured directly, can be calculated from other measurements. DGº = DHº - TDSº Use Hess’s Law with known reactions.

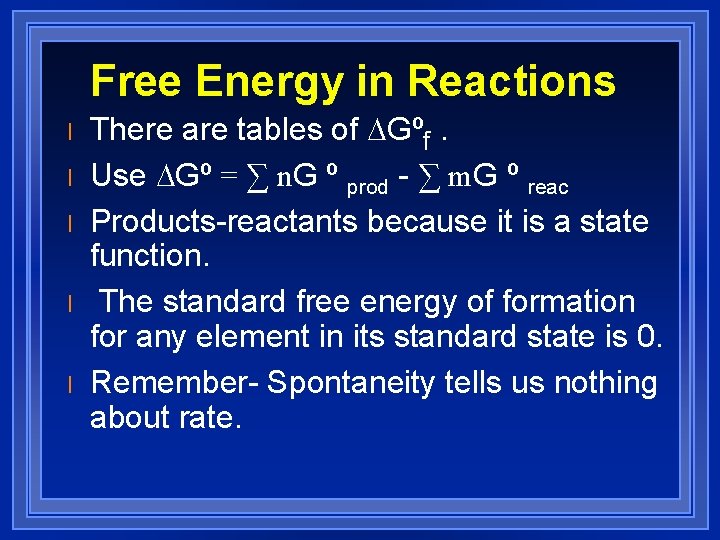
Free Energy in Reactions l l l There are tables of DGºf. Use DGº = ∑ n. G º prod - ∑ m. G º reac Products-reactants because it is a state function. The standard free energy of formation for any element in its standard state is 0. Remember- Spontaneity tells us nothing about rate.

Not Standard Conditions? l l Thus far, only reactions occurring under standard concentration or pressure conditions, as indicated by the superscript “°” have been considered. However, the concentration of each species in the equation does have an effect on the values of thermodynamic properties of the reaction.

l An increase in the concentration of the reactant species, or a decrease in the concentration of the product species, will provide a greater driving force to the reaction and will decrease the value of ΔGRxn from its value at standard concentrations.

l Similarly, an increase in product concentrations or a decrease in reactant concentrations will have the effect of increasing the value of ΔG Rxn from its value at standard concentrations.

Gibbs-Helmoltz Eqn l DG = DGº + RT ln(Q) where Q is the reaction quotient R is the gas constant R is 8. 31 x 10 -3 k. J /mole K

l Reactions that may occur spontaneously under standard concentrations (ΔGrxn < 0) may not occur spontaneously at concentrations in which Q is sufficiently great ΔG = ΔG° + RT(ln Q) > 0

Free energy and Pressure l DG = DGº + RT ln(Q) where Q is the reaction quotient CO(g) + 2 H 2(g) ® CH 3 OH(l) l Would the reaction be spontaneous at 25ºC with the H 2 pressure of 5. 0 atm and the CO pressure of 3. 0 atm? l DGºf CH 3 OH(l) = -166 k. J DGºf CO(g) = -137 k. J DGºf H 2(g) = 0 k. J l

How far will the free energy go? l l l DG tells us spontaneity at current conditions. When will it stop? It will go to the lowest possible free energy which may be an equilibrium position. At equilibrium DG = 0, Q = K ΔG = ΔG° + RT(ln Q) DGº = - RT ln. K

DGº = - RT ln K K DGº =1 >0 <0 =0 <0 >0 The connection between ∆Gº and K.

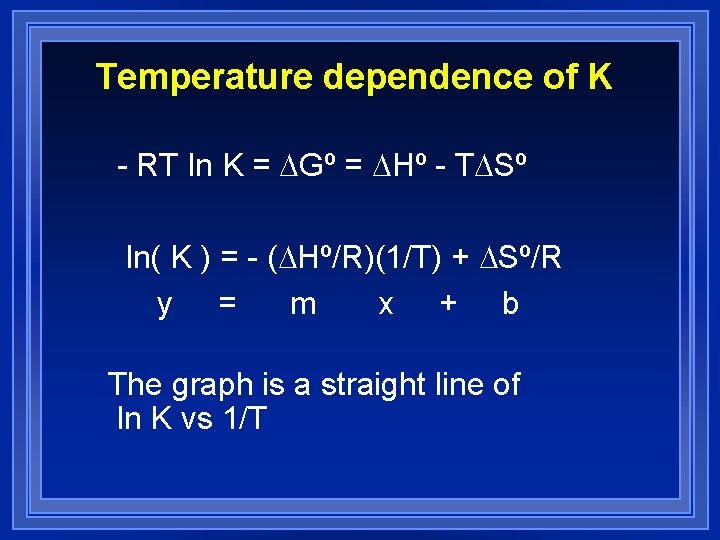
Temperature dependence of K - RT ln K = DGº = DHº - TDSº ln( K ) = - (DHº/R)(1/T) + DSº/R y = m x + b The graph is a straight line of ln K vs 1/T

ΔG = ΔG° + R T ln Q 0 = ΔG° + R T ln Keq ΔG° = - R T ln Keq or rearrange to: Keq = e –ΔG° ⁄R T
 Ap chemistry spontaneity entropy and free energy
Ap chemistry spontaneity entropy and free energy What is spontaneity in chemistry
What is spontaneity in chemistry Bpch21
Bpch21 Gibbs free energy and spontaneity
Gibbs free energy and spontaneity Formulas mcua
Formulas mcua Relationship between entropy and free energy
Relationship between entropy and free energy Entropy and gibbs free energy
Entropy and gibbs free energy Helmholtz free energy and gibbs free energy
Helmholtz free energy and gibbs free energy Is delta g spontaneous
Is delta g spontaneous Enthalpy entropy free energy
Enthalpy entropy free energy Gibbs free energy
Gibbs free energy Free gibbs energy
Free gibbs energy Predicting spontaneity
Predicting spontaneity Predicting spontaneity
Predicting spontaneity S
S Predicting spontaneity
Predicting spontaneity Complete the following table on reaction spontaneity
Complete the following table on reaction spontaneity Building redox tables
Building redox tables Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Unit 3 rev. statehood and westward expansion
Unit 3 rev. statehood and westward expansion That was then this is now summary
That was then this is now summary Optimistic poem
Optimistic poem Q system = -q surroundings
Q system = -q surroundings What is enthalpy and entropy
What is enthalpy and entropy Minimum enthalpy maximum entropy
Minimum enthalpy maximum entropy Entropy of the universe
Entropy of the universe Entropy order parameters and complexity
Entropy order parameters and complexity Rev 21:27
Rev 21:27 Ekskrementer fra hare
Ekskrementer fra hare Rev paul pediatr
Rev paul pediatr Nstm 300 revision 10
Nstm 300 revision 10 Slová z hlbín dávnych vekov rozbor
Slová z hlbín dávnych vekov rozbor Blinkin rev
Blinkin rev Radians
Radians Revelation 22 nasb
Revelation 22 nasb Who is rebecca nurse in the crucible
Who is rebecca nurse in the crucible Judge thomas danforth
Judge thomas danforth Revelation 1:1-3
Revelation 1:1-3 Remember the titans main characters
Remember the titans main characters Rev 21 16
Rev 21 16