Spontaneity Entropy and Free Energy Chapter 10 Email

















- Slides: 41

Spontaneity, Entropy and Free Energy Chapter 10 E-mail: benzene 4 president@gmail Web-site: http: //clas. sa. ucsb. edu/staff/terri/

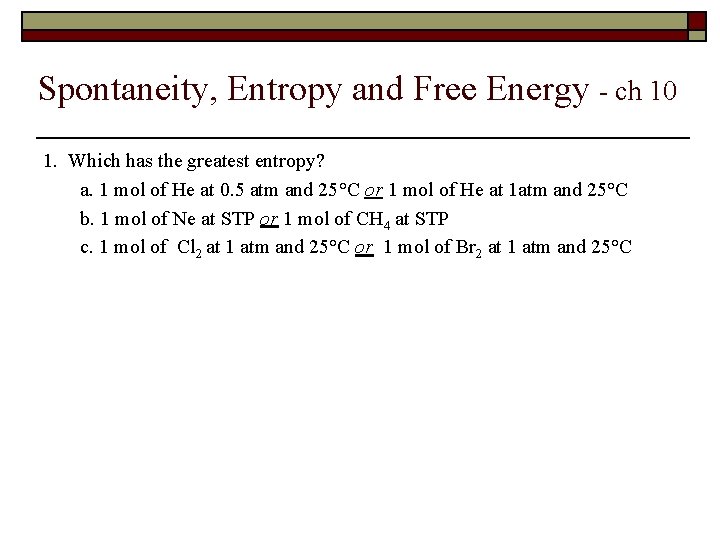
Spontaneity, Entropy and Free Energy - ch 10 1. Which has the greatest entropy? a. 1 mol of He at 0. 5 atm and 25°C or 1 mol of He at 1 atm and 25°C b. 1 mol of Ne at STP or 1 mol of CH 4 at STP c. 1 mol of Cl 2 at 1 atm and 25°C or 1 mol of Br 2 at 1 atm and 25°C

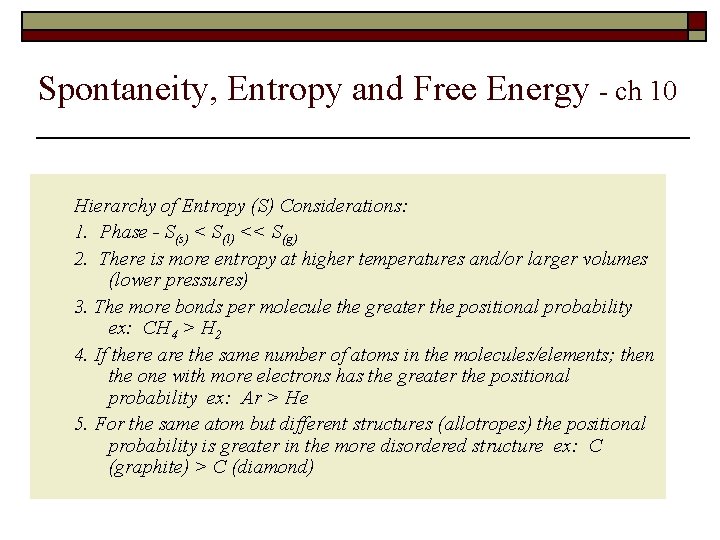
Spontaneity, Entropy and Free Energy - ch 10 Hierarchy of Entropy (S) Considerations: 1. Phase - S(s) < S(l) << S(g) 2. There is more entropy at higher temperatures and/or larger volumes (lower pressures) 3. The more bonds per molecule the greater the positional probability ex: CH 4 > H 2 4. If there are the same number of atoms in the molecules/elements; then the one with more electrons has the greater the positional probability ex: Ar > He 5. For the same atom but different structures (allotropes) the positional probability is greater in the more disordered structure ex: C (graphite) > C (diamond)

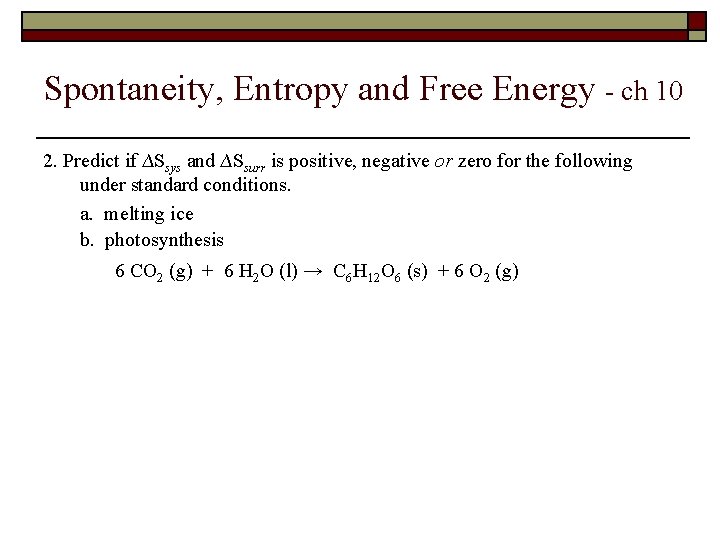
Spontaneity, Entropy and Free Energy - ch 10 2. Predict if ∆Ssys and ∆Ssurr is positive, negative or zero for the following under standard conditions. a. melting ice b. photosynthesis 6 CO 2 (g) + 6 H 2 O (l) → C 6 H 12 O 6 (s) + 6 O 2 (g)

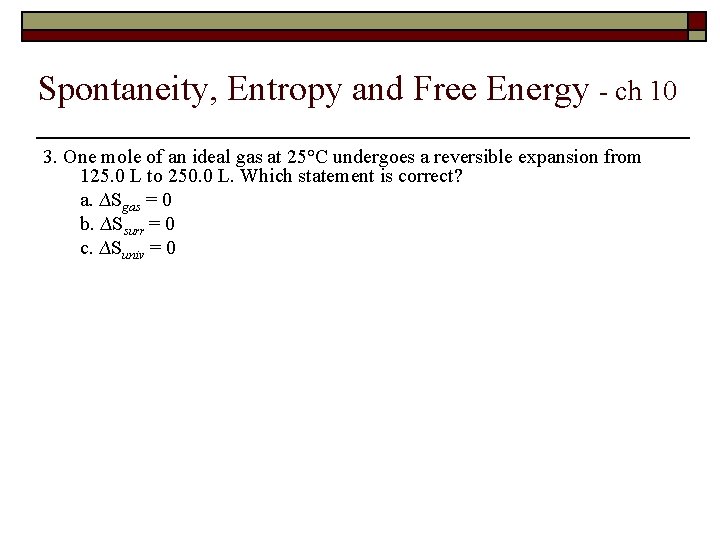
Spontaneity, Entropy and Free Energy - ch 10 3. One mole of an ideal gas at 25°C undergoes a reversible expansion from 125. 0 L to 250. 0 L. Which statement is correct? a. ∆Sgas = 0 b. ∆Ssurr = 0 c. ∆Suniv = 0

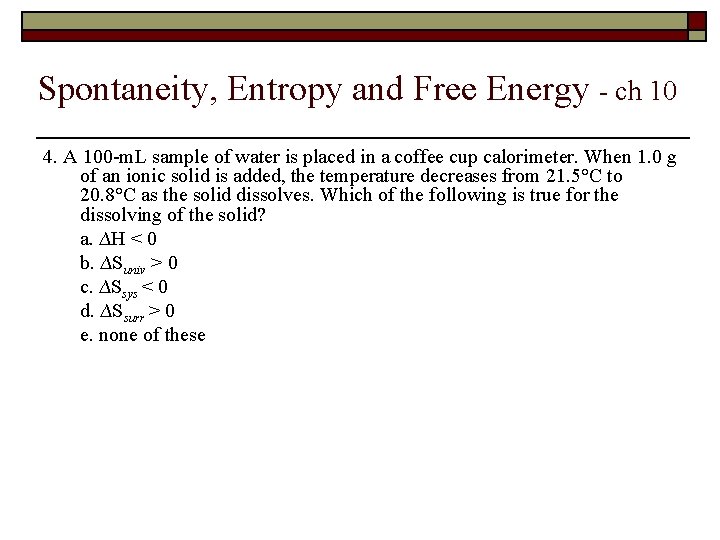
Spontaneity, Entropy and Free Energy - ch 10 4. A 100 -m. L sample of water is placed in a coffee cup calorimeter. When 1. 0 g of an ionic solid is added, the temperature decreases from 21. 5°C to 20. 8°C as the solid dissolves. Which of the following is true for the dissolving of the solid? a. ∆H < 0 b. ∆Suniv > 0 c. ∆Ssys < 0 d. ∆Ssurr > 0 e. none of these

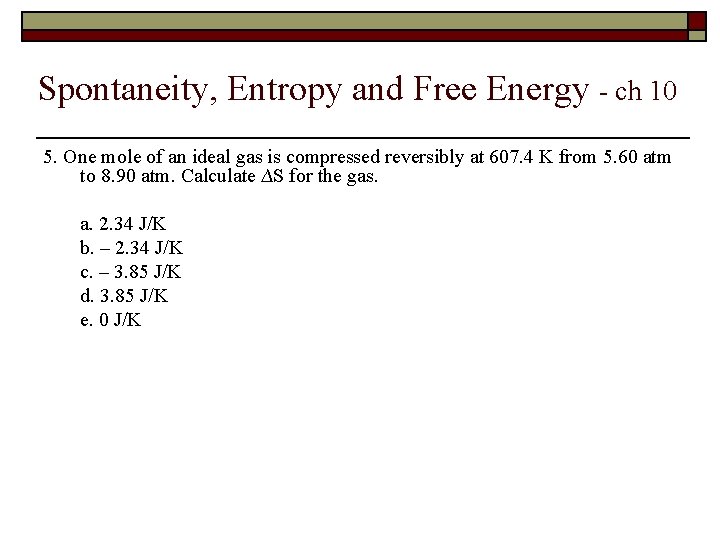
Spontaneity, Entropy and Free Energy - ch 10 5. One mole of an ideal gas is compressed reversibly at 607. 4 K from 5. 60 atm to 8. 90 atm. Calculate ∆S for the gas. a. 2. 34 J/K b. – 2. 34 J/K c. – 3. 85 J/K d. 3. 85 J/K e. 0 J/K

Spontaneity, Entropy and Free Energy - ch 10 6. Calculate the change in entropy (in J/K) for a process in which 54 g of ice at – 5 °C is mixed with 112 g of liquid water at 100°C in a perfectly insulated container. The specific heat capacities of ice and liquid water is 2. 03 J/g°C and 4. 18 J/g°C respectively. The heat of fusion for water is 6. 01 k. J/mol.

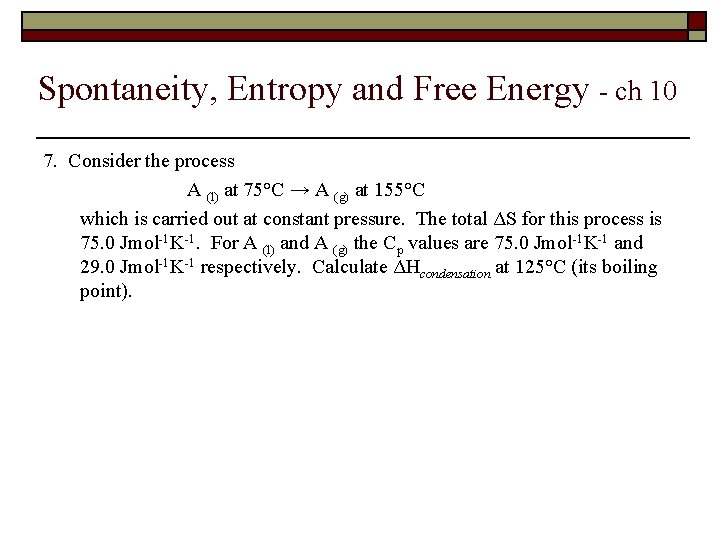
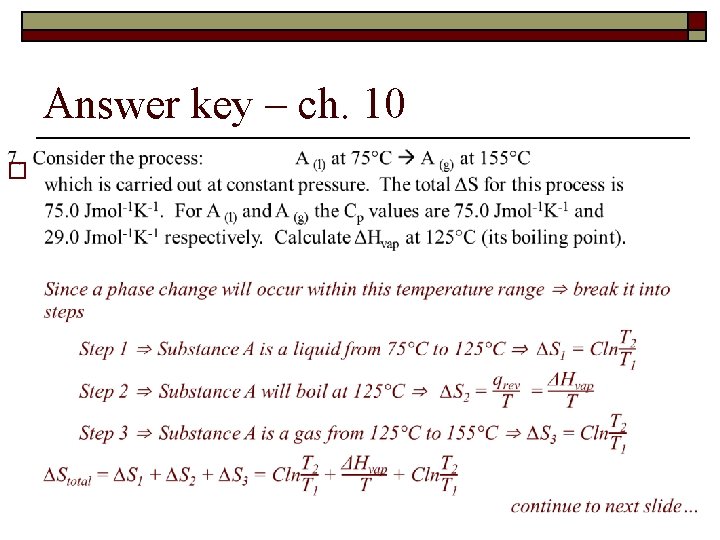
Spontaneity, Entropy and Free Energy - ch 10 7. Consider the process A (l) at 75°C → A (g) at 155°C which is carried out at constant pressure. The total ΔS for this process is 75. 0 Jmol-1 K-1. For A (l) and A (g) the Cp values are 75. 0 Jmol-1 K-1 and 29. 0 Jmol-1 K-1 respectively. Calculate ΔHcondensation at 125°C (its boiling point).

Spontaneity, Entropy and Free Energy - ch 10 8. Indicate true or false for each of the following statements: a. Spontaneous reactions must have a positive ΔSº for the reaction. b. When the change in free energy is less than zero for a chemical reaction, the reaction must be exothermic. For a spontaneous reaction, if ΔSº < 0 then the reaction must be exothermic.

Spontaneity, Entropy and Free Energy - ch 10 Spontaneous ⇒ wants to go forward on its own K > Q ∆Suniverse > 0 or ∆Gsystem < 0 Non-spontaneous ⇒ wants to go backward on its own K < Q ∆Suniverse < 0 or ∆Gsystem > 0 Equilibrium ⇒ doesn’t prefer one direction over the other K = Q ∆Suniverse = 0 or ∆Gsystem = 0

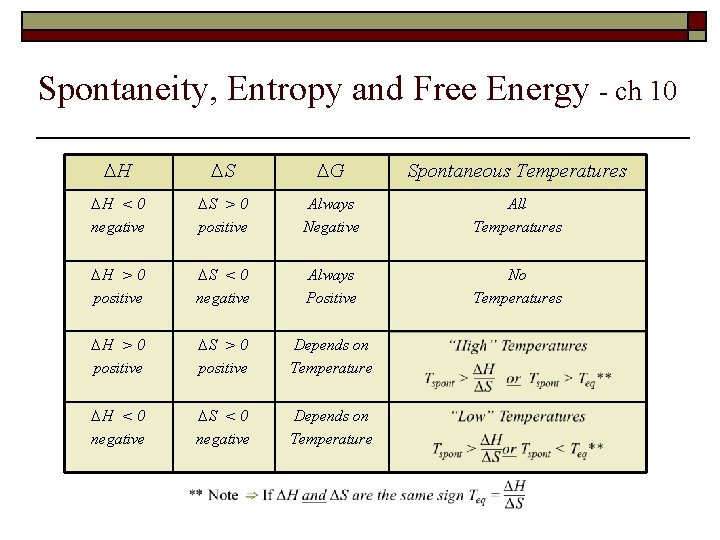
Spontaneity, Entropy and Free Energy - ch 10 ∆H ∆S ∆G Spontaneous Temperatures ∆H < 0 negative ∆S > 0 positive Always Negative All Temperatures ∆H > 0 positive ∆S < 0 negative Always Positive No Temperatures ∆H > 0 positive ∆S > 0 positive Depends on Temperature ∆H < 0 negative ∆S < 0 negative Depends on Temperature

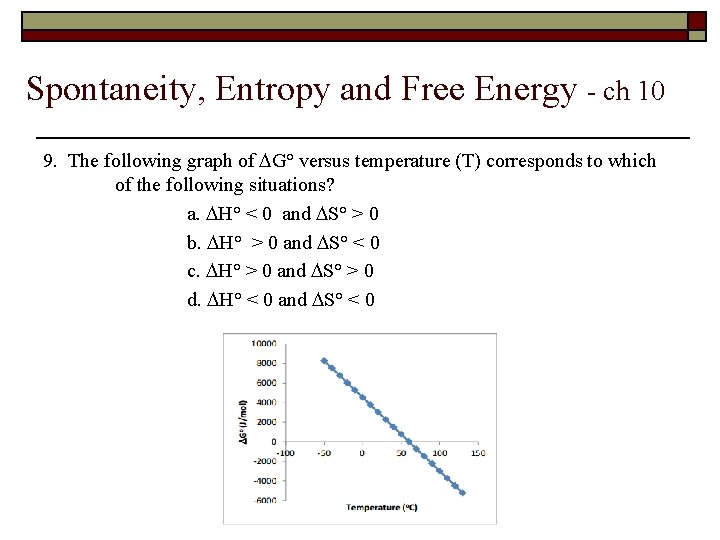
Spontaneity, Entropy and Free Energy - ch 10 9. The following graph of G° versus temperature (T) corresponds to which of the following situations? a. H° < 0 and S° > 0 b. H° > 0 and S° < 0 c. H° > 0 and S° > 0 d. H° < 0 and S° < 0

Spontaneity, Entropy and Free Energy - ch 10 10. Which of the following is true for the dissociation of fluorine? F 2 (g) → 2 F (g) a. spontaneous at all temperatures b. spontaneous at high temperatures c. spontaneous at low temperatures d. never spontaneous

Spontaneity, Entropy and Free Energy - ch 10 11. At 1 atm, the freezing point of mercury is – 39 °C. Which of the following is true regarding the freezing of mercury at – 30 °C and 1 atm? a. Ssurr > 0, Suniv > 0 b. Ssurr > 0, Suniv = 0 c. Ssurr < 0, Suniv > 0 d. Ssurr < 0, Suniv < 0 e. Ssurr > 0, Suniv < 0

Spontaneity, Entropy and Free Energy - ch 10 12. Given the following data, calculate the normal boiling point formic acid (HCOOH). ∆Hfo (k. J/mol) S° (J/K·mol) HCOOH (l) – 410. 130. HCOOH (g) – 363 251 a. 2. 57 K b. 1730°C c. 388°C d. 82°C e. 115°C

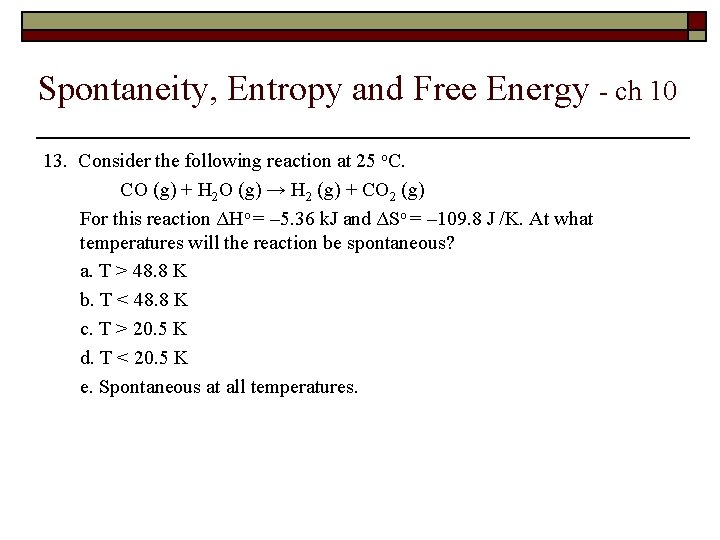
Spontaneity, Entropy and Free Energy - ch 10 13. Consider the following reaction at 25 o. C. CO (g) + H 2 O (g) → H 2 (g) + CO 2 (g) For this reaction ΔHo = – 5. 36 k. J and ΔSo = – 109. 8 J /K. At what temperatures will the reaction be spontaneous? a. T > 48. 8 K b. T < 48. 8 K c. T > 20. 5 K d. T < 20. 5 K e. Spontaneous at all temperatures.

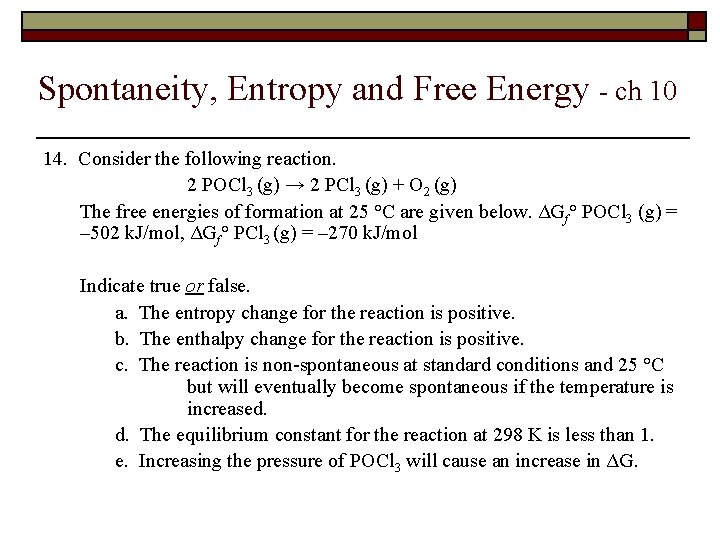
Spontaneity, Entropy and Free Energy - ch 10 14. Consider the following reaction. 2 POCl 3 (g) → 2 PCl 3 (g) + O 2 (g) The free energies of formation at 25 °C are given below. ΔGf° POCl 3 (g) = – 502 k. J/mol, ΔGf° PCl 3 (g) = – 270 k. J/mol Indicate true or false. a. The entropy change for the reaction is positive. b. The enthalpy change for the reaction is positive. c. The reaction is non-spontaneous at standard conditions and 25 °C but will eventually become spontaneous if the temperature is increased. The equilibrium constant for the reaction at 298 K is less than 1. e. Increasing the pressure of POCl 3 will cause an increase in ΔG.

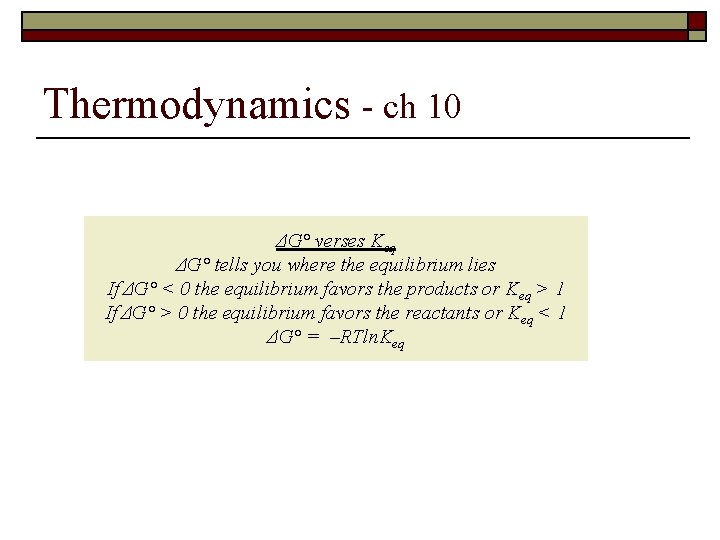
Thermodynamics - ch 10 ΔG° verses Keq ΔG° tells you where the equilibrium lies If ΔG° < 0 the equilibrium favors the products or Keq > 1 If ΔG° > 0 the equilibrium favors the reactants or Keq < 1 ΔG° = –RTln. Keq

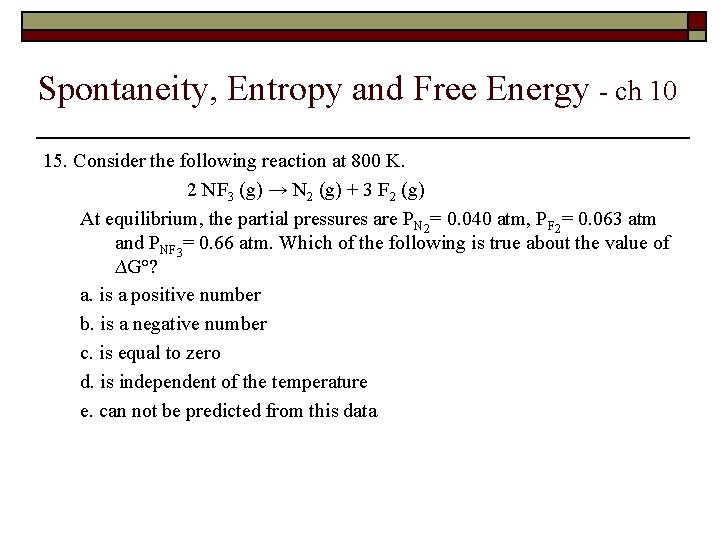
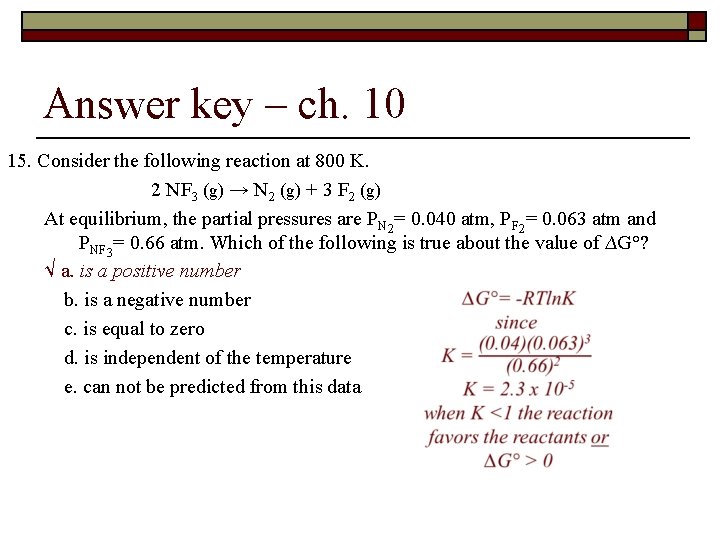
Spontaneity, Entropy and Free Energy - ch 10 15. Consider the following reaction at 800 K. 2 NF 3 (g) → N 2 (g) + 3 F 2 (g) At equilibrium, the partial pressures are PN 2= 0. 040 atm, PF 2= 0. 063 atm and PNF 3= 0. 66 atm. Which of the following is true about the value of ∆G°? a. is a positive number b. is a negative number c. is equal to zero d. is independent of the temperature e. can not be predicted from this data

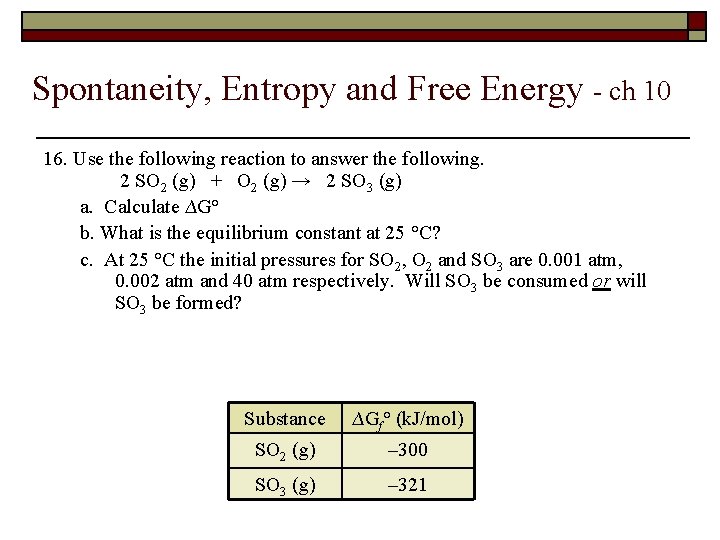
Spontaneity, Entropy and Free Energy - ch 10 16. Use the following reaction to answer the following. 2 SO 2 (g) + O 2 (g) → 2 SO 3 (g) a. Calculate ∆G° b. What is the equilibrium constant at 25 °C? c. At 25 °C the initial pressures for SO 2, O 2 and SO 3 are 0. 001 atm, 0. 002 atm and 40 atm respectively. Will SO 3 be consumed or will SO 3 be formed? Substance ∆Gf° (k. J/mol) SO 2 (g) – 300 SO 3 (g) – 321

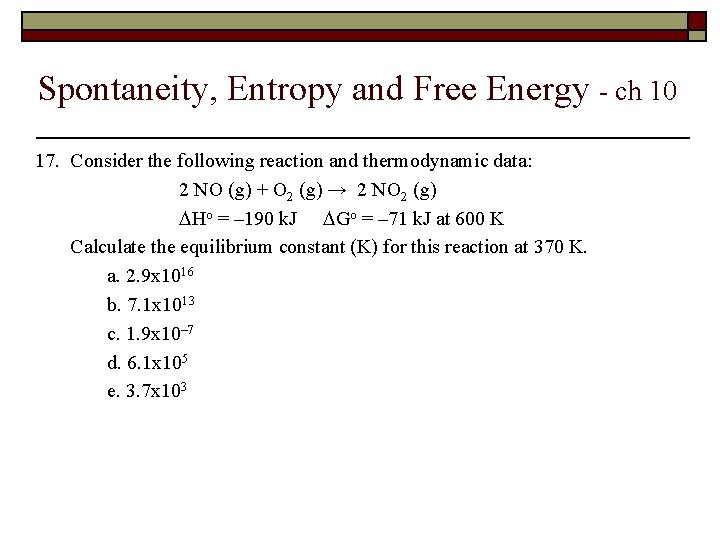
Spontaneity, Entropy and Free Energy - ch 10 17. Consider the following reaction and thermodynamic data: 2 NO (g) + O 2 (g) → 2 NO 2 (g) Ho = – 190 k. J Go = – 71 k. J at 600 K Calculate the equilibrium constant (K) for this reaction at 370 K. a. 2. 9 x 1016 b. 7. 1 x 1013 c. 1. 9 x 10– 7 d. 6. 1 x 105 e. 3. 7 x 103

Spontaneity, Entropy and Free Energy - ch 10 You have completed ch 10

Answer key – ch. 10 1. Which has the greatest entropy? a. 1 mol of He at 0. 5 atm and 25°C or 1 mol of He at 1 atm and 25°C as P↓ , V ↑ , S ↑ b. 1 mol of Ne at STP or 1 mol of CH 4 at STP as the number of atoms/molecule ↑ , S ↑ c. 1 mol of Cl 2 at 1 atm and 25°C or 1 mol of Br 2 at 1 atm and 25°C gases have more entropy than liquids

Answer key – ch. 10 2. Predict if ∆Ssys and ∆Ssurr is positive, negative or zero for the following under standard conditions. a. melting ice ⇒ H 2 O (s) H 2 O (l) ⇒ ∆Ssys >0 since Sliquid>Ssolid ⇒ ∆Hsys>0 since bonds are broken ⇒ ∆Ssurr<0 b. photosynthesis => 6 CO 2 (g) + 6 H 2 O (l) C 6 H 12 O 6 (s) + 6 O 2 (g) ∆Ssys<0 since the moles of gas are equal and considering the moles solids ↑ and liquids ↓ ⇒ ∆Hsys>0 because the reaction is combustion in reverse ⇒ ∆Ssurr<0

Answer key – ch. 10 3. One mole of an ideal gas at 25°C is expanded isothermally and reversibly from 125. 0 L to 250. 0 L. Which statement is correct? a. ∆Sgas = 0 b. ∆Ssurr = 0 ∆Sgas > 0 √ c. ∆Suniv = 0 since expanding a gas causes an increase in entropy qrev > 0 ⇒ ∆Ssurr < 0 ∆Suniv = 0 since reversible a. k. a. equilibrium

Answer key – ch. 10 4. A 100 -m. L sample of water is placed in a coffee cup calorimeter. When 1. 0 g of an ionic solid is added, the temperature decreases from 21. 5°C to 20. 8°C as the solid dissolves. Which of the following is true for the dissolving of the solid? a. ∆H < 0 false ⇒ it was observed that the T of the surroundings cooled √ b. ∆Suniv > 0 true ⇒ because the solid dissolved spontaneously c. ∆Ssys < 0 false ⇒ aqueous ions have more S than in a solid d. ∆Ssurr > 0 false ⇒ since the dissolving was endothermic e. none of these

Answer key – ch. 10 5. One mole of an ideal gas is compressed isothermally and reversibly at 607. 4 K from 5. 60 atm to 8. 90 atm. Calculate ∆S for the gas. a. 2. 34 J/K b. -2. 34 J/K ∆S = n. Rln. V 2/V 1 √ c. -3. 85 J/K since P 1 V 1=P 2 V 2 d. 3. 85 J/K ∆S = n. Rln. P 1/P 2 e. 0 J/K ∆S = (1 mol)(8. 314 J/mol. K)(ln(5. 6 atm/8. 9 atm)) ∆S = -3. 85 J/K

Answer key – ch. 10 6. Calculate the change in entropy for a process in which 3. 00 moles of liquid water at 0°C is mixed with 1. 00 mole of liquid water at 100°C in a perfectly insulated container. The molar heat capacity of liquid water is 75. 3 Jmol-l. K-1

Answer key – ch. 10 o

Answer key – ch. 10 o

Answer key – ch. 10 8. Indicate true or false for each of the following statements. a. Spontaneous reactions must have a positive ΔSº for the reaction. False, ΔSº can be either positive or negative in spontaneous reactions b. When the change in free energy is less than zero for a chemical reaction, the reaction must be exothermic. False, ΔHº can be either positive or negative in spontaneous reactions c. For a spontaneous reaction, if ΔSº < 0 then the reaction must be exothermic. True, ΔG = ΔH – TΔS

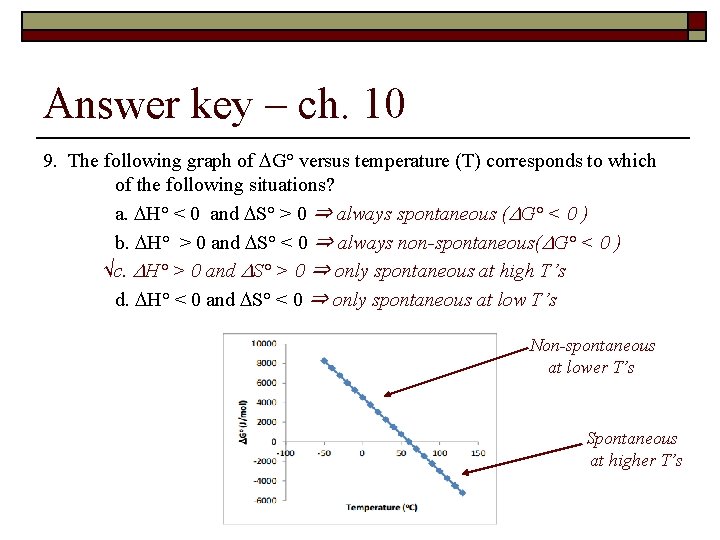
Answer key – ch. 10 9. The following graph of G° versus temperature (T) corresponds to which of the following situations? a. H° < 0 and S° > 0 ⇒ always spontaneous ( G° < 0 ) b. H° > 0 and S° < 0 ⇒ always non-spontaneous( G° < 0 ) √c. H° > 0 and S° > 0 ⇒ only spontaneous at high T’s d. H° < 0 and S° < 0 ⇒ only spontaneous at low T’s Non-spontaneous at lower T’s Spontaneous at higher T’s

Answer key – ch. 10 10. Which of the following is true for the dissociation of fluorine? F 2 (g) → 2 F (g) a. spontaneous at all temperatures √ b. spontaneous at high temperatures c. spontaneous at low temperatures d. never spontaneous

Answer key – ch. 10 11. At 1 atm, the freezing point of mercury is – 39 °C. Which of the following is true regarding the freezing of mercury at – 30 °C and 1 atm? √ a. Ssurr > 0, Suniv > 0 Ssurr > 0 b. Ssurr > 0, Suniv = 0 since freezing is exothermic due to c. Ssurr < 0, Suniv > 0 bonds being formed d. Ssurr < 0, Suniv < 0 Suniv > 0 since the T< FP and e. Ssurr > 0, Suniv < 0 therefore will spontaneously freeze

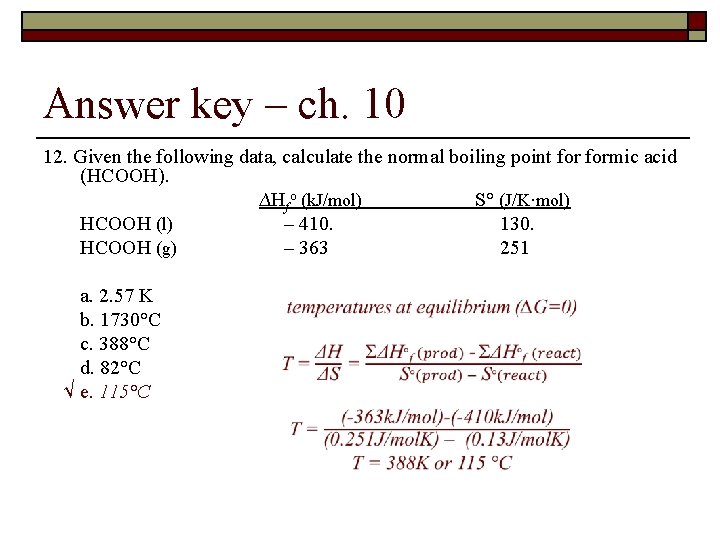
Answer key – ch. 10 12. Given the following data, calculate the normal boiling point formic acid (HCOOH). ∆Hfo (k. J/mol) S° (J/K·mol) HCOOH (l) – 410. 130. HCOOH (g) – 363 251 a. 2. 57 K b. 1730°C c. 388°C d. 82°C √ e. 115°C

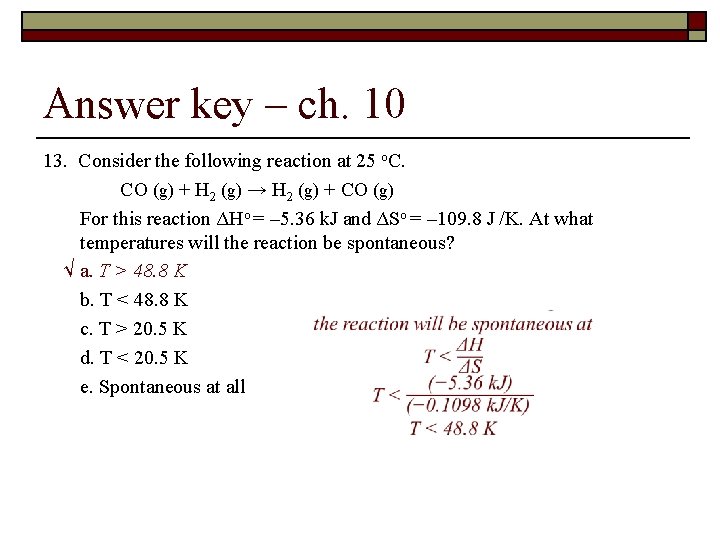
Answer key – ch. 10 13. Consider the following reaction at 25 o. C. CO (g) + H 2 (g) → H 2 (g) + CO (g) For this reaction ΔHo = – 5. 36 k. J and ΔSo = – 109. 8 J /K. At what temperatures will the reaction be spontaneous? √ a. T > 48. 8 K b. T < 48. 8 K c. T > 20. 5 K d. T < 20. 5 K e. Spontaneous at all temperatures.

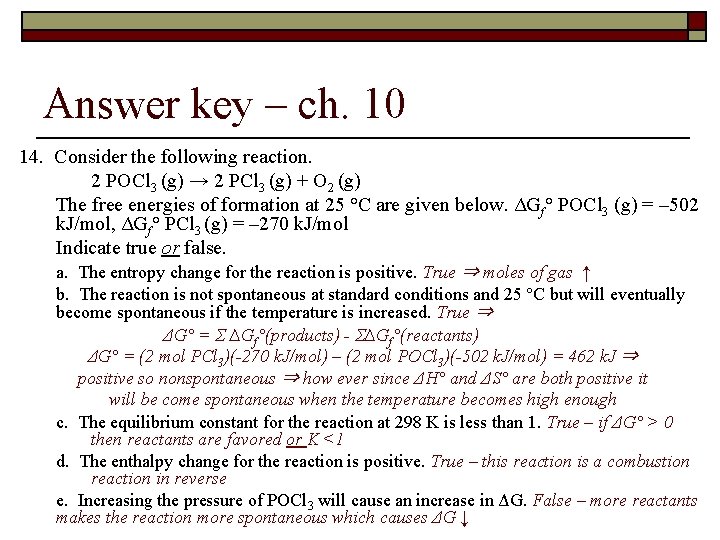
Answer key – ch. 10 14. Consider the following reaction. 2 POCl 3 (g) → 2 PCl 3 (g) + O 2 (g) The free energies of formation at 25 °C are given below. ΔGf° POCl 3 (g) = – 502 k. J/mol, ΔGf° PCl 3 (g) = – 270 k. J/mol Indicate true or false. a. The entropy change for the reaction is positive. True ⇒ moles of gas ↑ b. The reaction is not spontaneous at standard conditions and 25 °C but will eventually become spontaneous if the temperature is increased. True ⇒ ΔG° = Σ ∆Gf°(products) - Σ∆Gf°(reactants) ΔG° = (2 mol PCl 3)(-270 k. J/mol) – (2 mol POCl 3)(-502 k. J/mol) = 462 k. J ⇒ positive so nonspontaneous ⇒ how ever since ΔH° and ΔS° are both positive it will be come spontaneous when the temperature becomes high enough c. The equilibrium constant for the reaction at 298 K is less than 1. True – if ΔG° > 0 then reactants are favored or K <1 d. The enthalpy change for the reaction is positive. True – this reaction is a combustion reaction in reverse e. Increasing the pressure of POCl 3 will cause an increase in ΔG. False – more reactants makes the reaction more spontaneous which causes ΔG ↓

Answer key – ch. 10 15. Consider the following reaction at 800 K. 2 NF 3 (g) → N 2 (g) + 3 F 2 (g) At equilibrium, the partial pressures are PN 2= 0. 040 atm, PF 2= 0. 063 atm and PNF 3= 0. 66 atm. Which of the following is true about the value of ∆G°? √ a. is a positive number b. is a negative number c. is equal to zero d. is independent of the temperature e. can not be predicted from this data

Answer key – ch. 10 o

Answer key – ch. 10 17. Consider the following reaction and thermodynamic data: 2 NO (g) + O 2 (g) → 2 NO 2 (g) Ho = – 190 k. J Go = – 71 k. J at 600 K Calculate the equilibrium constant (K) for this reaction at 370 K. √ a. 2. 9 x 1016 b. 7. 1 x 1013 c. 1. 9 x 10– 7 d. 6. 1 x 105 e. 3. 7 x 103
 Ap chemistry spontaneity entropy and free energy
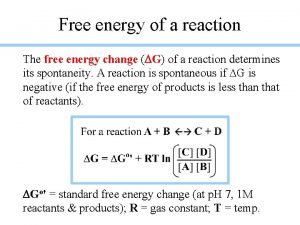
Ap chemistry spontaneity entropy and free energy Standard free energy change
Standard free energy change How to calculate gibbs free energy with partial pressures
How to calculate gibbs free energy with partial pressures How to calculate gibbs free energy
How to calculate gibbs free energy Absolute entropy
Absolute entropy Entropy and gibbs free energy
Entropy and gibbs free energy Helmholtz free energy
Helmholtz free energy Delta g positive or negative
Delta g positive or negative Enthalpy entropy free energy
Enthalpy entropy free energy Unit of entropy
Unit of entropy Gibbs free energy unit
Gibbs free energy unit Thermodynamics ppt
Thermodynamics ppt Predicting spontaneity
Predicting spontaneity Half cell reaction
Half cell reaction Predicting spontaneity
Predicting spontaneity Predicting spontaneity
Predicting spontaneity Is the redox spontaneity rule empirical
Is the redox spontaneity rule empirical Formal vs informal email
Formal vs informal email Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Plot story of an hour
Plot story of an hour Happy isles ulysses
Happy isles ulysses Q system = -q surroundings
Q system = -q surroundings What is enthalpy and entropy
What is enthalpy and entropy Entropy vs enthalpy
Entropy vs enthalpy Entropy of the universe
Entropy of the universe Entropy order parameters and complexity
Entropy order parameters and complexity Chapter 7 energy conservation of energy
Chapter 7 energy conservation of energy Allocation map
Allocation map Free free absorption
Free free absorption Phosphoanhydride group
Phosphoanhydride group Entropy equation
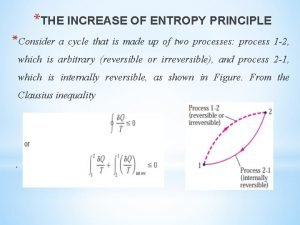
Entropy equation The increase of entropy principle
The increase of entropy principle Reverse entropy
Reverse entropy In a spontaneous irreversible process
In a spontaneous irreversible process Entropy change formula
Entropy change formula Entropy is scalar or vector
Entropy is scalar or vector δhsys
δhsys Thermodynamics
Thermodynamics Entropy change formula
Entropy change formula Entropy in bits
Entropy in bits Huffman code entropy
Huffman code entropy