Zumdahls Chapter 16 Spontaneity Entropy Free Energy and















- Slides: 30

Zumdahl’s Chapter 16 Spontaneity, Entropy, Free Energy, and Why All Things Happen … “The Universe Becomes Less Predictable”

Chapter Contents ¬ Spontaneous Process and Entropy, S ¬ 2 nd Law of Thermodynamics, Suniv 0 ¬ Entropy’s Change with Temperature ¬ Change in S During Chemical Reactions ¬ “Free Energy”, G, & Chemical Reactions ¬ G’s Dependence on Pressure ¬ Pointing the Way to Equilibrium ¬ G’s Relation to K ¬ Non-PV Work & G

Spontaneity • “Sponte” is Latin for “voluntarily. ” ¬We’re willing to concede that highly exothermic reactions are spontaneous. – While the First Law assures that the enthalpy released could be used to resurrect reactants, we know from experience that hot things cool off, and disperse q to the environment, so that it is unavailable to reverse the reaction. – But why do some endothermic reactions go?

Punctuality ¬For that matter, why do some highly exothermic reactions hesitate, requiring a kick start, to do their spontaneous thing? – Or proceed lethargically once started? ¬While last slide’s question is one Thermo can address, the questions above lie in the realm of later chemical topics, viz. , Kinetics and Dynamics.

Norse Mythology ¬Valhalla is the abode of the Norse gods. – But, contrary to many other mythologies, Norse gods are not immortal. – Valhalla is held up by a giant tree, the roots of which are being gnawed by a serpent. – The serpent will succeed, and when it does, Valhalla and the Universe will fall. ¬The serpent’s name is haos

Universal Chaos, Suniv ¬The Norsemen were right! – There is Chaos growing in the Universe all the time at the expense of Order. It is now a fundamental principle of Science. – It’s called “entropy, ” S, and is a state function that must always increase for the Universe as a whole, but some System’s S may decrease. – It is a (logarithmic) measure of the combinations of wave functions available to the Universe!

S = k loge. W (Boltzmann’s Headstone!) ¬S = k ln W in modern symbolism. – W is an actual count of how many different ways the Universe could be arranged without being detectably different macroscopically. • And it is usually enormous! • For example, how many different poker hands might be in some player’s possession? • W (52)(51)(50)(49)(48) / 5! or 2, 598, 960. • For 4 players, that’s ~1. 48 1024 different games. • Over twice Avogadro’s Number!

Poker Microstates ¬One microstate in poker might be a flush; all cards of the same suit. – Wflush = 4(13)(12)(11)(10)(9) / 5! = 5148 as the number of ways to get a flush on the deal. – But Wflush / Wtotal gives ~505: 1 odds against. – So flushes-on-the-deal are fairly ignorable. ¬In k ln W, the most likely microstate is used to calculate W*. It overwhelms others.

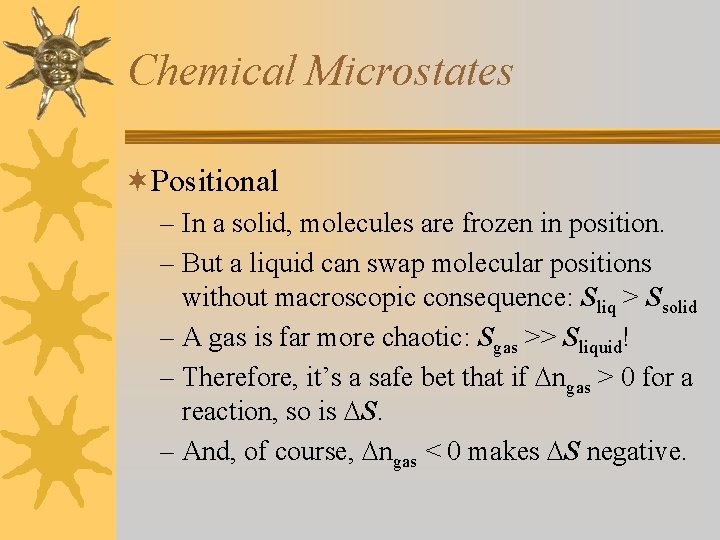
Chemical Microstates ¬Positional – In a solid, molecules are frozen in position. – But a liquid can swap molecular positions without macroscopic consequence: Sliq > Ssolid – A gas is far more chaotic: Sgas >> Sliquid! – Therefore, it’s a safe bet that if ngas > 0 for a reaction, so is S. – And, of course, ngas < 0 makes S negative.

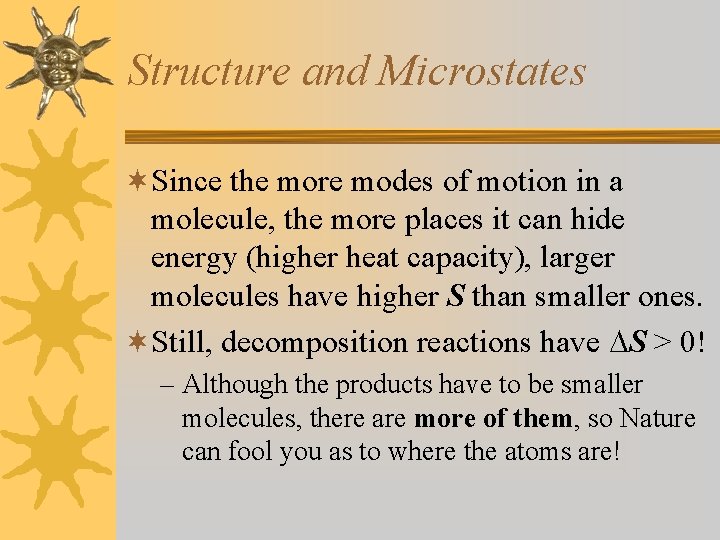
Structure and Microstates ¬Since the more modes of motion in a molecule, the more places it can hide energy (higher heat capacity), larger molecules have higher S than smaller ones. ¬Still, decomposition reactions have S > 0! – Although the products have to be smaller molecules, there are more of them, so Nature can fool you as to where the atoms are!

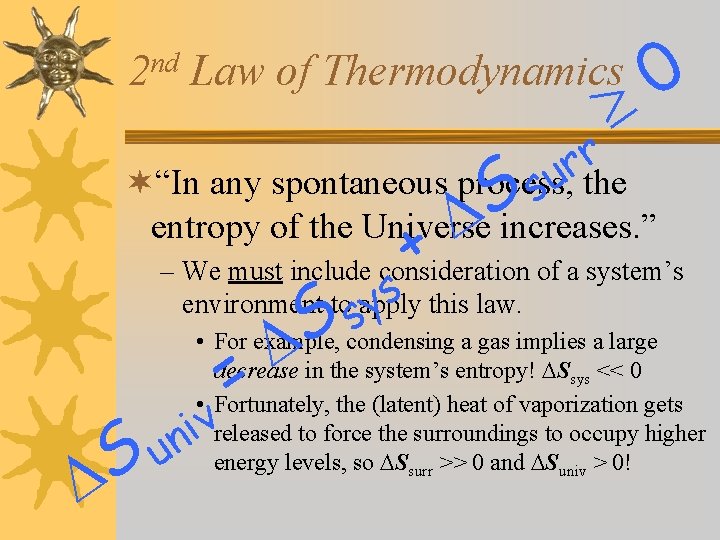
nd 2 0 Law of Thermodynamics r r u ¬“In any spontaneous process, the s S + entropy of the Universe increases. ” – We must include consideration of a system’s environment to apply ys this law. s S condensing a gas implies a large • For example, = decrease in the system’s entropy! Ssys << 0 • Fortunately, the (latent) heat of vaporization gets released to force the surroundings to occupy higher energy levels, so Ssurr >> 0 and Suniv > 0! v i n Su

Entropy Rules Everywhere ¬Photosynthesis makes few large molecules (CH 2 O)n from smaller ones (CO 2 & H 2 O). – So definitely Ssys < 0 – But the absorption of light releases heat into the environment. More importantly … – It then casts many long IR photons into the universe having absorbed fewer short VIS. – So even growth of Life makes Suniv > 0

Perhaps even where it shouldn’t ¬Over a century ago, Darwin published The Origin of Species and coined “the survival of the fittest. ” (…condemning us to Reality TV) – Social Darwinism used that to excuse all the excesses of predatory Capitalism. ¬Economists are turning to Ilya Prigogine. – His notion that processes win that make S grow most quickly is ripe for similar abuse.

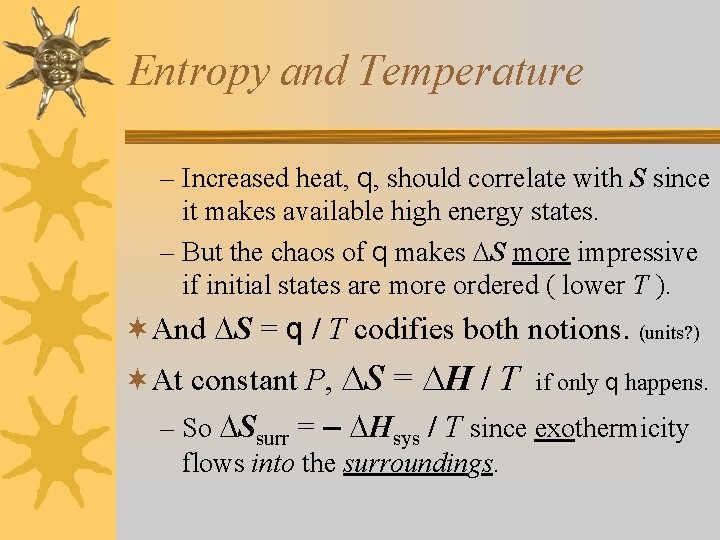
Entropy and Temperature – Increased heat, q, should correlate with S since it makes available high energy states. – But the chaos of q makes S more impressive if initial states are more ordered ( lower T ). ¬And S = q / T codifies both notions. (units? ) ¬At constant P, S = H / T if only q happens. – So Ssurr = – Hsys / T since exothermicity flows into the surroundings.

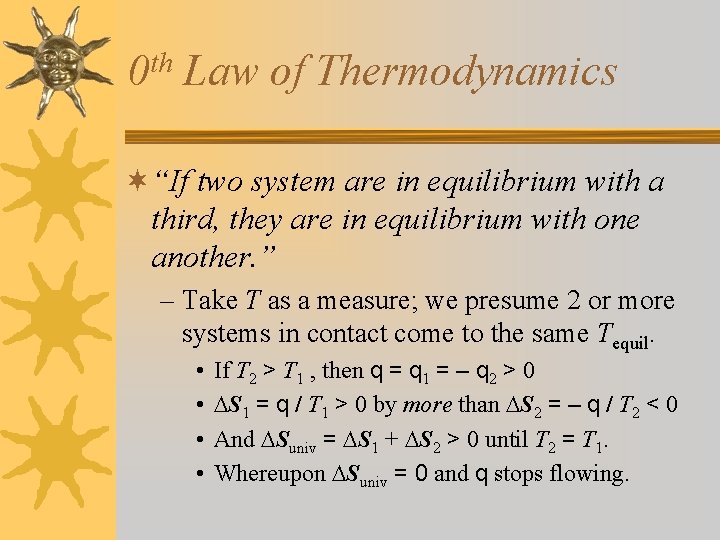
th 0 Law of Thermodynamics ¬“If two system are in equilibrium with a third, they are in equilibrium with one another. ” – Take T as a measure; we presume 2 or more systems in contact come to the same Tequil. • • If T 2 > T 1 , then q = q 1 = – q 2 > 0 S 1 = q / T 1 > 0 by more than S 2 = – q / T 2 < 0 And Suniv = S 1 + S 2 > 0 until T 2 = T 1. Whereupon Suniv = 0 and q stops flowing.

Le Châtlier Confirmed! ¬Suppose a reaction has an exothermicity of H. Then a qsurr = – H > 0 ¬And Ssurr = qsurr / T > 0 aids spontaneity. ¬Le Châtlier claims that higher T makes such a reaction less spontaneous! ¬ Assuming q varies insignificantly with T (true), then higher T makes Ssurr a smaller value! Le Châtlier Confirmed!

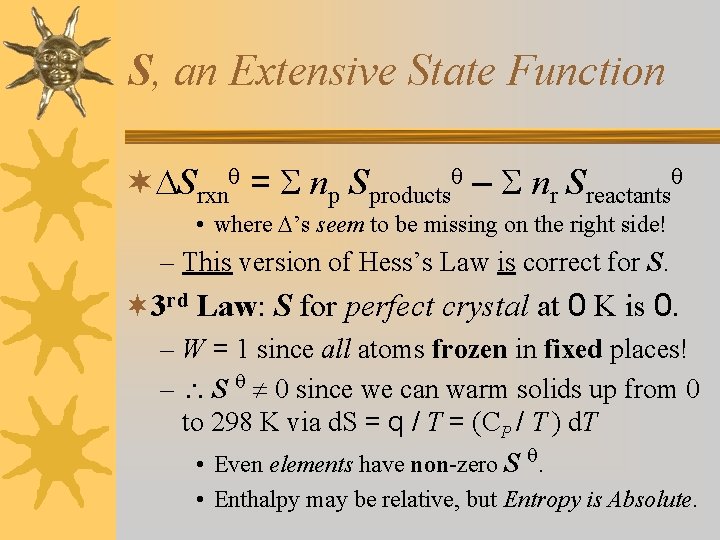
S, an Extensive State Function ¬ Srxn = np Sproducts – nr Sreactants • where ’s seem to be missing on the right side! – This version of Hess’s Law is correct for S. ¬ 3 rd Law: S for perfect crystal at 0 K is 0. – W = 1 since all atoms frozen in fixed places! – S 0 since we can warm solids up from 0 to 298 K via d. S = q / T = (CP / T ) d. T • Even elements have non-zero S . • Enthalpy may be relative, but Entropy is Absolute.

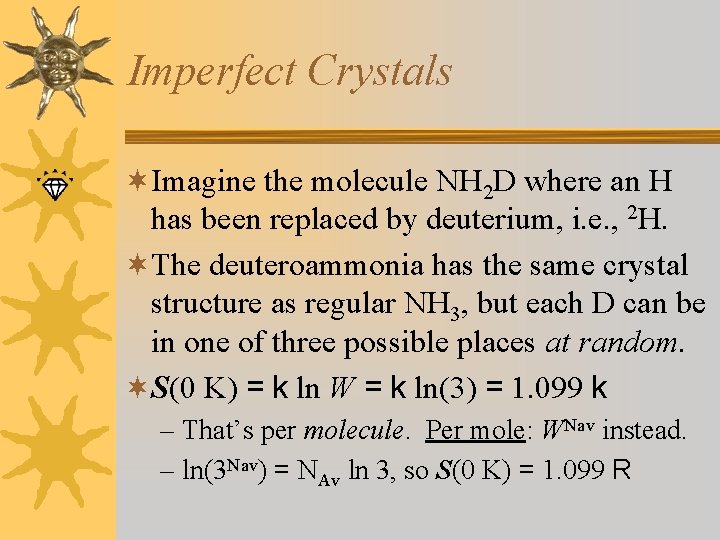
Imperfect Crystals ¬Imagine the molecule NH 2 D where an H has been replaced by deuterium, i. e. , 2 H. ¬The deuteroammonia has the same crystal structure as regular NH 3, but each D can be in one of three possible places at random. ¬S(0 K) = k ln W = k ln(3) = 1. 099 k – That’s per molecule. Per mole: WNav instead. – ln(3 Nav) = NAv ln 3, so S(0 K) = 1. 099 R

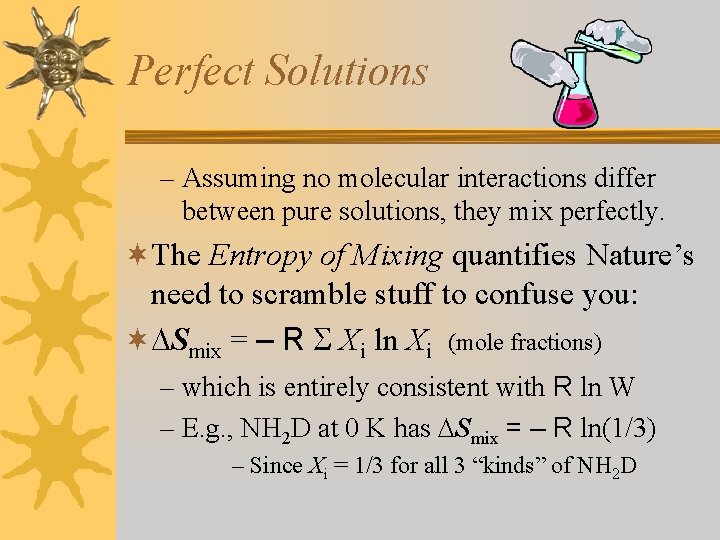
Perfect Solutions – Assuming no molecular interactions differ between pure solutions, they mix perfectly. ¬The Entropy of Mixing quantifies Nature’s need to scramble stuff to confuse you: ¬ Smix = – R Xi ln Xi (mole fractions) – which is entirely consistent with R ln W – E. g. , NH 2 D at 0 K has Smix = – R ln(1/3) – Since Xi = 1/3 for all 3 “kinds” of NH 2 D

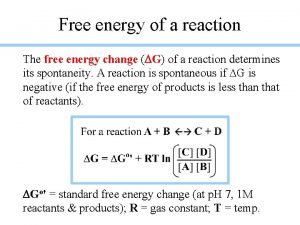
Hiding the Surroundings ¬Since Ssurr = – Hsys / T, and ¬ Suniv = Ssys + Ssurr 0, and therefore ¬T Suniv = T Ssys + T Ssurr 0, then ¬T Ssys – Hsys 0 is also the 2 nd Law. ¬ Hsys – T Ssys 0 is too. ¬ Gsys Hsys – T Ssys 0 is our choice! ¬Gibb’s Free Energy, G H – TS

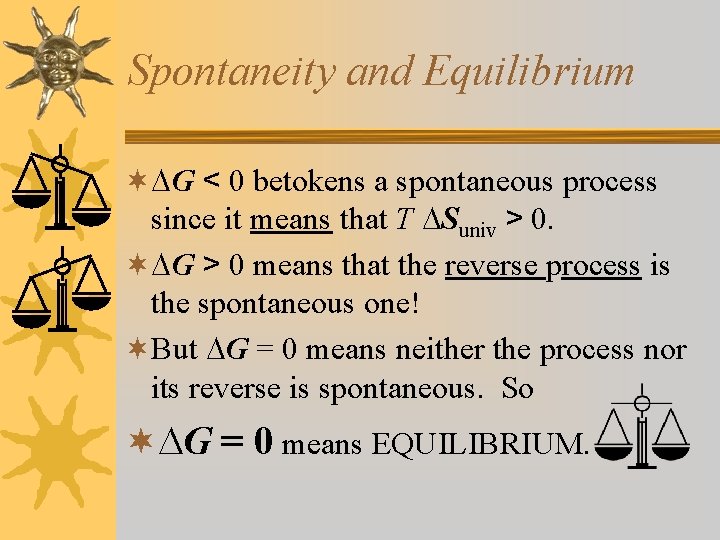
Spontaneity and Equilibrium ¬ G < 0 betokens a spontaneous process since it means that T Suniv > 0. ¬ G > 0 means that the reverse process is the spontaneous one! ¬But G = 0 means neither the process nor its reverse is spontaneous. So ¬ G = 0 means EQUILIBRIUM.

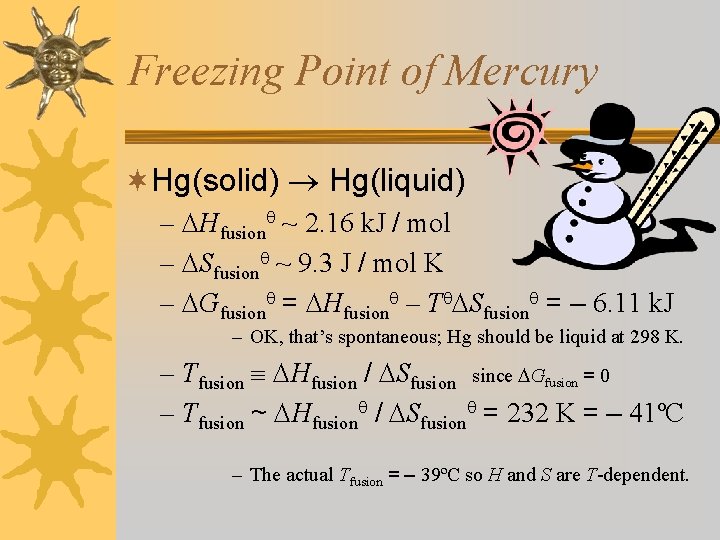
Freezing Point of Mercury ¬Hg(solid) Hg(liquid) – Hfusion ~ 2. 16 k. J / mol – Sfusion ~ 9. 3 J / mol K – Gfusion = Hfusion – T Sfusion = – 6. 11 k. J – OK, that’s spontaneous; Hg should be liquid at 298 K. – Tfusion Hfusion / Sfusion since Gfusion = 0 – Tfusion ~ Hfusion / Sfusion = 232 K = – 41ºC – The actual Tfusion = – 39ºC so H and S are T-dependent.

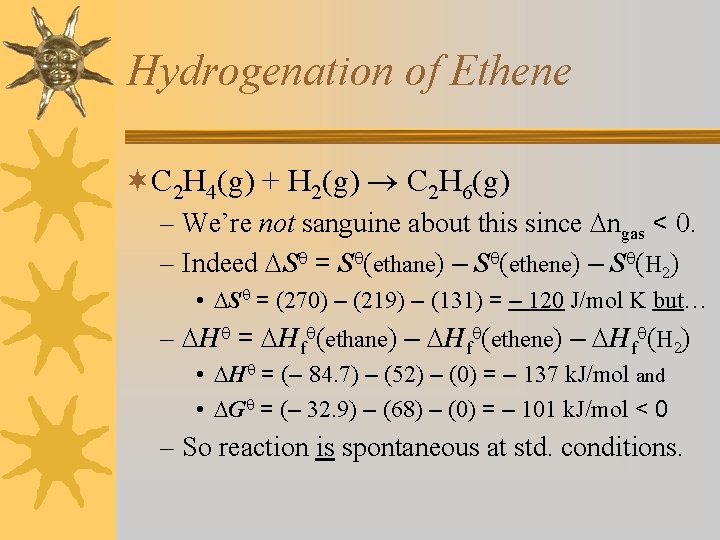
Hydrogenation of Ethene ¬C 2 H 4(g) + H 2(g) C 2 H 6(g) – We’re not sanguine about this since ngas < 0. – Indeed S = S (ethane) – S (ethene) – S (H 2) • S = (270) – (219) – (131) = – 120 J/mol K but… – H = Hf (ethane) – Hf (ethene) – Hf (H 2) • H = (– 84. 7) – (52) – (0) = – 137 k. J/mol and • G = (– 32. 9) – (68) – (0) = – 101 k. J/mol < 0 – So reaction is spontaneous at std. conditions.

Improving Le Châtlier’s Odds ¬Since H < 0, we don’t want to heat the reaction, or we’d reduce spontaneity. – We would expect G to be increased. ¬But since ngas < 0, we do want to apply additional pressure to drive it to products. – We’d expect G to become more negative. ¬So what was that again about G’s pressure dependence?

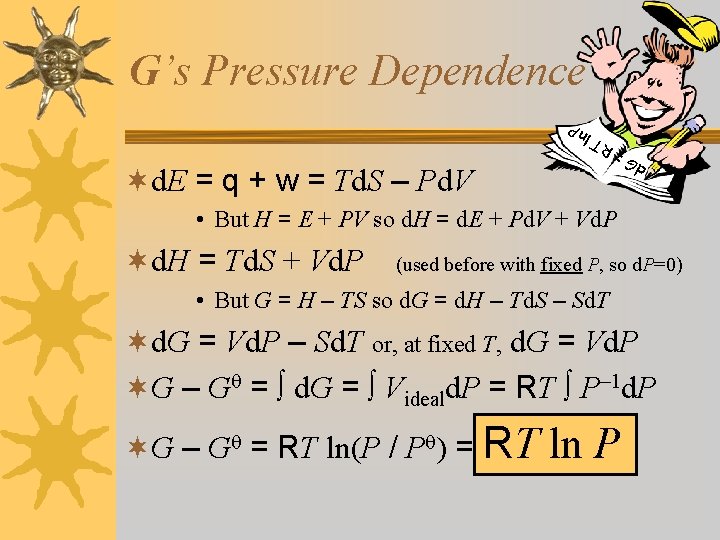
G’s Pressure Dependence d. G = n. P R T l ¬d. E = q + w = Td. S – Pd. V • But H = E + PV so d. H = d. E + Pd. V + Vd. P ¬d. H = Td. S + Vd. P (used before with fixed P, so d. P=0) • But G = H – TS so d. G = d. H – Td. S – Sd. T ¬d. G = Vd. P – Sd. T or, at fixed T, d. G = Vd. P ¬G – G = d. G = Videald. P = RT P– 1 d. P ¬G – G = RT ln(P / P ) = RT ln P

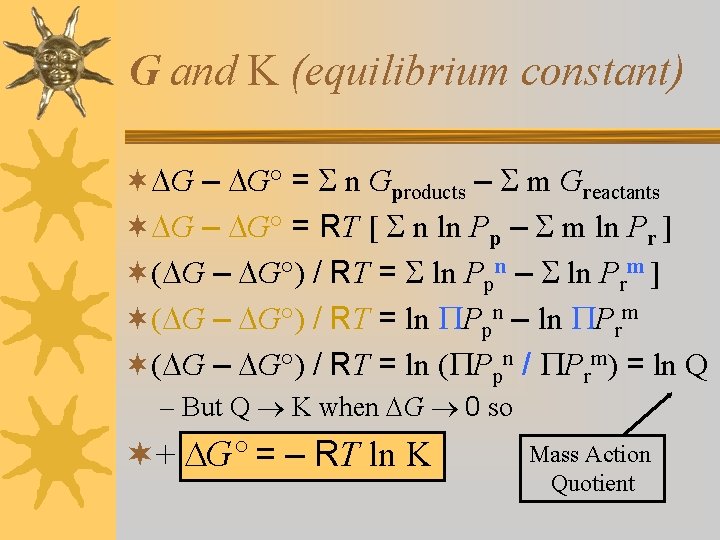
G and K (equilibrium constant) ¬ G – G° = n Gproducts – m Greactants ¬ G – G° = RT [ n ln Pp – m ln Pr ] ¬( G – G°) / RT = ln Ppn – ln Prm ] ¬( G – G°) / RT = ln Ppn – ln Prm ¬( G – G°) / RT = ln ( Ppn / Prm) = ln Q – But Q K when G 0 so ¬+ G° = – RT ln K Mass Action Quotient

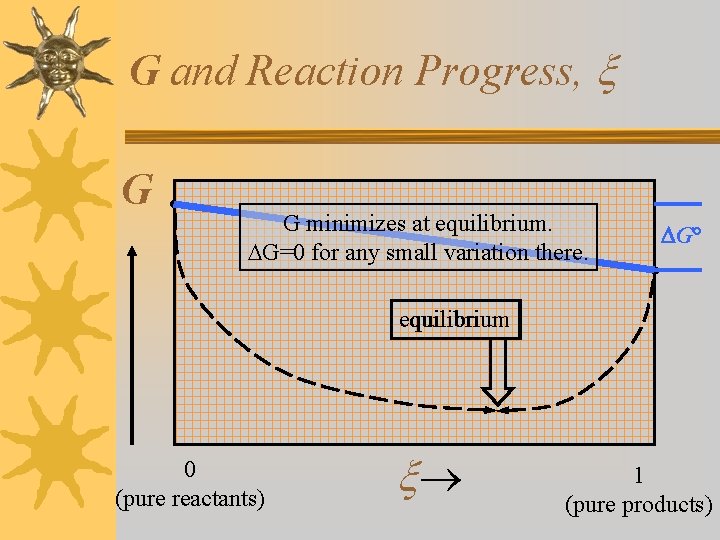
G and Reaction Progress, G G minimizes at equilibrium. G=0 for any small variation there. G° equilibrium 0 (pure reactants) 1 (pure products)

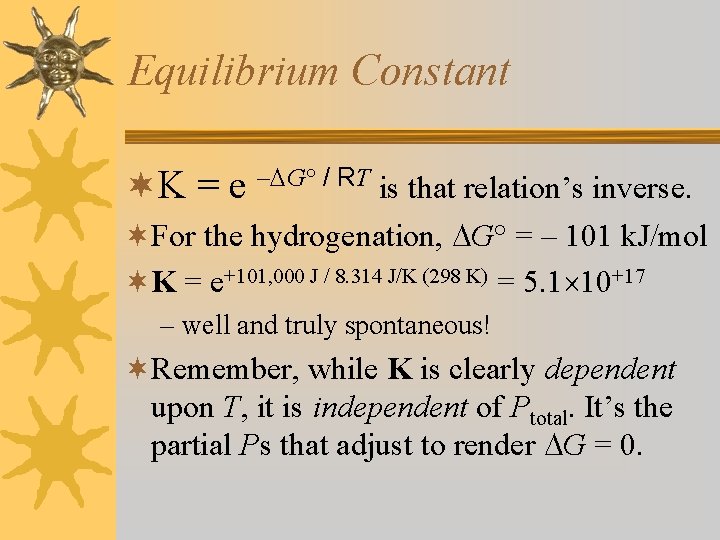
Equilibrium Constant ¬K = e – G° / RT is that relation’s inverse. ¬For the hydrogenation, G° = – 101 k. J/mol ¬K = e+101, 000 J / 8. 314 J/K (298 K) = 5. 1 10+17 – well and truly spontaneous! ¬Remember, while K is clearly dependent upon T, it is independent of Ptotal. It’s the partial Ps that adjust to render G = 0.

K’s Temperature Dependence ¬ln K = – G°/RT = – H°/RT + S°/R ¬ln K = – ( H°/R)T – 1 + ( S°/R) – We expect a plot of ln K vs. 1/T to be ~ linear. • That’s if H and S are weak functions of T themselves. True if we don’t change T much. ¬d(ln. K) = + ( H°/R)T – 2 d. T (van’t Hoff) • It says that ln K increases with T when the reaction is endothermic; decreases otherwise. – Le Châtlier! • But the increase becomes less impressive at high T.

Maximizing Work ¬ G = Vd. P – Sd. T + wnon-PV • We’ve been ignoring the non-PV work all this time, but it’s really been there in E, H, and G. – Here it means that at fixed P & T, the first two terms vanish, and G = wnon-PV, the maximum (non-PV) work of which the system is capable. • If you want maximum total w, the physicists need to tell you about A. (A = E – TS, the “work function. ”) In either case, we must be so gentle as to be at equilibrium all the time; “reversible work!”
 Ap chemistry entropy and free energy
Ap chemistry entropy and free energy Gibbs free energy and spontaneity
Gibbs free energy and spontaneity Gibbs free energy and spontaneity
Gibbs free energy and spontaneity Gibbs free energy unit
Gibbs free energy unit Absolute entropy
Absolute entropy Gibbs free energy spontaneous
Gibbs free energy spontaneous Helmholtz free energy and gibbs free energy
Helmholtz free energy and gibbs free energy Delta g positive or negative
Delta g positive or negative Enthalpy entropy free energy
Enthalpy entropy free energy Gibbs free energy equation
Gibbs free energy equation Delta g
Delta g Predicting spontaneity
Predicting spontaneity Predicting spontaneity
Predicting spontaneity Spontaneity of redox reactions
Spontaneity of redox reactions Predicting spontaneity
Predicting spontaneity Predicting spontaneity
Predicting spontaneity Is the redox spontaneity rule empirical
Is the redox spontaneity rule empirical Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis The story of an hour summary
The story of an hour summary Happy isles ulysses
Happy isles ulysses Q system = -q surroundings
Q system = -q surroundings What is enthalpy and entropy
What is enthalpy and entropy Enthalpy
Enthalpy Entropy of system and surroundings
Entropy of system and surroundings Entropy order parameters and complexity
Entropy order parameters and complexity Chapter 7 energy conservation of energy
Chapter 7 energy conservation of energy Allocating kernel memory in os
Allocating kernel memory in os Free-free absorption
Free-free absorption Atp hydrolysis and free energy change
Atp hydrolysis and free energy change Entropy equation
Entropy equation