Chapter 16 Spontaneity entropy and free energy Spontaneous




















- Slides: 25

Chapter 16 Spontaneity, entropy and free energy

Spontaneous l. A reaction that will occur without outside intervention. l We can’t determine how fast. l We need both thermodynamics and kinetics to describe a reaction completely. l Thermodynamics compares initial and final states. l Kinetics describes pathway between.

Thermodynamics l 1 st Law- the energy of the universe is constant. l Keeps track of thermodynamics doesn’t correctly predict spontaneity. l Entropy (S) – Number of ways things can be arranged – Looks like disorder or randomness l 2 nd Law the entropy of the universe increases in any change

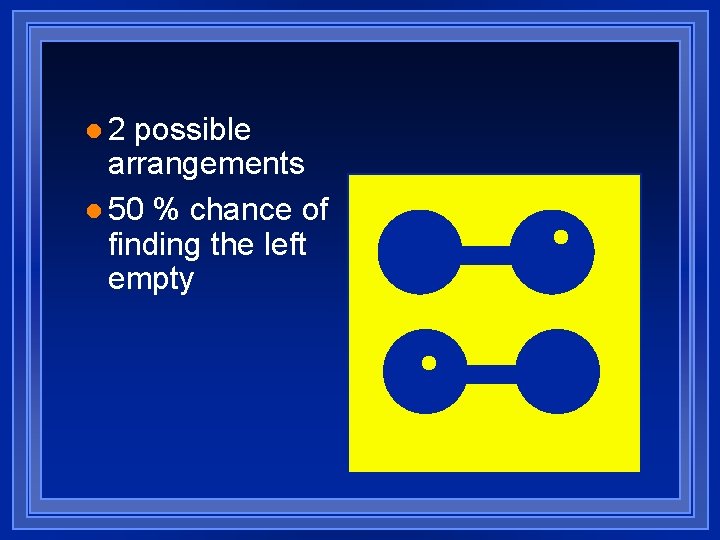
Entropy l Defined in terms of probability. l Substances take the arrangement that is most likely. l The most likely is the most random. l Calculate the number of arrangements for a system.

l 2 possible arrangements l 50 % chance of finding the left empty

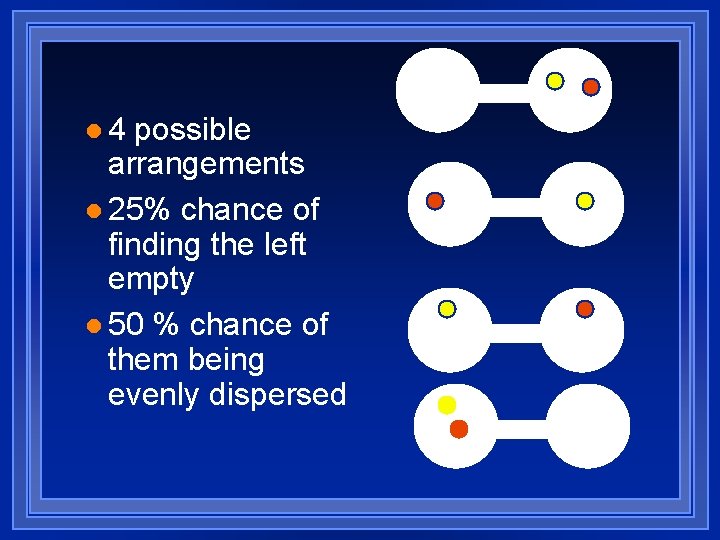
l 4 possible arrangements l 25% chance of finding the left empty l 50 % chance of them being evenly dispersed

l 4 atoms l 8% chance of finding the left empty l 50 % chance of them being evenly dispersed

Gases l Gases completely fill their chamber because there are many more ways to do that than to leave half empty. l. Ssolid <Sliquid <<Sgas l there are many more ways for the molecules to be arranged as a liquid than a solid. l Gases have a huge number of positions possible.

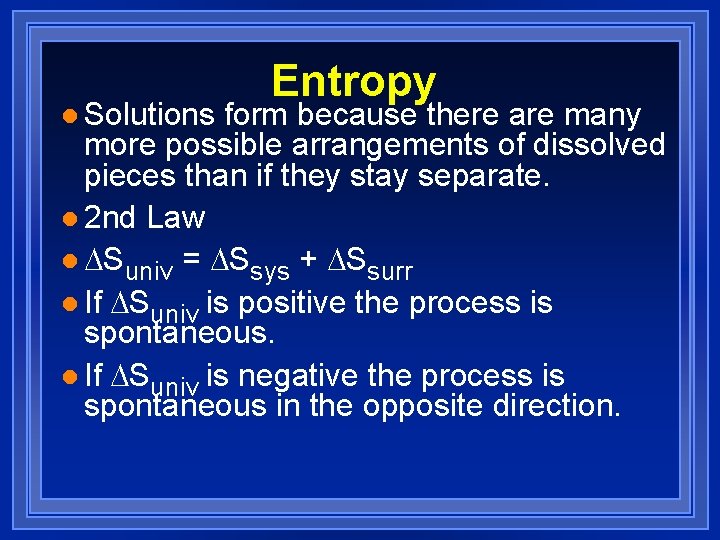
l Solutions Entropy form because there are many more possible arrangements of dissolved pieces than if they stay separate. l 2 nd Law l Suniv = Ssys + Ssurr l If Suniv is positive the process is spontaneous. l If Suniv is negative the process is spontaneous in the opposite direction.

exothermic processes Ssurr is positive. l For endothermic processes Ssurr is negative. l Consider this process H 2 O(l)® H 2 O(g) l Ssys is positive l Ssurr is negative l Suniv depends on temperature. l For

Temperature and Spontaneity l Entropy changes in the surroundings are determined by the heat flow. l An exothermic process is favored because by giving up heat the entropy of the surroundings increases. l The size of Ssurr depends on temperature l Ssurr = - H/T

Ssys - H/T Ssurr Suniv + + + No, Reverse + - ? At High temp. - + ? At Low temp. Spontaneous? Yes

Gibb's Free Energy l G=H-TS l Never used this way. l G= H-T S at constant temperature l Divide by -T l - G/T = - H/T- S l - G/T = Ssurr + S l - G/T = Suniv l If G is negative at constant T and P, the Process is spontaneous.

Let’s Check the reaction H 2 O(s) ® H 2 O(l) l Sº = 22. 1 J/K mol Hº =6030 J/mol l Calculate G at 10ºC and -10ºC l When does it become spontaneous? l Look at the equation G= H-T S l Spontaneity can be predicted from the sign of H and S. l For

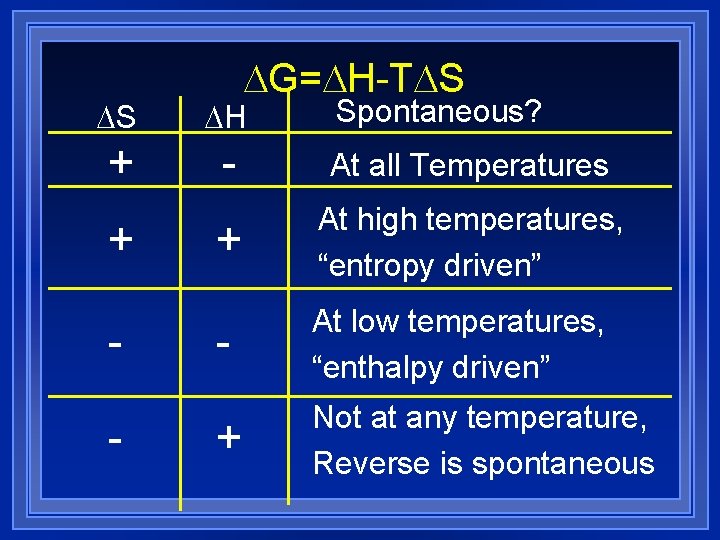
G= H-T S Spontaneous? S H + - At all Temperatures + At high temperatures, “entropy driven” - At low temperatures, “enthalpy driven” + Not at any temperature, Reverse is spontaneous + -

Third Law of Thermo l The entropy of a pure crystal at 0 K is 0. l Gives us a starting point. l All others must be>0. l Standard Entropies Sº ( at 298 K and 1 atm) of substances are listed. l Products - reactants to find Sº (a state function). l More complex molecules higher Sº.

Free Energy in Reactions l Gº = standard free energy change. l Free energy change that will occur if reactants in their standard state turn to products in their standard state. l Can’t be measured directly, can be calculated from other measurements. l Gº= Hº-T Sº l Use Hess’s Law with known reactions.

Free Energy in Reactions are tables of Gºf. l Products-reactants because it is a state function. l The standard free energy of formation for any element in its standard state is 0. l Remember- Spontaneity tells us nothing about rate. l There

Free energy and Pressure l G = Gº +RTln(Q) where Q is the reaction quotients (P of the products /P of the reactants). l CO(g) + 2 H 2(g) ® CH 3 OH(l) l Would the reaction be spontaneous at 25ºC with the H 2 pressure of 5. 0 atm and the CO pressure of 3. 0 atm? l Gºf CH 3 OH(l) = -166 k. J l Gºf CO(g) = -137 k. J Gºf H 2(g) = 0 k. J

How far? l G tells us spontaneity at current conditions. When will it stop? l It will go to the lowest possible free energy which may be an equilibrium. l At equilibrium G = 0, Q = K l Gº = -RTln. K

Gº =0 <0 >0 K =1 >1 <1 Gº = -RTln. K

At 1500°C for the reaction CO(g) + 2 H 2(g) → CH 3 OH(g) the equilibrium constant is Kp = 1. 4 x 10 -7. Is H° at this temperature: A. positive B. negative C. zero D. can not be determined

The standard free energy ( Grxn 0 for the reaction N 2(g) + 3 H 2(g) → 2 NH 3(g) is -32. 9 k. J. Calculate the equilibrium constant for this reaction at 25 o. C. A. 13. 3 B. 5. 8 x 105 C. 2. 5 D. 4. 0 x 10 -6 E. 9. 1 x 108

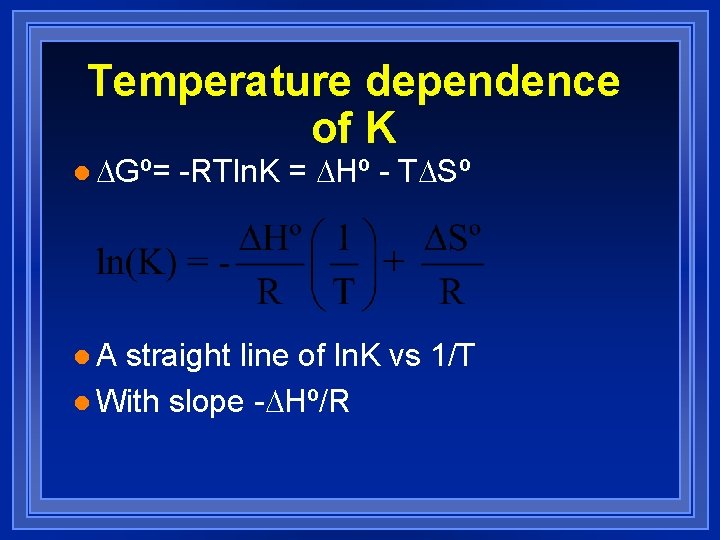
Temperature dependence of K l Gº= l. A -RTln. K = Hº - T Sº straight line of ln. K vs 1/T l With slope - Hº/R

Free energy And Work l Free energy is that energy free to do work. l The maximum amount of work possible at a given temperature and pressure. l E = q + w l Never really achieved because some of the free energy is changed to heat during a change, so it can’t be used to do work. l Can’t be 100% efficient
 Ap chemistry entropy and free energy
Ap chemistry entropy and free energy Standard free energy change
Standard free energy change How to calculate gibbs free energy with partial pressures
How to calculate gibbs free energy with partial pressures Gibbs free energy equation
Gibbs free energy equation Relationship between entropy and free energy
Relationship between entropy and free energy Enthalpy entropy free energy
Enthalpy entropy free energy Helmholtz free energy and gibbs free energy
Helmholtz free energy and gibbs free energy Unit of entropy
Unit of entropy If gibbs free energy is negative
If gibbs free energy is negative Enthalpy entropy free energy
Enthalpy entropy free energy Gibbs free energy unit
Gibbs free energy unit Thermodynamics ppt
Thermodynamics ppt Predicting spontaneity
Predicting spontaneity Concentration of cells
Concentration of cells Predicting spontaneity
Predicting spontaneity Complete the following table on reaction spontaneity
Complete the following table on reaction spontaneity Is the redox spontaneity rule empirical
Is the redox spontaneity rule empirical Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Free body and soul free
Free body and soul free Happy isles ulysses
Happy isles ulysses Are nonspontaneous processes impossible
Are nonspontaneous processes impossible Negotiated short term financing
Negotiated short term financing Q system = -q surroundings
Q system = -q surroundings What is enthalpy and entropy
What is enthalpy and entropy Minimum enthalpy and maximum entropy
Minimum enthalpy and maximum entropy