Spontaneity Entropy and Free Energy Spontaneous Processes and








- Slides: 15

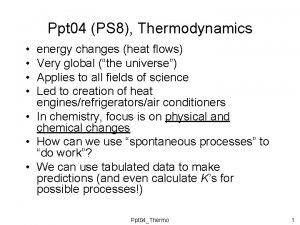
Spontaneity, Entropy and Free Energy

Spontaneous Processes and Entropy q. First Law • “Energy can neither be created nor destroyed" • The energy of the universe is constant q. Spontaneous Processes • Processes that occur without outside intervention • Spontaneous processes may be fast or slow – Many forms of combustion are fast – Conversion of diamond to graphite is slow

Entropy (S) Ø A measure of the randomness or disorder Ø The driving force for a spontaneous process is an increase in the entropy of the universe Ø Entropy is a thermodynamic function describing the number of arrangements that are available to a system Ø Nature proceeds toward the states that have the highest probabilities of existing

Positional Entropy § The probability of occurrence of a particular state depends on the number of ways (microstates) in which that arrangement can be achieved Ssolid < Sliquid << Sgas

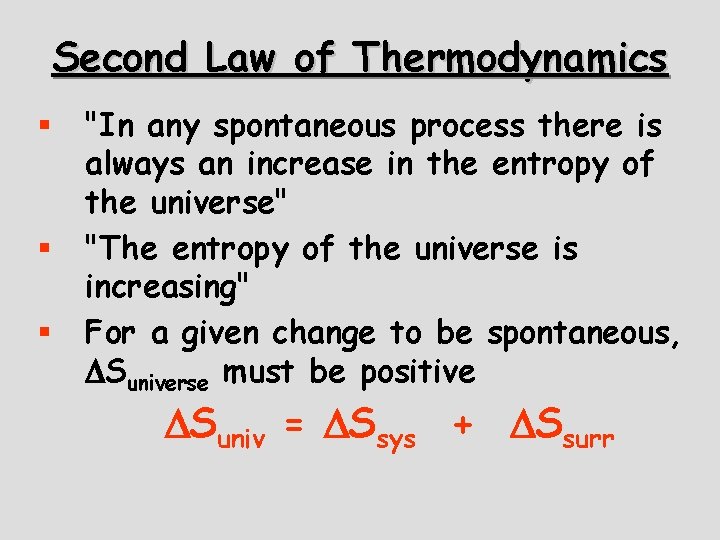
Second Law of Thermodynamics § § § "In any spontaneous process there is always an increase in the entropy of the universe" "The entropy of the universe is increasing" For a given change to be spontaneous, Suniverse must be positive Suniv = Ssys + Ssurr

H, S, G and Spontaneity G = H - T S H is enthalpy, T is Kelvin temperature Value of H Value of T S Value of Spontaneity G Negative Positive Negative Spontaneous Negative Positive Nonspontaneous Negative ? ? ? Spontaneous if the absolute value of H is greater than the absolute value of T S (low temperature) Positive ? ? ? Spontaneous if the absolute value of T S is greater than the absolute value of H (high temperature)

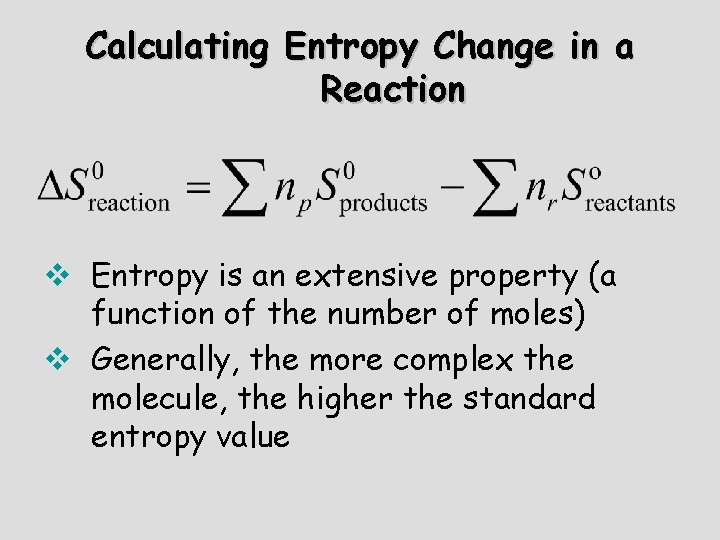
Calculating Entropy Change in a Reaction v Entropy is an extensive property (a function of the number of moles) v Generally, the more complex the molecule, the higher the standard entropy value

Standard Free Energy Change v G 0 is the change in free energy that will occur if the reactants in their standard states are converted to the products in their standard states v G 0 cannot be measured directly v The more negative the value for G 0, the farther to the right the reaction will proceed in order to achieve equilibrium v Equilibrium is the lowest possible free energy position for a reaction

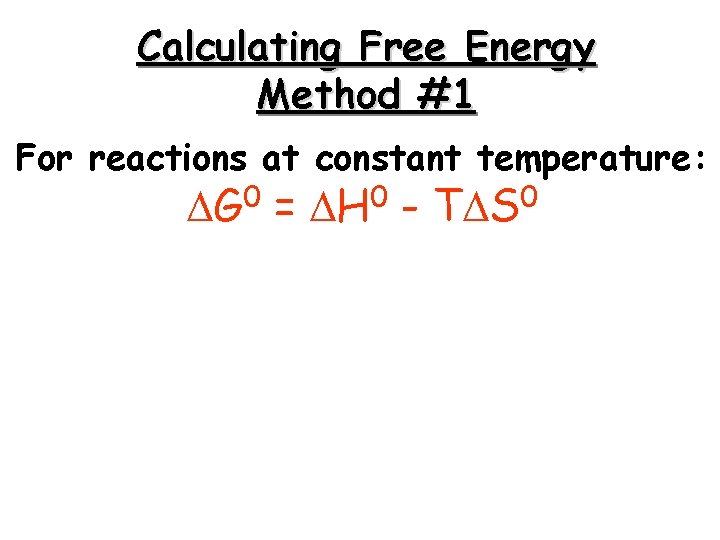
Calculating Free Energy Method #1 For reactions at constant temperature: 0 G = 0 H - 0 T S

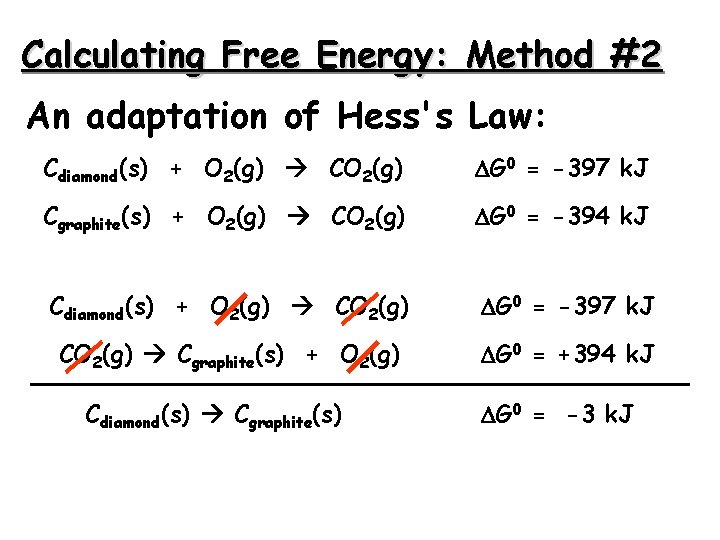
Calculating Free Energy: Method #2 An adaptation of Hess's Law: Cdiamond(s) + O 2(g) CO 2(g) G 0 = -397 k. J Cgraphite(s) + O 2(g) CO 2(g) G 0 = -394 k. J Cdiamond(s) + O 2(g) CO 2(g) G 0 = -397 k. J CO 2(g) Cgraphite(s) + O 2(g) G 0 = +394 k. J Cdiamond(s) Cgraphite(s) G 0 = -3 k. J

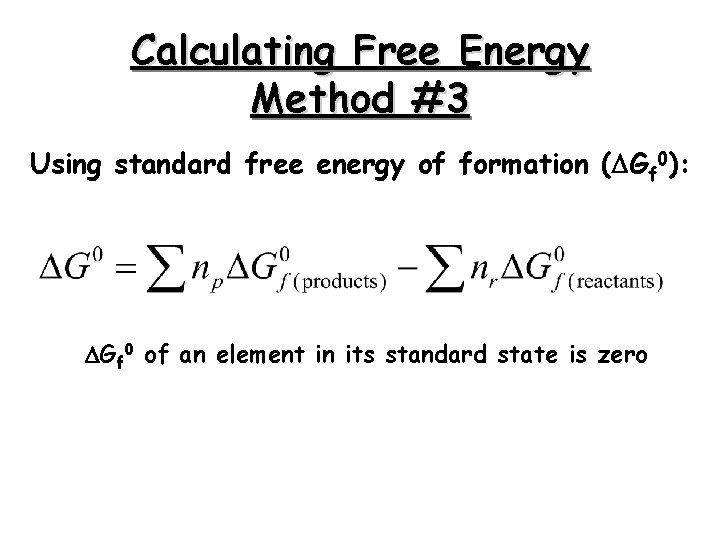
Calculating Free Energy Method #3 Using standard free energy of formation ( Gf 0): Gf 0 of an element in its standard state is zero

The Dependence of Free Energy on Pressure q Enthalpy, H, is not pressure dependent q Entropy, S Ø entropy depends on volume, so it also depends on pressure Slarge volume > Ssmall volume Slow pressure > Shigh pressure

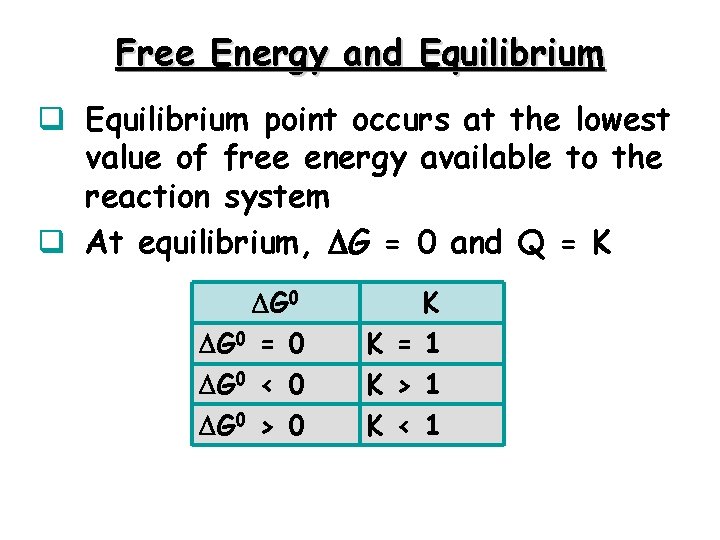
Free Energy and Equilibrium q Equilibrium point occurs at the lowest value of free energy available to the reaction system q At equilibrium, G = 0 and Q = K G 0 = 0 G 0 < 0 G 0 > 0 K K = 1 K > 1 K < 1

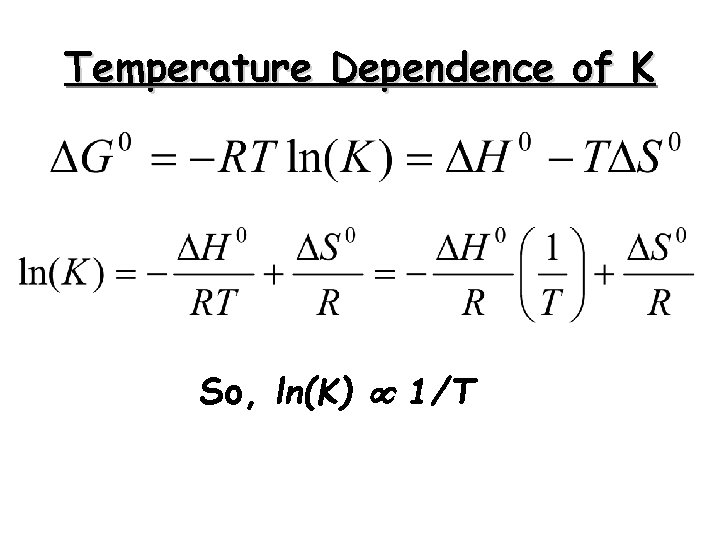
Temperature Dependence of K So, ln(K) 1/T

Free Energy and Work q The maximum possible useful work obtainable from a process at constant temperature and pressure is equal to the change in free energy q The amount of work obtained is always less than the maximum q Henry Bent's First Two Laws of Thermodynamics q First law: You can't win, you can only break even q Second law: You can't break even
 Entropy ap chem
Entropy ap chem Gibbs free energy and spontaneity
Gibbs free energy and spontaneity Gibbs free energy practice problems
Gibbs free energy practice problems Gibbs free energy and spontaneity
Gibbs free energy and spontaneity Enthalpy vs entropy
Enthalpy vs entropy Enthalpy entropy free energy
Enthalpy entropy free energy Gibbs free energy spontaneous
Gibbs free energy spontaneous Difference between entropy and enthalpy
Difference between entropy and enthalpy Enthalpy entropy free energy
Enthalpy entropy free energy Helmholtz free energy and gibbs free energy
Helmholtz free energy and gibbs free energy Delta g = delta g not + rtlnq
Delta g = delta g not + rtlnq Concurrent in os
Concurrent in os Thermodynamics ppt
Thermodynamics ppt Predicting spontaneity
Predicting spontaneity Half cell reaction
Half cell reaction Predicting spontaneity
Predicting spontaneity