Chapter Sixteen SPONTANEITY ENTROPY AND FREE ENERGY Spontaneous


















- Slides: 53

Chapter Sixteen: SPONTANEITY, ENTROPY, AND FREE ENERGY

Spontaneous Processes and Entropy

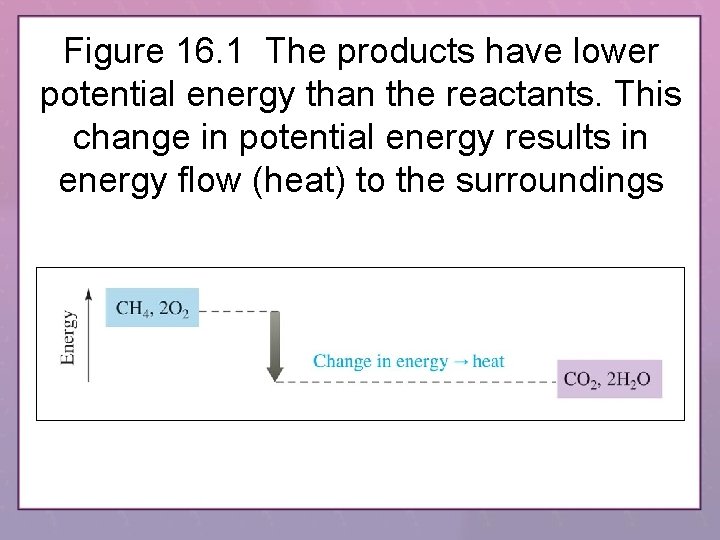
Figure 16. 1 The products have lower potential energy than the reactants. This change in potential energy results in energy flow (heat) to the surroundings

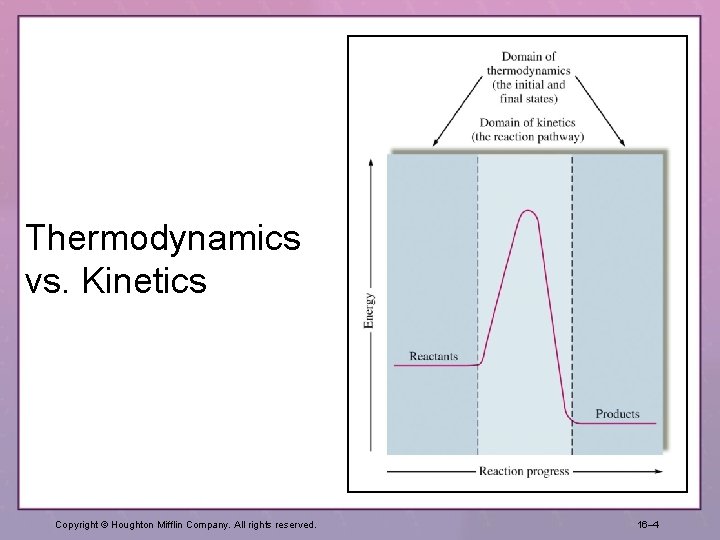
Thermodynamics vs. Kinetics Copyright © Houghton Mifflin Company. All rights reserved. 16– 4

Plant materials burn to form CO 2 and H 2 O

Spontaneous Processes and Entropy • Thermodynamics lets us predict whether a process will occur but gives no information about the amount of time required for the process. • A spontaneous process is one that occurs without outside intervention. Copyright © Houghton Mifflin Company. All rights reserved. 16– 6

A Disordered Pile of Playing Cards

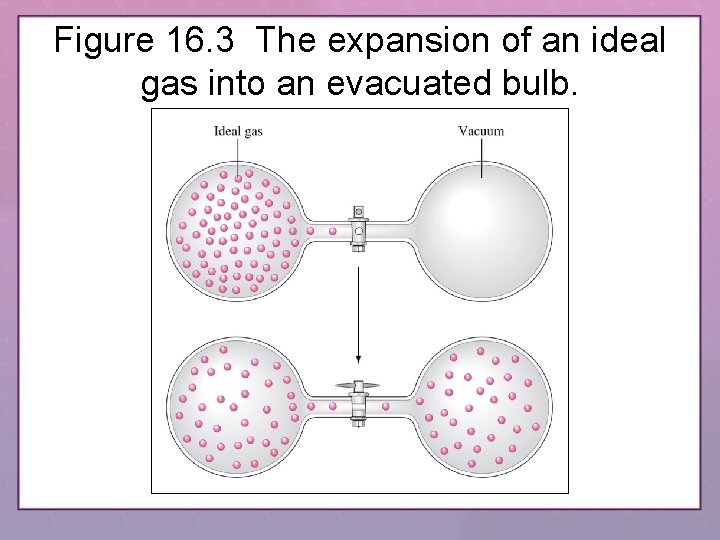
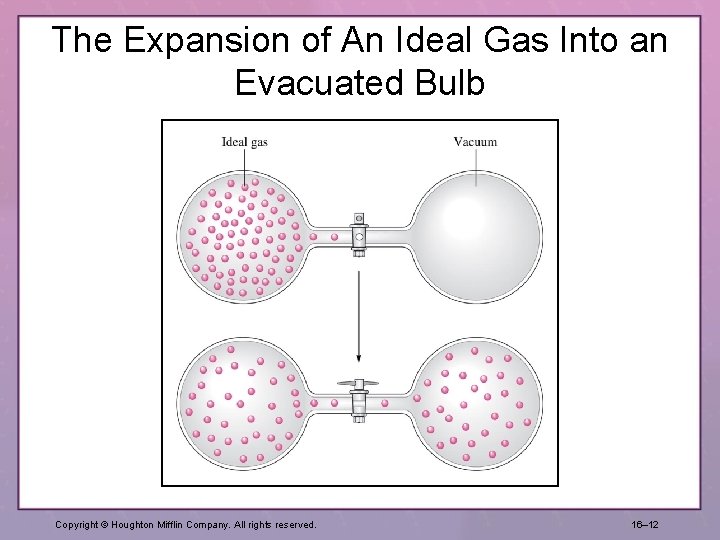
Figure 16. 3 The expansion of an ideal gas into an evacuated bulb.

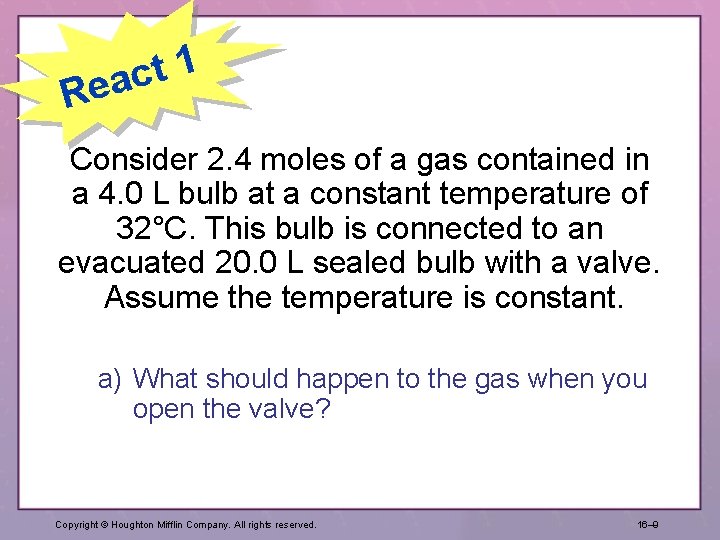
1 t eac R Consider 2. 4 moles of a gas contained in a 4. 0 L bulb at a constant temperature of 32°C. This bulb is connected to an evacuated 20. 0 L sealed bulb with a valve. Assume the temperature is constant. a) What should happen to the gas when you open the valve? Copyright © Houghton Mifflin Company. All rights reserved. 16– 9

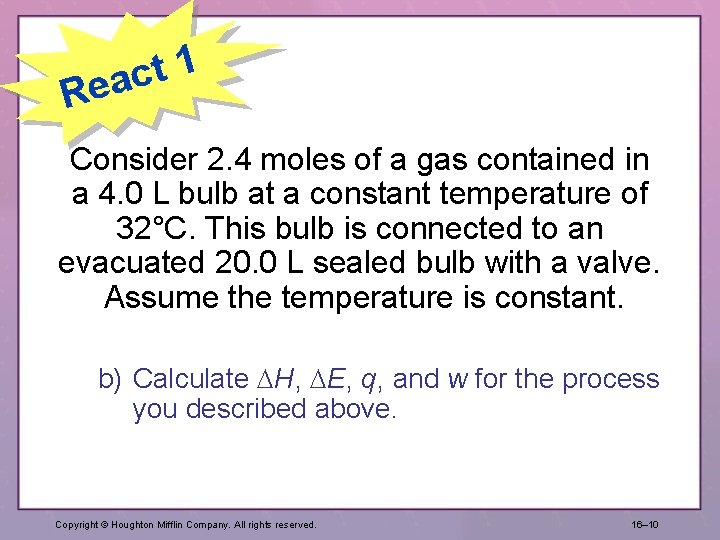
1 t eac R Consider 2. 4 moles of a gas contained in a 4. 0 L bulb at a constant temperature of 32°C. This bulb is connected to an evacuated 20. 0 L sealed bulb with a valve. Assume the temperature is constant. b) Calculate H, E, q, and w for the process you described above. Copyright © Houghton Mifflin Company. All rights reserved. 16– 10

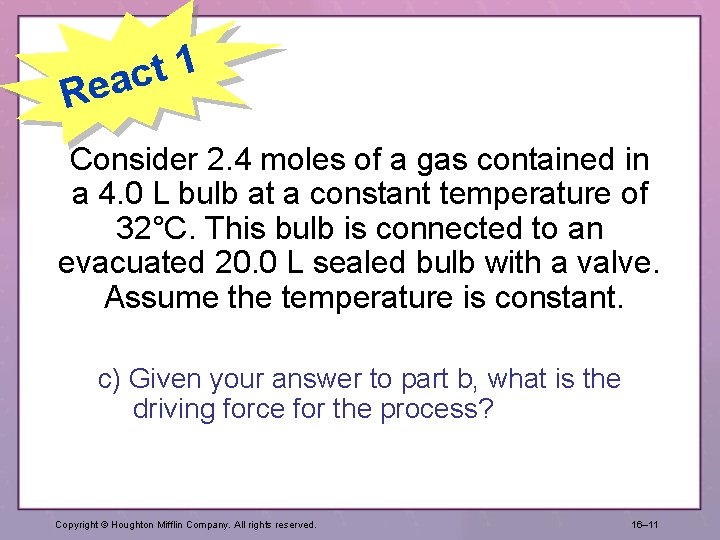
1 t eac R Consider 2. 4 moles of a gas contained in a 4. 0 L bulb at a constant temperature of 32°C. This bulb is connected to an evacuated 20. 0 L sealed bulb with a valve. Assume the temperature is constant. c) Given your answer to part b, what is the driving force for the process? Copyright © Houghton Mifflin Company. All rights reserved. 16– 11

The Expansion of An Ideal Gas Into an Evacuated Bulb Copyright © Houghton Mifflin Company. All rights reserved. 16– 12

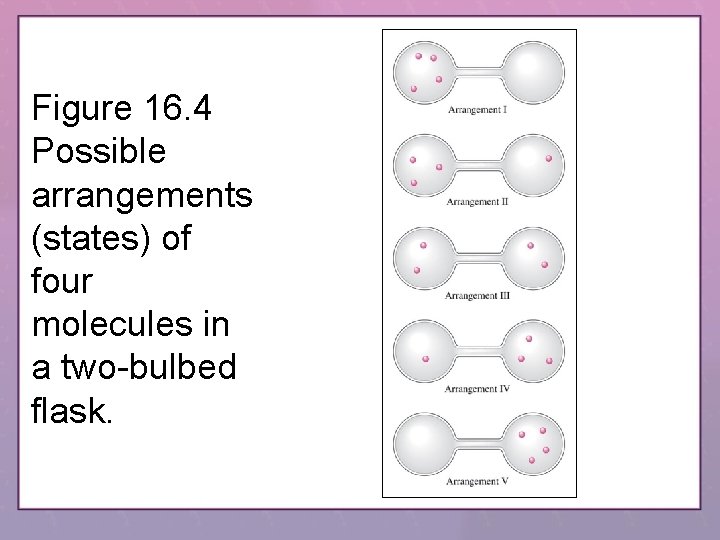
Figure 16. 4 Possible arrangements (states) of four molecules in a two-bulbed flask.

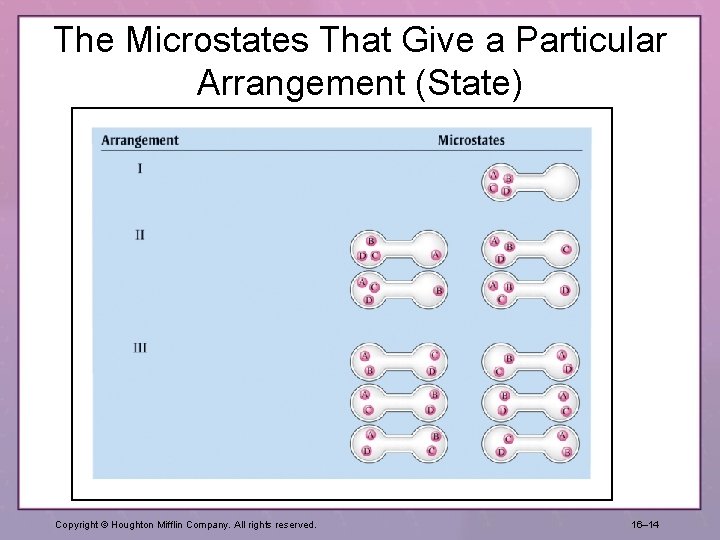
The Microstates That Give a Particular Arrangement (State) Copyright © Houghton Mifflin Company. All rights reserved. 16– 14

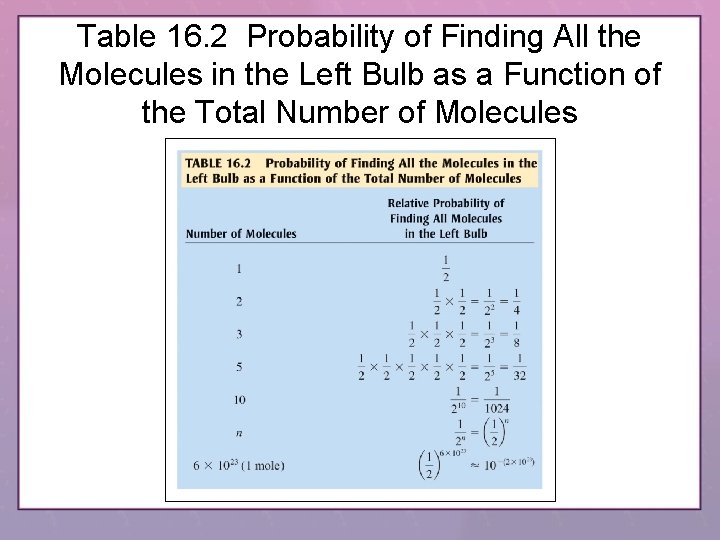
Table 16. 2 Probability of Finding All the Molecules in the Left Bulb as a Function of the Total Number of Molecules

Boiling Water to Form Steam Increases Its Volume and Thus Its Entropy

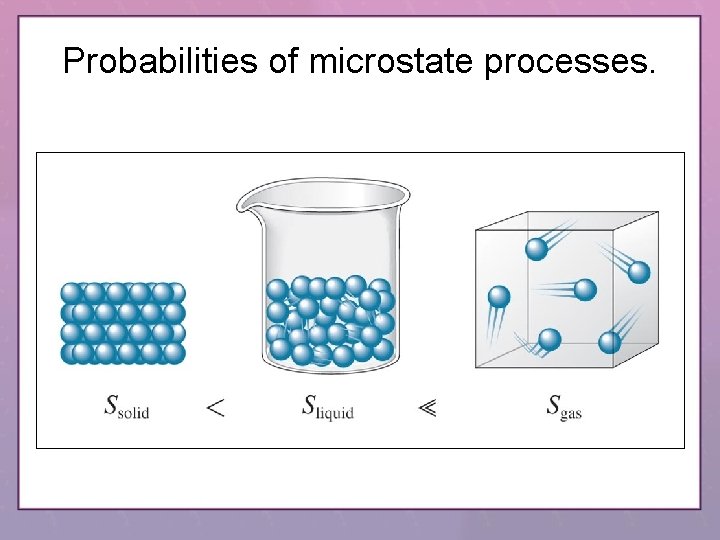
Probabilities of microstate processes.

Positional Entropy • A gas expands into a vacuum because the expanded state has the highest positional probability of states available to the system. • Therefore, Ssolid < Sliquid << Sgas Copyright © Houghton Mifflin Company. All rights reserved. 16– 18

Molecule positioning.

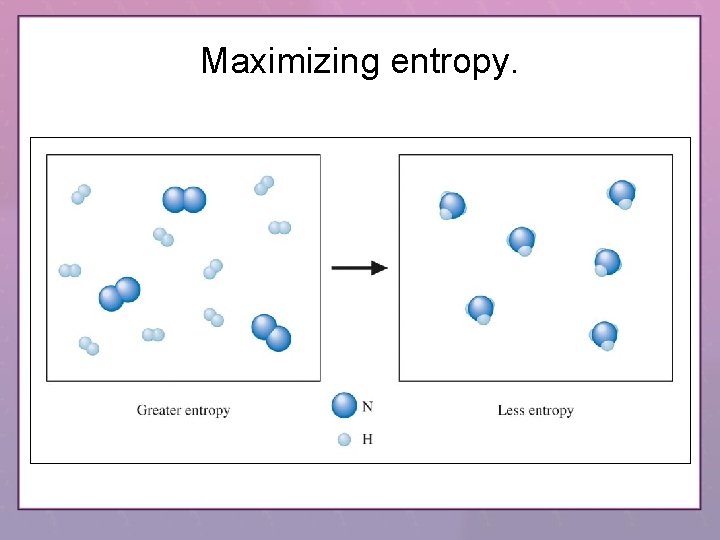
Maximizing entropy.

2 t eac R Predict the sign of S for each of the following, and explain: a) the evaporation of alcohol b) the freezing of water c) compressing an ideal gas at constant temperature d) heating an ideal gas at constant pressure e) dissolving Na. Cl in water Copyright © Houghton Mifflin Company. All rights reserved. 16– 21

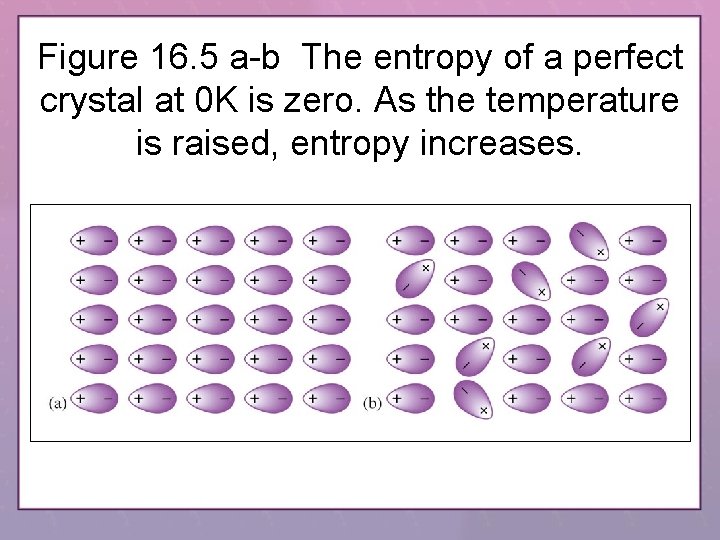
Figure 16. 5 a-b The entropy of a perfect crystal at 0 K is zero. As the temperature is raised, entropy increases.

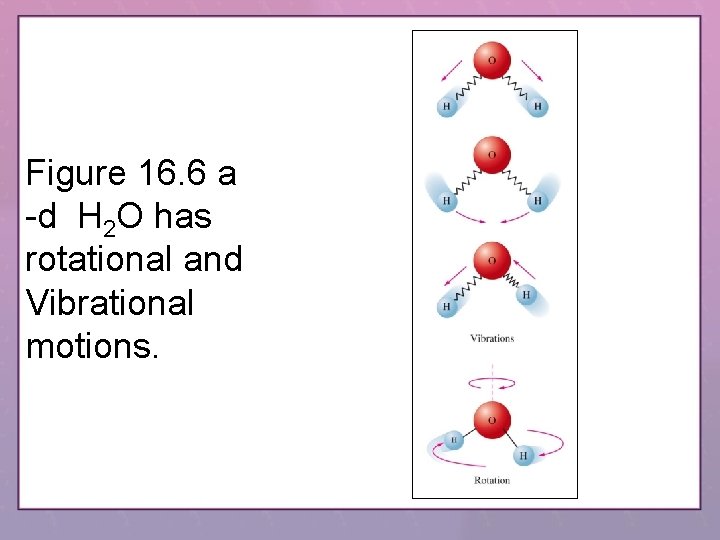
Figure 16. 6 a -d H 2 O has rotational and Vibrational motions.

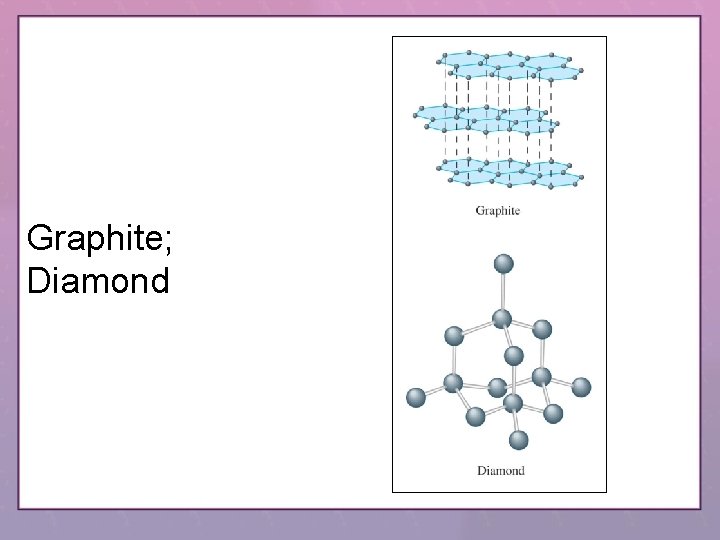
Graphite; Diamond

The Effects of Temperature on Spontaneity

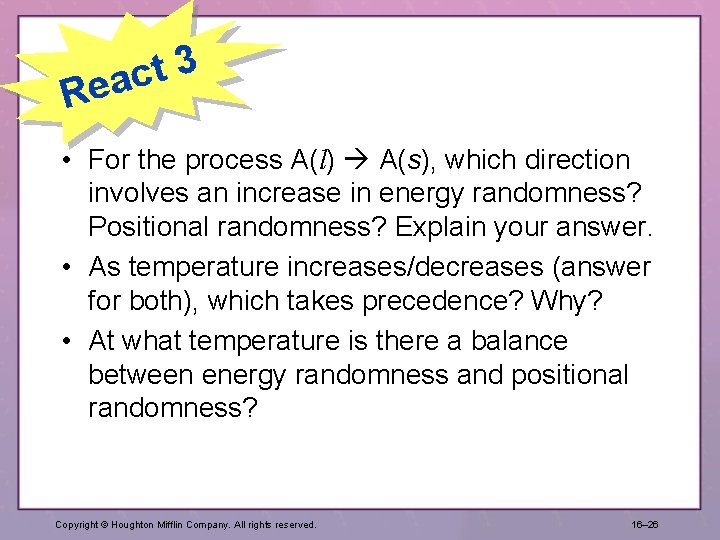
3 t eac R • For the process A(l) A(s), which direction involves an increase in energy randomness? Positional randomness? Explain your answer. • As temperature increases/decreases (answer for both), which takes precedence? Why? • At what temperature is there a balance between energy randomness and positional randomness? Copyright © Houghton Mifflin Company. All rights reserved. 16– 26

4 t eac R • • Describe the following as spontaneous/ nonspontaneous/cannot tell, and explain. A reaction that is a) b) c) d) e) Exothermic and becomes more positionally random Exothermic and becomes less positionally random Endothermic and becomes more positionally random Endothermic and becomes less positionally random Explain how temperature affects your answers. Provide a real example of two of the items above. Copyright © Houghton Mifflin Company. All rights reserved. 16– 27

5 t eac R Use the ideas of energy randomness and positional randomness in your discussion of the following: a) Under what conditions is the freezing of water spontaneous? Why? b) Under what conditions is the melting of water spontaneous? Why? c) Under what conditions is the freezing of water as likely as the melting of water? Why? Copyright © Houghton Mifflin Company. All rights reserved. 16– 28

6 t eac R A liquid is vaporized at its boiling point. Predict the signs of w, q, H, Ssurr and G. Explain your answers. Copyright © Houghton Mifflin Company. All rights reserved. 16– 29

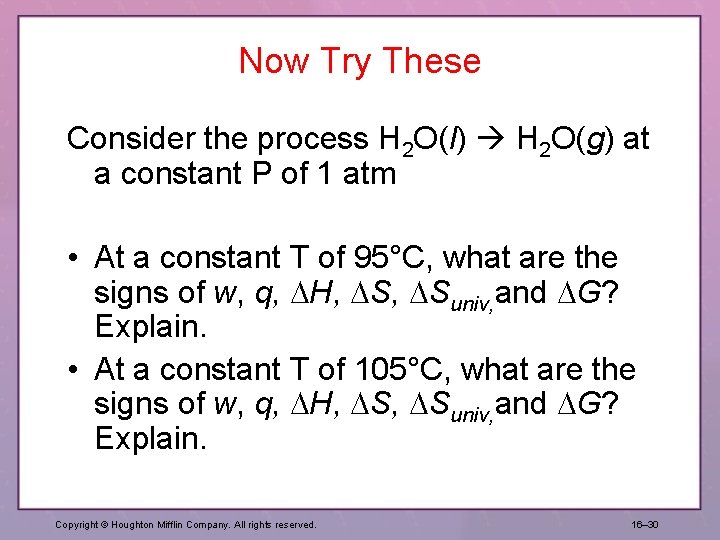
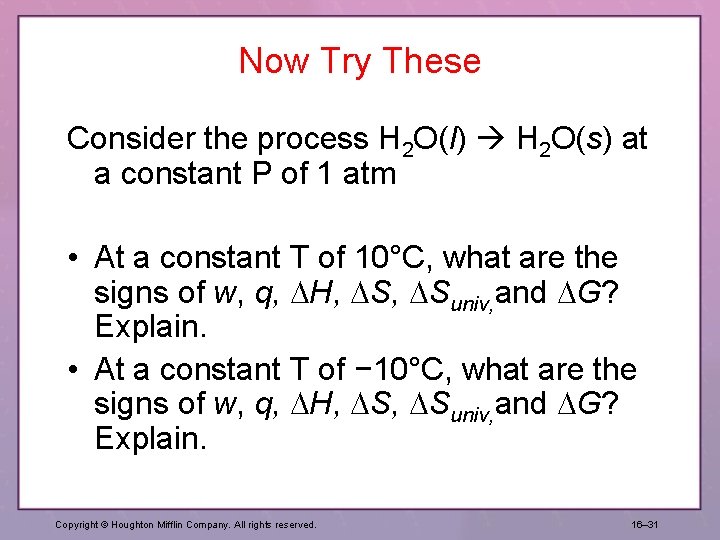
Now Try These Consider the process H 2 O(l) H 2 O(g) at a constant P of 1 atm • At a constant T of 95°C, what are the signs of w, q, H, Suniv, and G? Explain. • At a constant T of 105°C, what are the signs of w, q, H, Suniv, and G? Explain. Copyright © Houghton Mifflin Company. All rights reserved. 16– 30

Now Try These Consider the process H 2 O(l) H 2 O(s) at a constant P of 1 atm • At a constant T of 10°C, what are the signs of w, q, H, Suniv, and G? Explain. • At a constant T of − 10°C, what are the signs of w, q, H, Suniv, and G? Explain. Copyright © Houghton Mifflin Company. All rights reserved. 16– 31

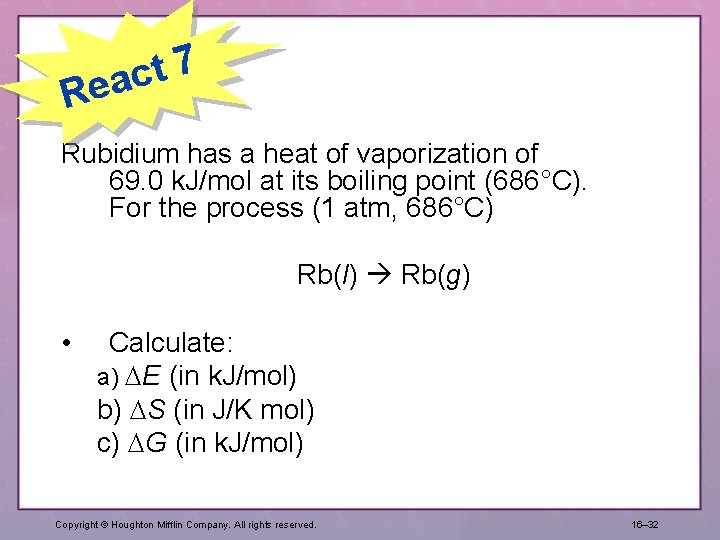
7 t eac R Rubidium has a heat of vaporization of 69. 0 k. J/mol at its boiling point (686°C). For the process (1 atm, 686°C) Rb(l) Rb(g) • Calculate: a) E (in k. J/mol) b) S (in J/K mol) c) G (in k. J/mol) Copyright © Houghton Mifflin Company. All rights reserved. 16– 32

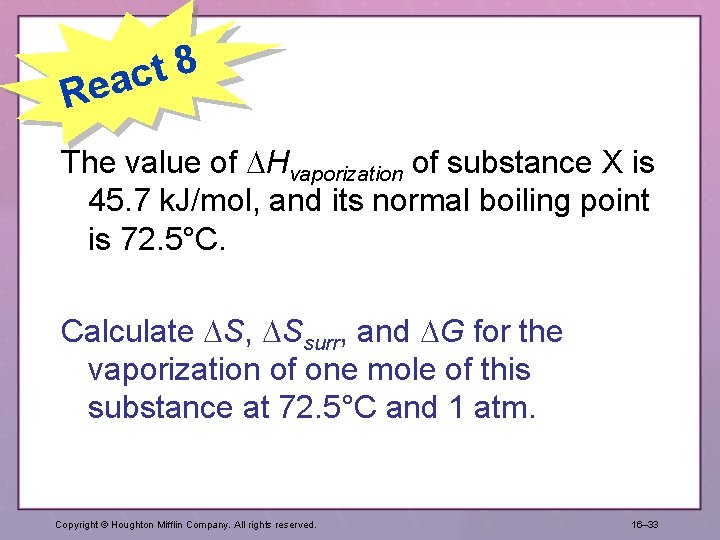
8 t eac R The value of Hvaporization of substance X is 45. 7 k. J/mol, and its normal boiling point is 72. 5°C. Calculate S, Ssurr, and G for the vaporization of one mole of this substance at 72. 5°C and 1 atm. Copyright © Houghton Mifflin Company. All rights reserved. 16– 33

The Mineral Stibnite Contains Sb 2 S 3

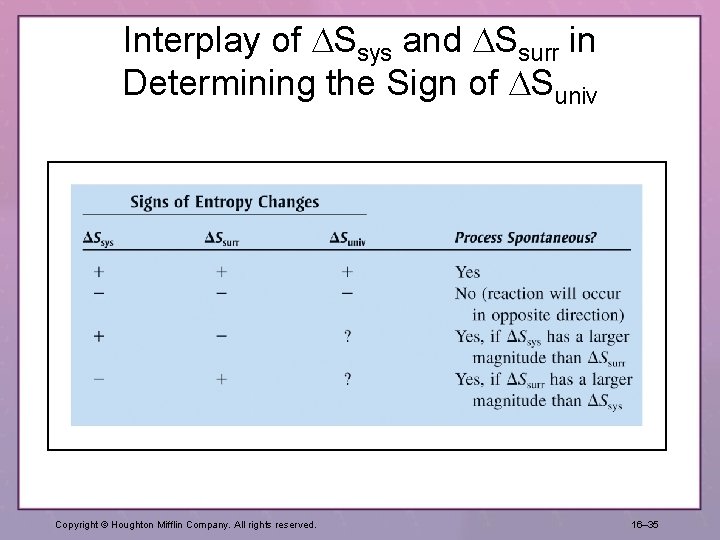
Interplay of Ssys and Ssurr in Determining the Sign of Suniv Copyright © Houghton Mifflin Company. All rights reserved. 16– 35

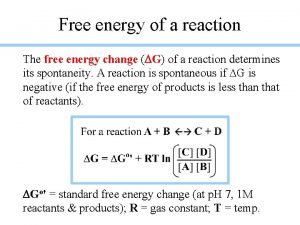
Free Energy

Spontaneous Reactions Copyright © Houghton Mifflin Company. All rights reserved. 16– 37

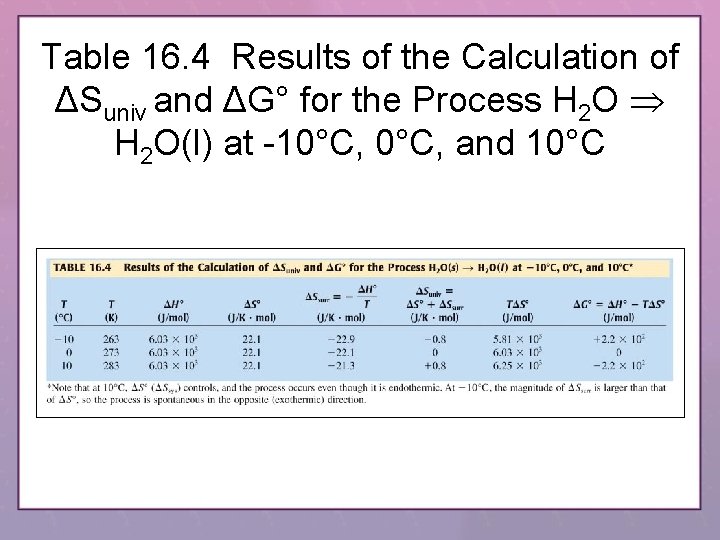
Table 16. 4 Results of the Calculation of ΔSuniv and ΔG° for the Process H 2 O(l) at -10°C, and 10°C

Table 16. 5 Various Possible Combinations of ΔH and Δs for a Process and the Resulting Dependence of Spontaneity on Temperature

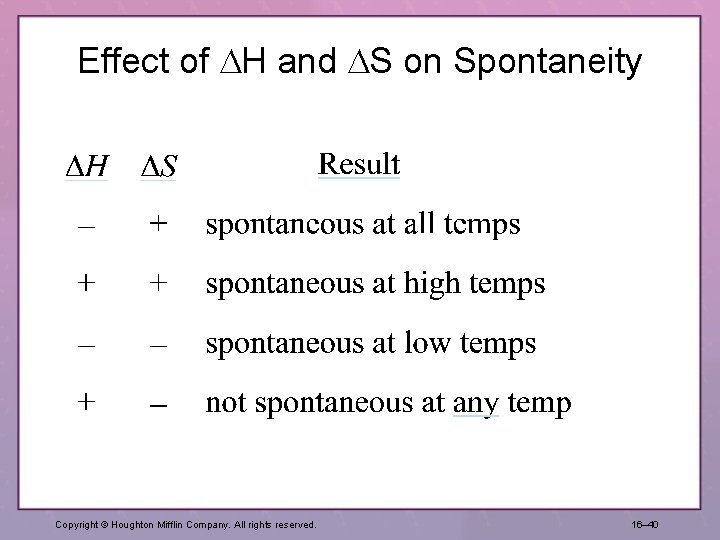
Effect of H and S on Spontaneity Copyright © Houghton Mifflin Company. All rights reserved. 16– 40

Entropy Changes and Free Energy in Chemical Reactions

9 t eac R Gas A 2 reacts with gas B 2 to form gas AB at constant temperature and pressure. The bond energy of AB is much greater than that of either reactant. Predict the signs of: H Ssurr S Explain. Copyright © Houghton Mifflin Company. All rights reserved. Suniv 16– 42

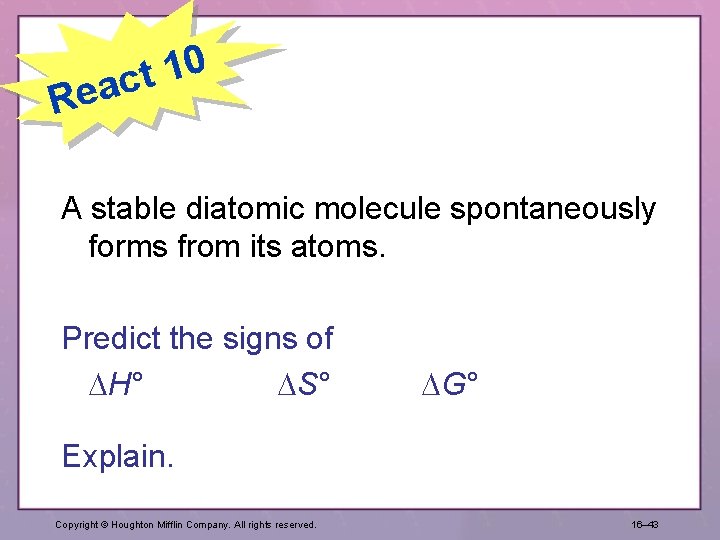
Re 0 1 act A stable diatomic molecule spontaneously forms from its atoms. Predict the signs of H° S° G° Explain. Copyright © Houghton Mifflin Company. All rights reserved. 16– 43

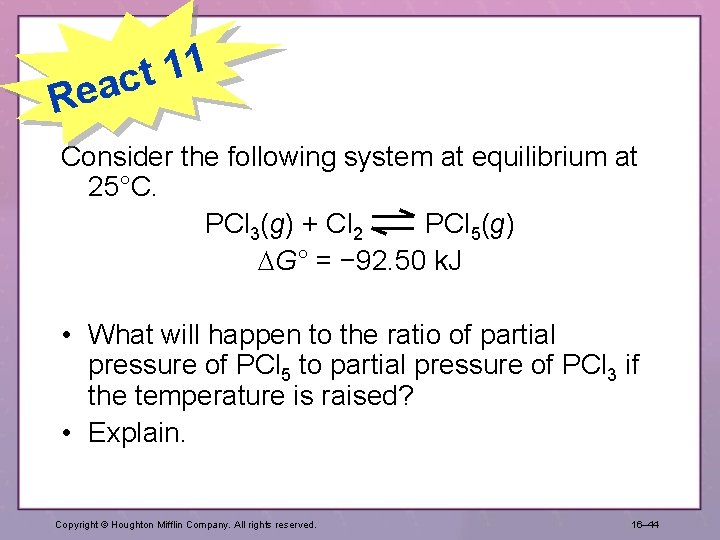
1 1 t eac R Consider the following system at equilibrium at 25°C. PCl 3(g) + Cl 2 PCl 5(g) G° = − 92. 50 k. J • What will happen to the ratio of partial pressure of PCl 5 to partial pressure of PCl 3 if the temperature is raised? • Explain. Copyright © Houghton Mifflin Company. All rights reserved. 16– 44

2 1 t eac R Sketch graphs of 1. G vs. P 2. H vs. P 3. ln(K) vs. 1/T (for both endothermic and exothermic cases) Copyright © Houghton Mifflin Company. All rights reserved. 16– 45

Free Energy and Equilibrium

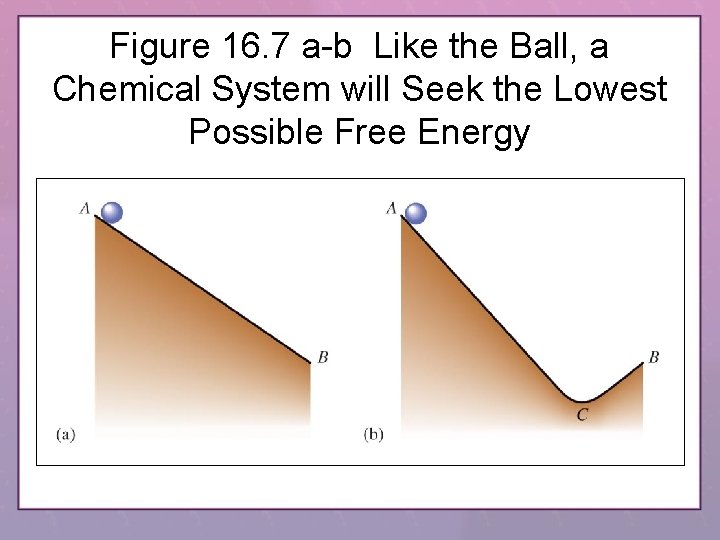
Figure 16. 7 a-b Like the Ball, a Chemical System will Seek the Lowest Possible Free Energy

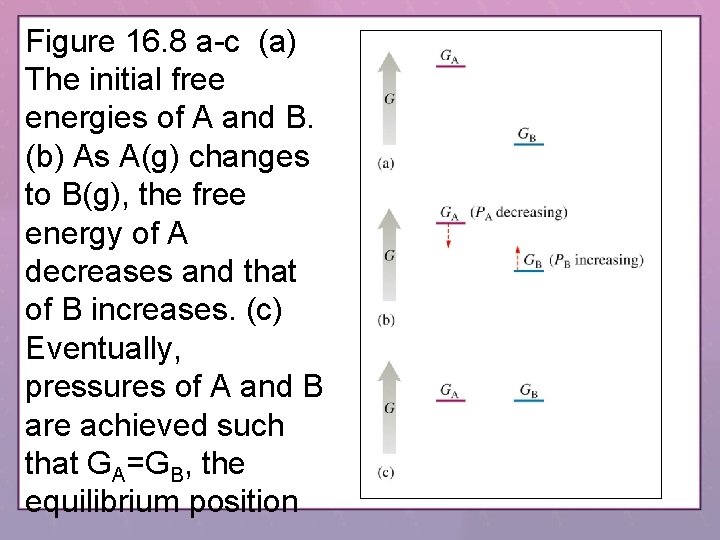
Figure 16. 8 a-c (a) The initial free energies of A and B. (b) As A(g) changes to B(g), the free energy of A decreases and that of B increases. (c) Eventually, pressures of A and B are achieved such that GA=GB, the equilibrium position

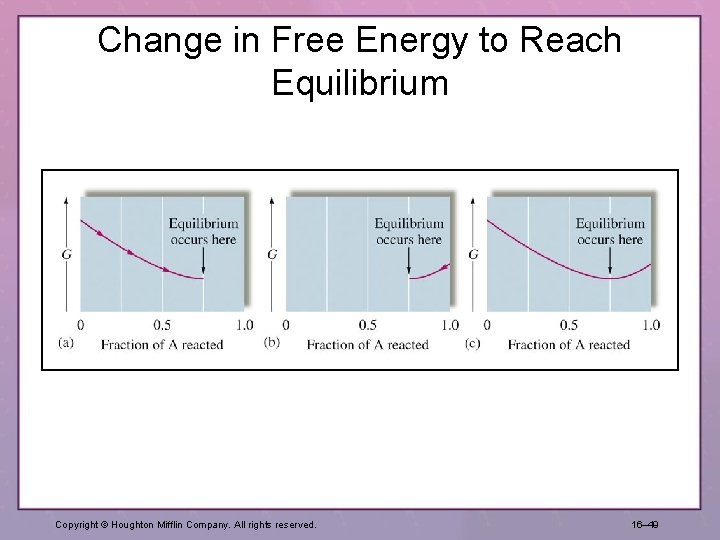
Change in Free Energy to Reach Equilibrium Copyright © Houghton Mifflin Company. All rights reserved. 16– 49

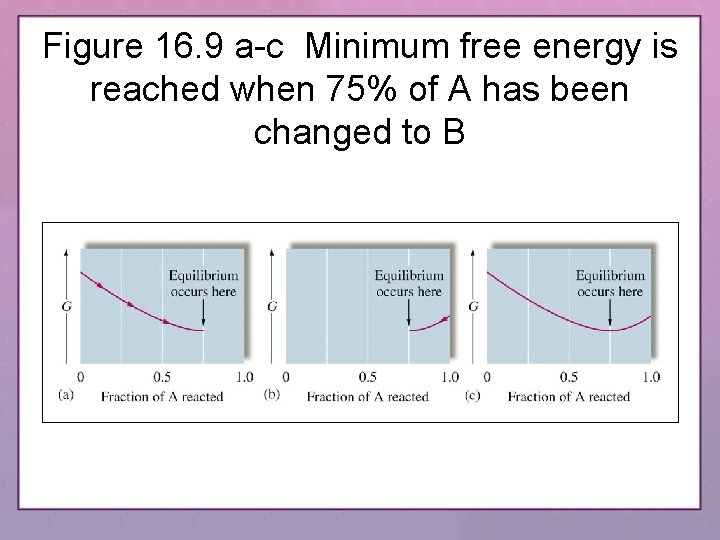
Figure 16. 9 a-c Minimum free energy is reached when 75% of A has been changed to B

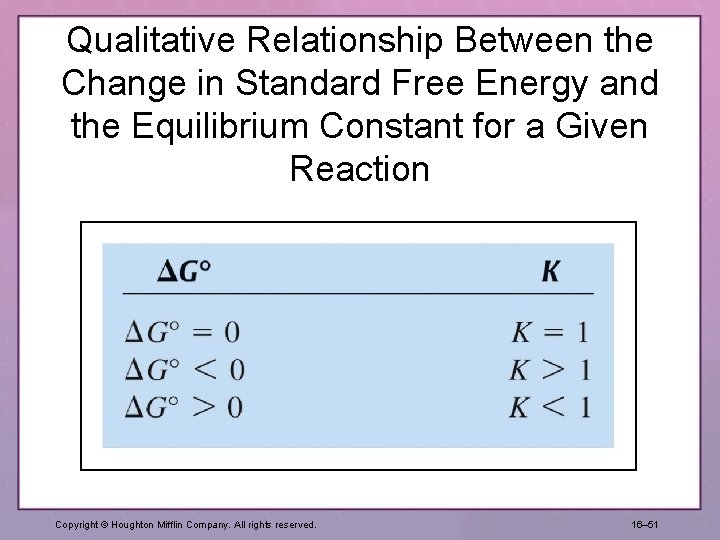
Qualitative Relationship Between the Change in Standard Free Energy and the Equilibrium Constant for a Given Reaction Copyright © Houghton Mifflin Company. All rights reserved. 16– 51

Formation of Rust on Bare Steel is a Spontaneous Process

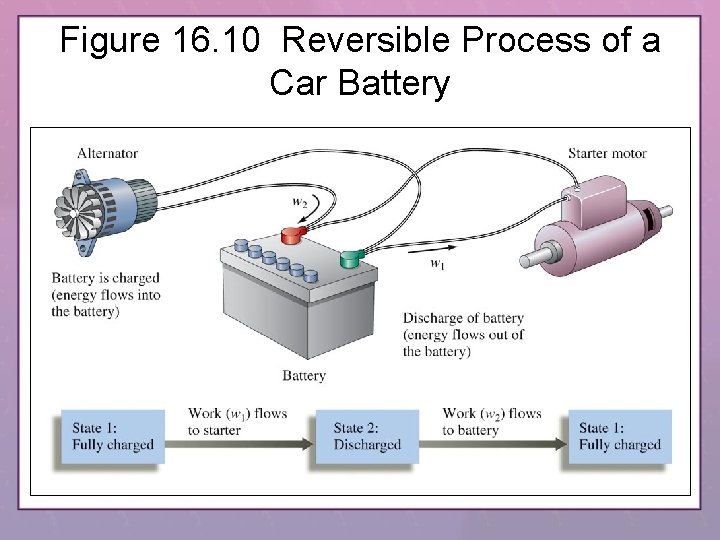
Figure 16. 10 Reversible Process of a Car Battery
 Ap chemistry spontaneity entropy and free energy
Ap chemistry spontaneity entropy and free energy Chemistry microstates
Chemistry microstates Gibbs free energy chart
Gibbs free energy chart Gibbs free energy and spontaneity
Gibbs free energy and spontaneity Relationship between entropy and free energy
Relationship between entropy and free energy Enthalpy entropy free energy
Enthalpy entropy free energy Helmholtz free energy and gibbs free energy
Helmholtz free energy and gibbs free energy Gibbs energy and equilibrium
Gibbs energy and equilibrium If gibbs free energy is negative
If gibbs free energy is negative Enthalpy entropy free energy
Enthalpy entropy free energy Delta g units
Delta g units 16-point
16-point Whats half past 9
Whats half past 9 The sixteen career clusters
The sixteen career clusters Sixteen year old carrie is babysitting
Sixteen year old carrie is babysitting Sixteen securities inc
Sixteen securities inc Thermodynamics ppt
Thermodynamics ppt Predicting spontaneity
Predicting spontaneity Zn cu
Zn cu Predicting spontaneity
Predicting spontaneity Complete the following table on reaction spontaneity
Complete the following table on reaction spontaneity Predicting redox reactions
Predicting redox reactions Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis A story of an hour summary
A story of an hour summary Free hearts free foreheads you and i are
Free hearts free foreheads you and i are Spontaneous and nonspontaneous process in thermodynamics
Spontaneous and nonspontaneous process in thermodynamics Negotiated financing
Negotiated financing Q system = -q surroundings
Q system = -q surroundings What is enthalpy and entropy
What is enthalpy and entropy Enthalpy
Enthalpy Entropy system and surroundings
Entropy system and surroundings Entropy order parameters and complexity
Entropy order parameters and complexity Chapter 7 energy conservation of energy
Chapter 7 energy conservation of energy The allocation map
The allocation map Free free absorption
Free free absorption Phosphoanhydrid
Phosphoanhydrid Delta g spontaneous
Delta g spontaneous What is the change of entropy in an irreversible process
What is the change of entropy in an irreversible process Light's criteria
Light's criteria Spontaneous transmutation
Spontaneous transmutation Odyssey of the mind spontaneous
Odyssey of the mind spontaneous Spontaneous emission
Spontaneous emission Spontaneous fission definition
Spontaneous fission definition K=e^-g/rt
K=e^-g/rt Behavioral contrast
Behavioral contrast Spontaneous recovery psychology
Spontaneous recovery psychology Tgcsa grading plaque
Tgcsa grading plaque Is a negative delta g spontaneous
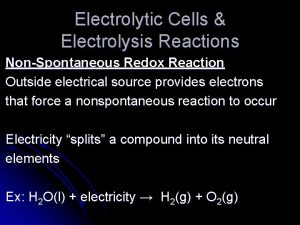
Is a negative delta g spontaneous Electrolysis spontaneous or nonspontaneous
Electrolysis spontaneous or nonspontaneous Learning is permanent
Learning is permanent Smyth model of reflection
Smyth model of reflection Real life example of classical conditioning
Real life example of classical conditioning Spontaneous reaction chemistry
Spontaneous reaction chemistry