Electrochemistry Spontaneity of Redox Reactions 21 1 Electrochemistry




![Application of Concentration Cell: p. H meter The glass electrode monitors the [H+] of Application of Concentration Cell: p. H meter The glass electrode monitors the [H+] of](https://slidetodoc.com/presentation_image_h/f998de9add2cf19829efb771db85852e/image-51.jpg)

- Slides: 77

Electrochemistry Spontaneity of Redox Reactions 21 -1

Electrochemistry: Chemical Change and Electrical Work Redox Reactions and Electrochemical Cells Voltaic Cells: Using Spontaneous Reactions to Generate Electrical Energy Cell Potential: Output of a Voltaic Cell Free Energy and Electrical Work Electrochemical Processes in Batteries Corrosion: An Environmental Voltaic Cell Electrolytic Cells: Using Electrical Energy to Drive Nonspontaneous Reactions 21 -2

Overview of Redox Reactions Chemistry magic (vid): Copper coin “Silver” “Gold” Oxidation: Loss of electrons Reduction: Gain of electrons. These processes occur simultaneously. Oxidation results in an increase in Oxidation Numbers (O. N. ). while reduction results in a decrease in O. N. Oxidizing agent takes electrons from the substance being oxidized. The oxidizing agent is therefore reduced. Reducing agent takes electrons from the substance being oxidized. The reducing agent is therefore oxidized. 21 -3

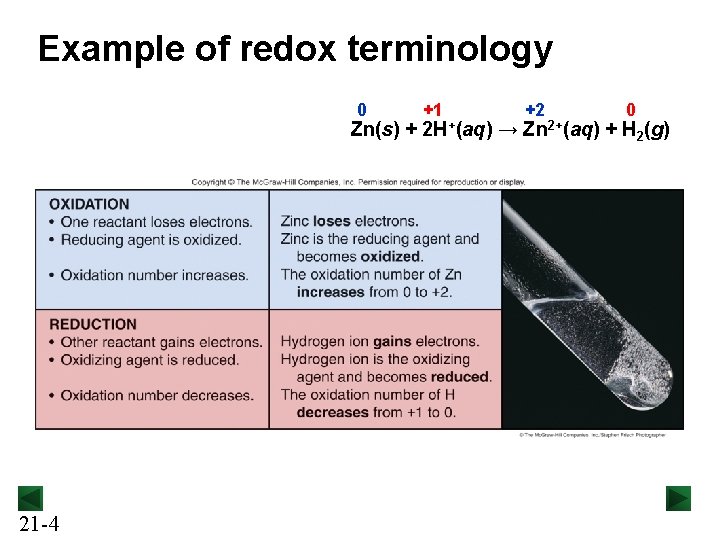
Example of redox terminology 0 +1 +2 0 Zn(s) + 2 H+(aq) → Zn 2+(aq) + H 21 -4 2(g)

Half-Reaction Method for Balancing Redox Reactions The half-reaction method divides a redox reaction into its oxidation and reduction half-reactions. - This reflects their physical separation in electrochemical cells. This method does not require assigning oxidation numbers (O. N. s). The half-reaction method is easier to apply to reactions in acidic or basic solutions. 21 -5

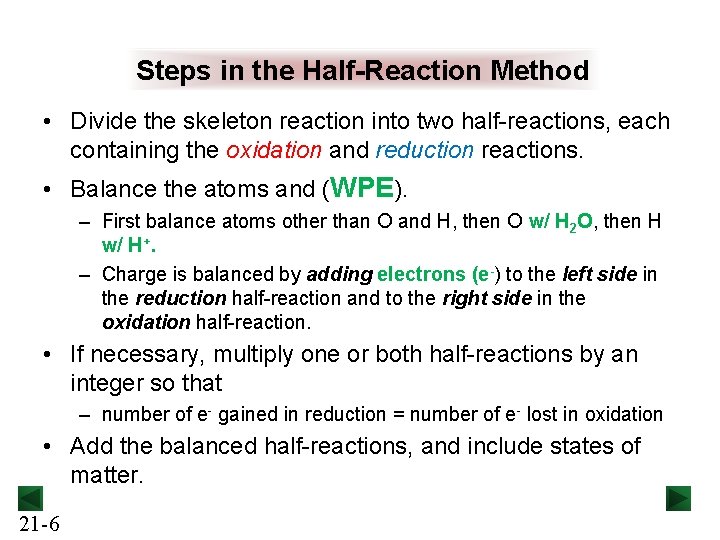
Steps in the Half-Reaction Method • Divide the skeleton reaction into two half-reactions, each containing the oxidation and reduction reactions. • Balance the atoms and (WPE). – First balance atoms other than O and H, then O w/ H 2 O, then H w/ H+. – Charge is balanced by adding electrons (e-) to the left side in the reduction half-reaction and to the right side in the oxidation half-reaction. • If necessary, multiply one or both half-reactions by an integer so that – number of e- gained in reduction = number of e- lost in oxidation • Add the balanced half-reactions, and include states of matter. 21 -6

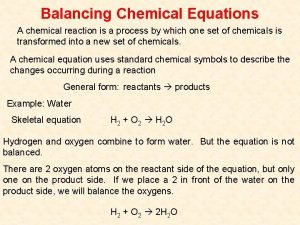
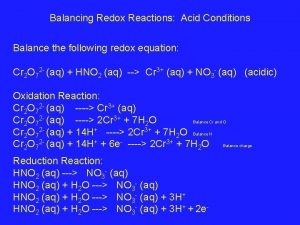
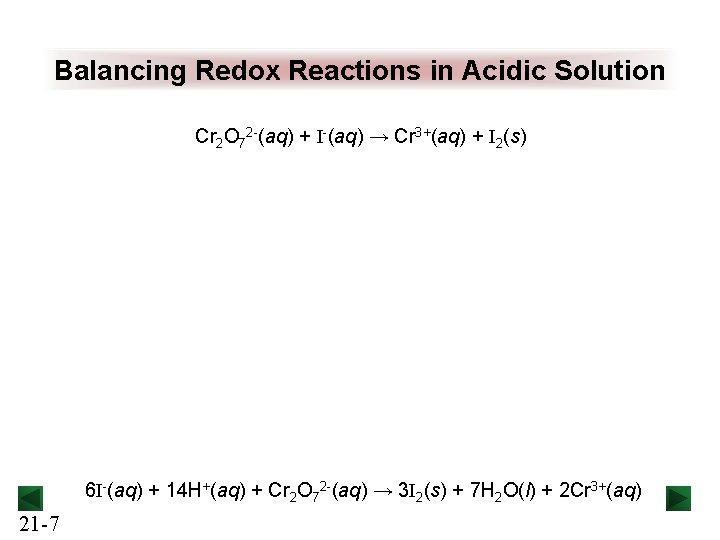
Balancing Redox Reactions in Acidic Solution Cr 2 O 72 -(aq) + I-(aq) → Cr 3+(aq) + I 2(s) 6 I-(aq) + 14 H+(aq) + Cr 2 O 72 -(aq) → 3 I 2(s) + 7 H 2 O(l) + 2 Cr 3+(aq) 21 -7

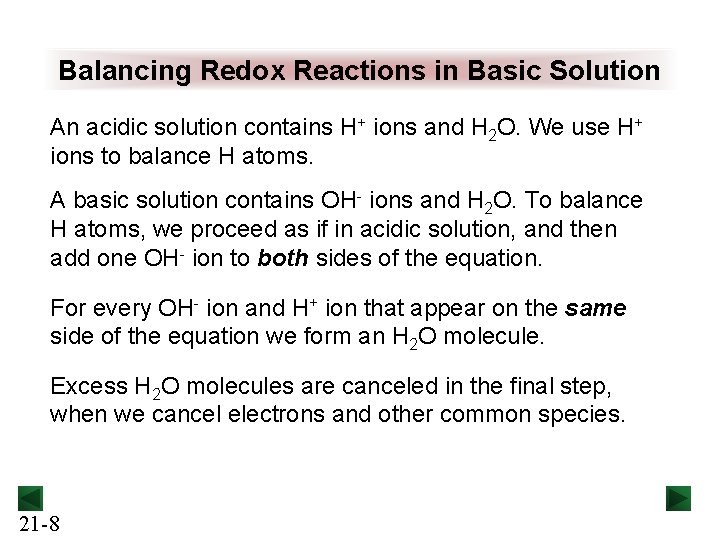
Balancing Redox Reactions in Basic Solution An acidic solution contains H+ ions and H 2 O. We use H+ ions to balance H atoms. A basic solution contains OH- ions and H 2 O. To balance H atoms, we proceed as if in acidic solution, and then add one OH- ion to both sides of the equation. For every OH- ion and H+ ion that appear on the same side of the equation we form an H 2 O molecule. Excess H 2 O molecules are canceled in the final step, when we cancel electrons and other common species. 21 -8

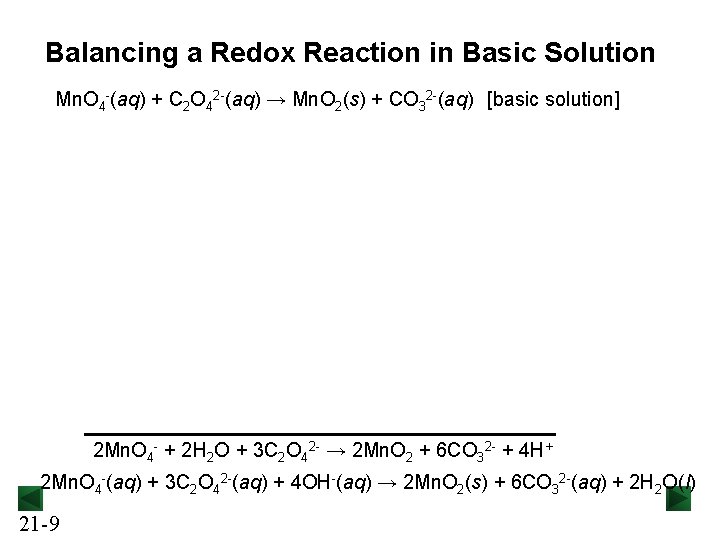
Balancing a Redox Reaction in Basic Solution Mn. O 4 -(aq) + C 2 O 42 -(aq) → Mn. O 2(s) + CO 32 -(aq) [basic solution] 2 Mn. O 4 - + 2 H 2 O + 3 C 2 O 42 - → 2 Mn. O 2 + 6 CO 32 - + 4 H+ 2 Mn. O 4 -(aq) + 3 C 2 O 42 -(aq) + 4 OH-(aq) → 2 Mn. O 2(s) + 6 CO 32 -(aq) + 2 H 2 O(l) 21 -9

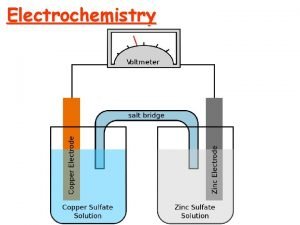
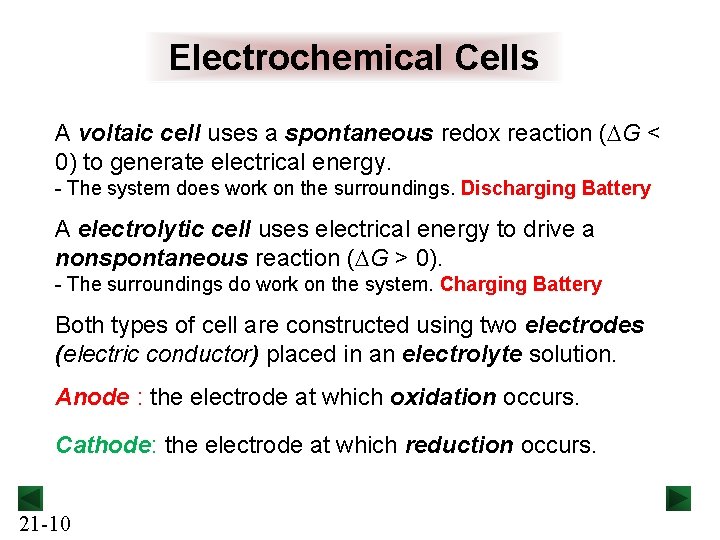
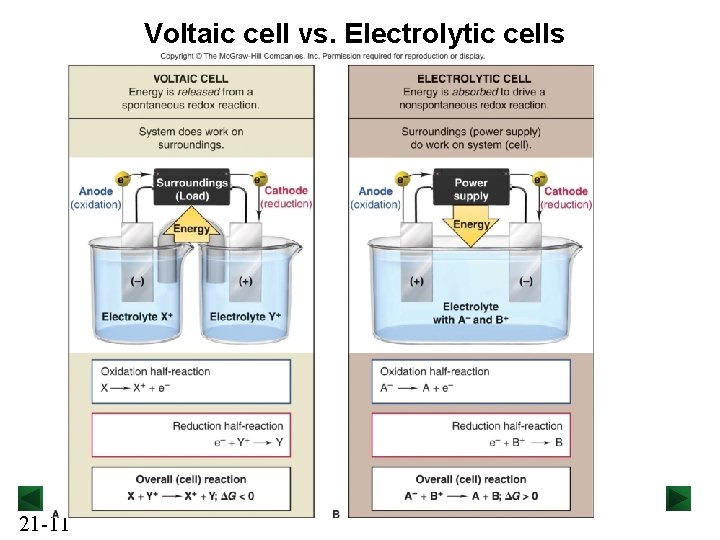
Electrochemical Cells A voltaic cell uses a spontaneous redox reaction (DG < 0) to generate electrical energy. - The system does work on the surroundings. Discharging Battery A electrolytic cell uses electrical energy to drive a nonspontaneous reaction (DG > 0). - The surroundings do work on the system. Charging Battery Both types of cell are constructed using two electrodes (electric conductor) placed in an electrolyte solution. Anode : the electrode at which oxidation occurs. Cathode: the electrode at which reduction occurs. 21 -10

Voltaic cell vs. Electrolytic cells 21 -11

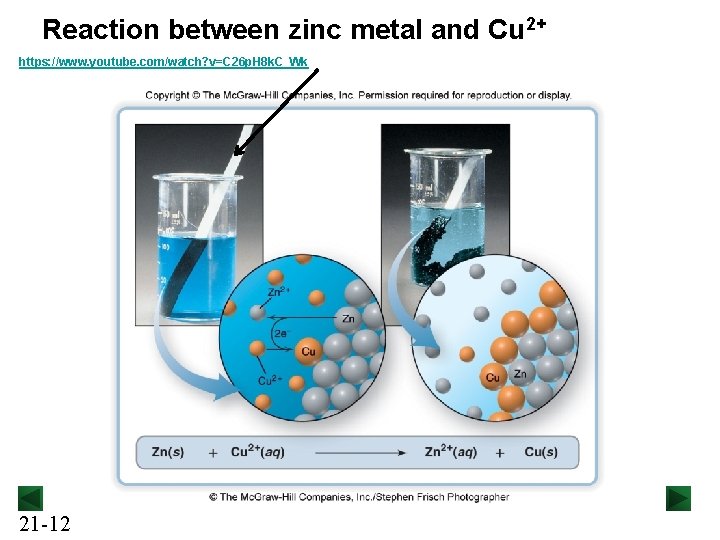
Reaction between zinc metal and Cu 2+ https: //www. youtube. com/watch? v=C 26 p. H 8 k. C_Wk 21 -12

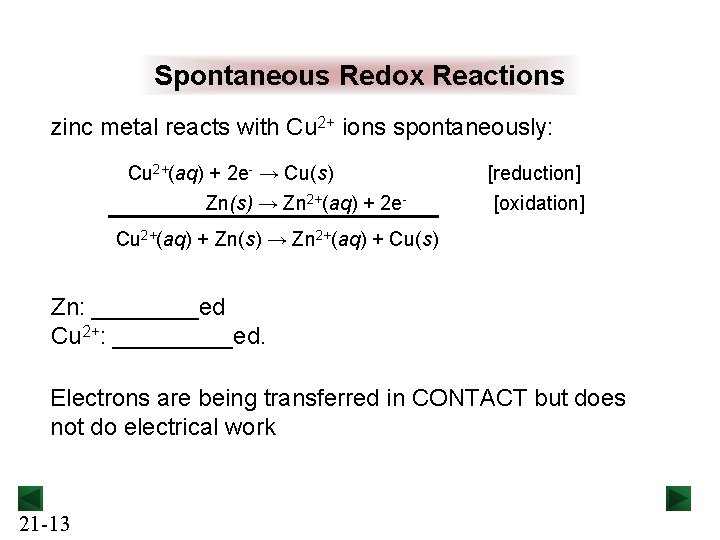
Spontaneous Redox Reactions zinc metal reacts with Cu 2+ ions spontaneously: Cu 2+(aq) + 2 e- → Cu(s) Zn(s) → Zn 2+(aq) + 2 e- [reduction] [oxidation] Cu 2+(aq) + Zn(s) → Zn 2+(aq) + Cu(s) Zn: ____ed Cu 2+: _____ed. Electrons are being transferred in CONTACT but does not do electrical work 21 -13

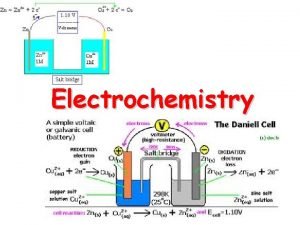
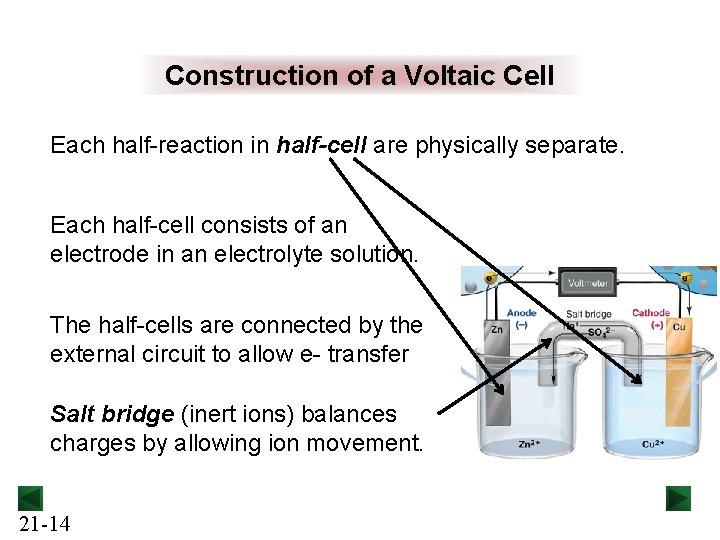
Construction of a Voltaic Cell Each half-reaction in half-cell are physically separate. Each half-cell consists of an electrode in an electrolyte solution. The half-cells are connected by the external circuit to allow e- transfer Salt bridge (inert ions) balances charges by allowing ion movement. 21 -14

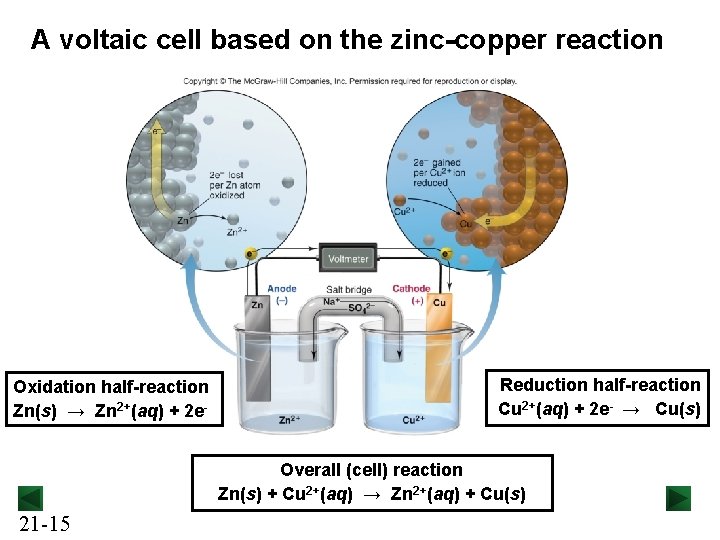
A voltaic cell based on the zinc-copper reaction Oxidation half-reaction Zn(s) → Zn 2+(aq) + 2 e- Reduction half-reaction Cu 2+(aq) + 2 e- → Cu(s) Overall (cell) reaction Zn(s) + Cu 2+(aq) → Zn 2+(aq) + Cu(s) 21 -15

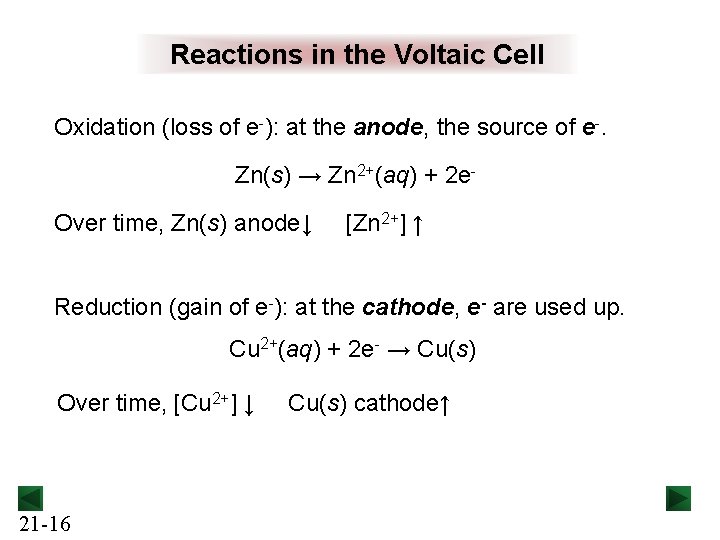
Reactions in the Voltaic Cell Oxidation (loss of e-): at the anode, the source of e-. Zn(s) → Zn 2+(aq) + 2 e. Over time, Zn(s) anode↓ [Zn 2+] ↑ Reduction (gain of e-): at the cathode, e- are used up. Cu 2+(aq) + 2 e- → Cu(s) Over time, [Cu 2+] ↓ 21 -16 Cu(s) cathode↑

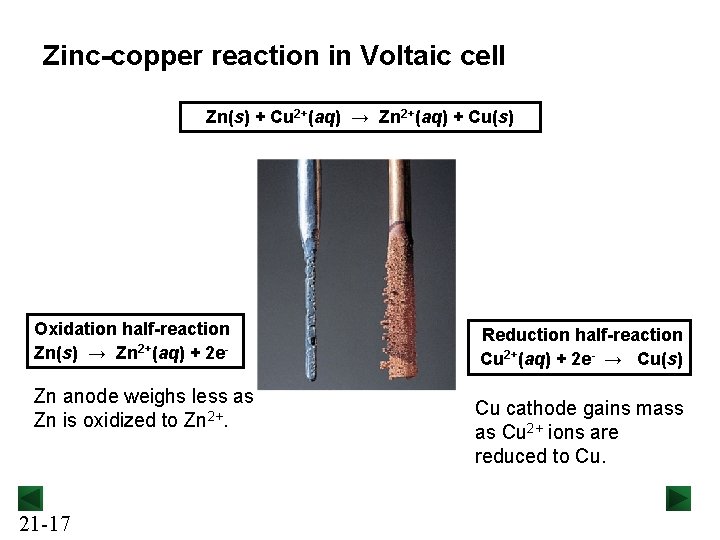
Zinc-copper reaction in Voltaic cell Zn(s) + Cu 2+(aq) → Zn 2+(aq) + Cu(s) Oxidation half-reaction Zn(s) → Zn 2+(aq) + 2 e- Zn anode weighs less as Zn is oxidized to Zn 2+. 21 -17 Reduction half-reaction Cu 2+(aq) + 2 e- → Cu(s) Cu cathode gains mass as Cu 2+ ions are reduced to Cu.

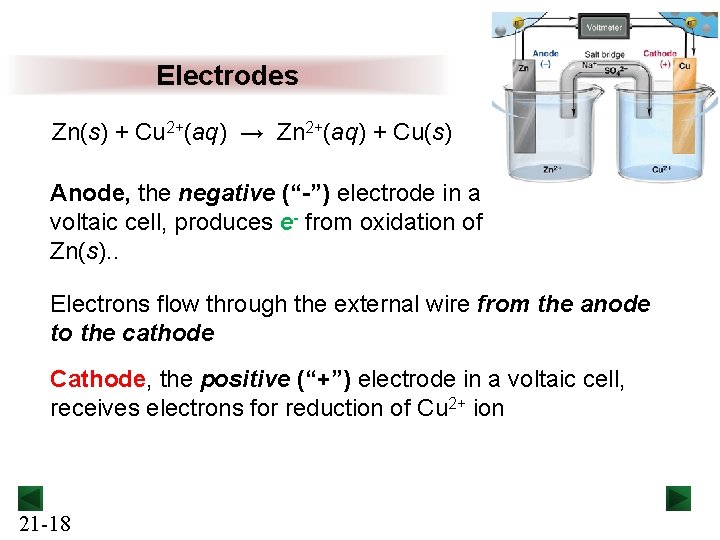
Electrodes Zn(s) + Cu 2+(aq) → Zn 2+(aq) + Cu(s) Anode, the negative (“-”) electrode in a voltaic cell, produces e- from oxidation of Zn(s). . Electrons flow through the external wire from the anode to the cathode Cathode, the positive (“+”) electrode in a voltaic cell, receives electrons for reduction of Cu 2+ ion 21 -18

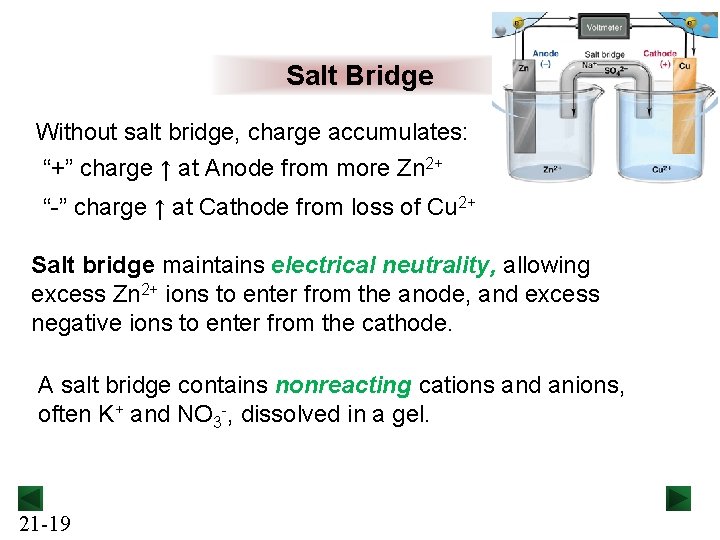
Salt Bridge Without salt bridge, charge accumulates: “+” charge ↑ at Anode from more Zn 2+ “-” charge ↑ at Cathode from loss of Cu 2+ Salt bridge maintains electrical neutrality, allowing excess Zn 2+ ions to enter from the anode, and excess negative ions to enter from the cathode. A salt bridge contains nonreacting cations and anions, often K+ and NO 3 -, dissolved in a gel. 21 -19

Active and Inactive Electrodes Active electrode involved in the half-reaction (as a reactant or product in the overall reaction). Example: Zinc-Copper voltaic cell -Copper Inactive electrode provides a surface for the reaction and completes the circuit. It does not participate in the overall reaction. - Inactive electrodes are necessary when none of the reaction components can be used as an electrode. Inactive electrodes are usually unreactive substances such as graphite or platinum. 21 -20

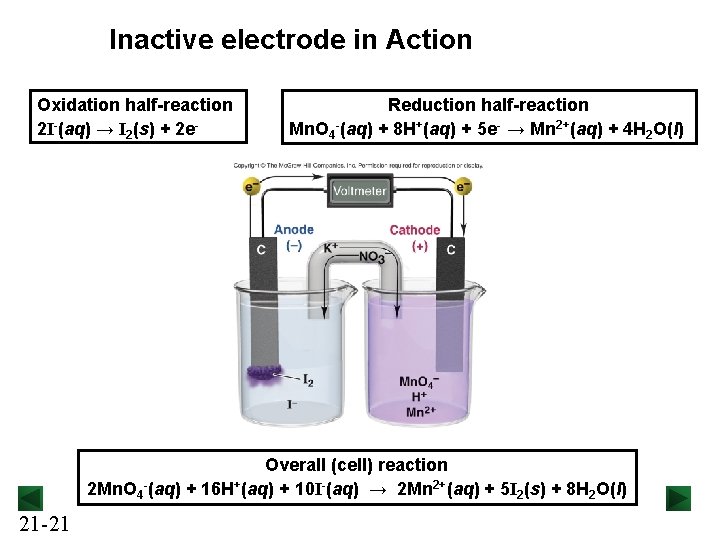
Inactive electrode in Action Oxidation half-reaction 2 I-(aq) → I 2(s) + 2 e- Reduction half-reaction Mn. O 4 -(aq) + 8 H+(aq) + 5 e- → Mn 2+(aq) + 4 H 2 O(l) Overall (cell) reaction 2 Mn. O 4 -(aq) + 16 H+(aq) + 10 I-(aq) → 2 Mn 2+(aq) + 5 I 2(s) + 8 H 2 O(l) 21 -21

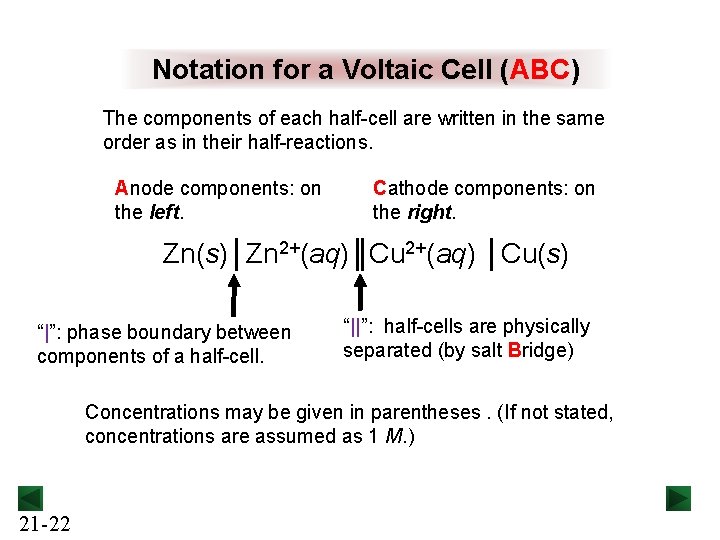
Notation for a Voltaic Cell (ABC) The components of each half-cell are written in the same order as in their half-reactions. Anode components: on the left. Cathode components: on the right. Zn(s)│Zn 2+(aq)║Cu 2+(aq) │Cu(s) “|”: phase boundary between components of a half-cell. “||”: half-cells are physically separated (by salt Bridge) Concentrations may be given in parentheses. (If not stated, concentrations are assumed as 1 M. ) 21 -22

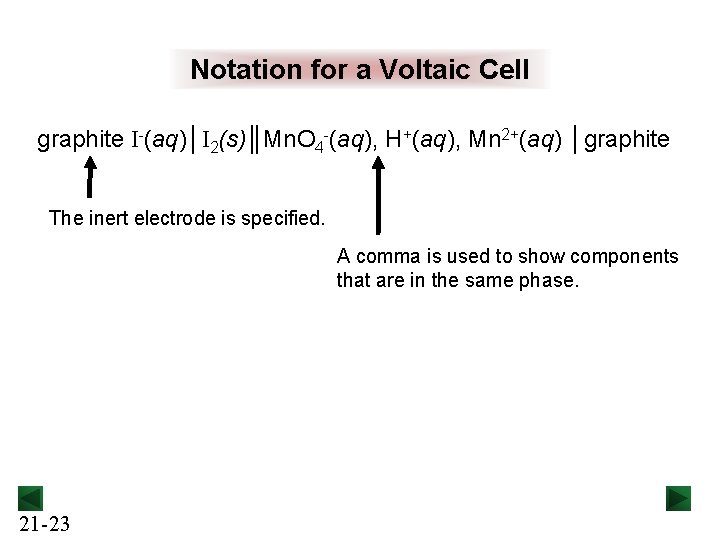
Notation for a Voltaic Cell graphite I-(aq)│I 2(s)║Mn. O 4 -(aq), H+(aq), Mn 2+(aq) │graphite The inert electrode is specified. A comma is used to show components that are in the same phase. 21 -23

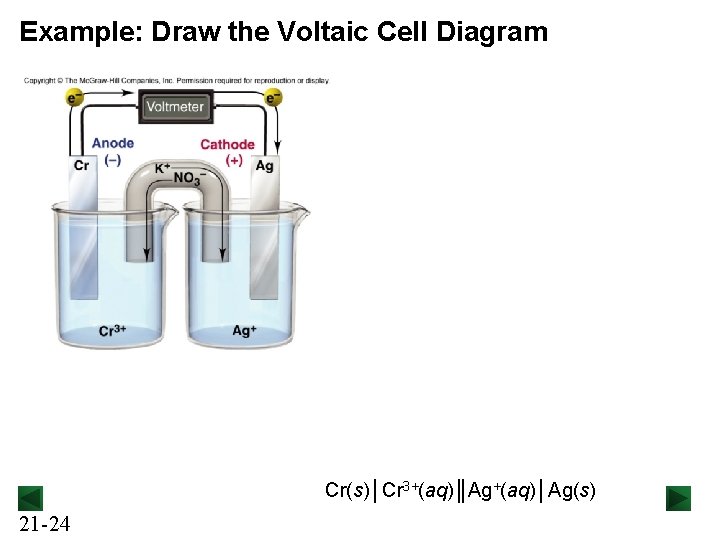
Example: Draw the Voltaic Cell Diagram Cr(s)│Cr 3+(aq)║Ag+(aq)│Ag(s) 21 -24

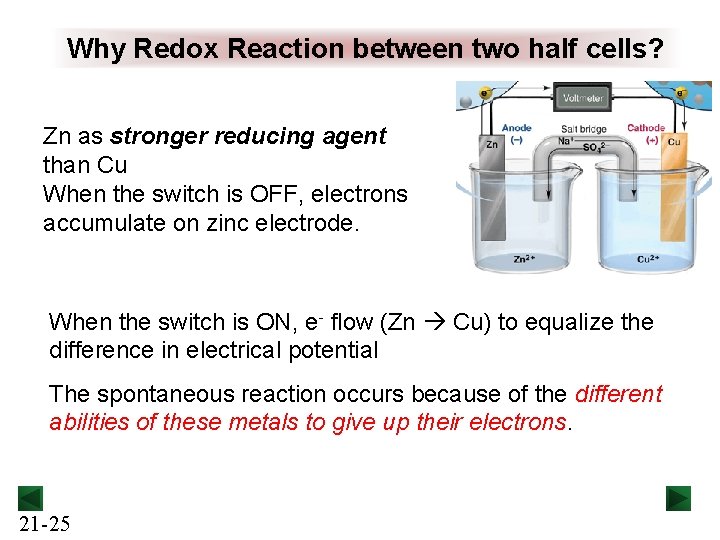
Why Redox Reaction between two half cells? Zn as stronger reducing agent than Cu When the switch is OFF, electrons accumulate on zinc electrode. When the switch is ON, e- flow (Zn Cu) to equalize the difference in electrical potential The spontaneous reaction occurs because of the different abilities of these metals to give up their electrons. 21 -25

Cell Potential Cell potential (Ecell, Voltage) depends on the different abilities of these metals to give up their electrons Ecell can be measured using voltammeter (one of many functions in a multimeter). Ecell > 0 for a spontaneous process. 21 -26

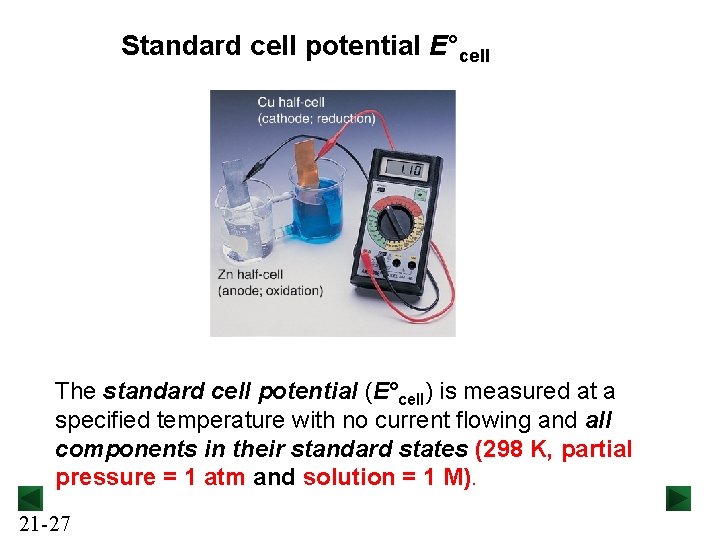
Standard cell potential E°cell The standard cell potential (E°cell) is measured at a specified temperature with no current flowing and all components in their standard states (298 K, partial pressure = 1 atm and solution = 1 M). 21 -27

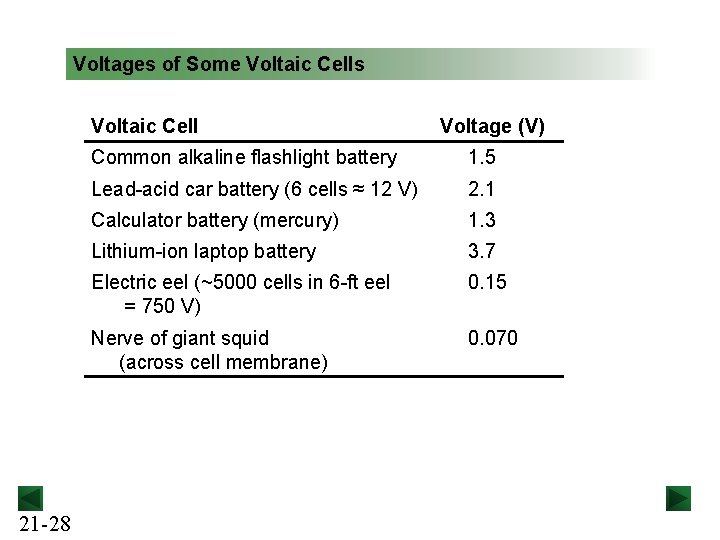
Voltages of Some Voltaic Cells Voltaic Cell 21 -28 Voltage (V) Common alkaline flashlight battery 1. 5 Lead-acid car battery (6 cells ≈ 12 V) 2. 1 Calculator battery (mercury) 1. 3 Lithium-ion laptop battery 3. 7 Electric eel (~5000 cells in 6 -ft eel = 750 V) 0. 15 Nerve of giant squid (across cell membrane) 0. 070

Standard Hydrogen Electrode (SHE) The standard hydrogen electrode has a standard electrode potential defined as zero (E°reference = 0. 00 V). Component: a Pt electrode, H 2(g, 1 atm) bubbling through it. The Pt electrode is in 1 M strong acid. 2 H+(aq; 1 M) + 2 e- H 2(g; 1 atm) E°ref = 0. 00 V Half-cell potentials are measured relative to SHE 21 -29

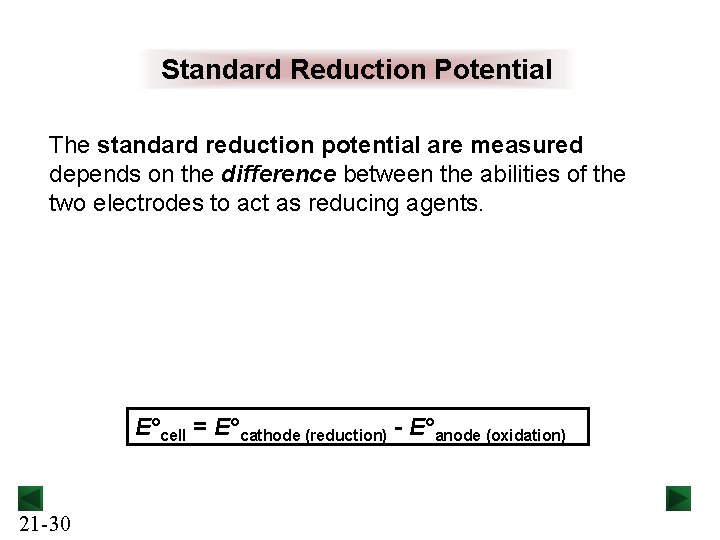
Standard Reduction Potential The standard reduction potential are measured depends on the difference between the abilities of the two electrodes to act as reducing agents. E°cell = E°cathode (reduction) - E°anode (oxidation) 21 -30

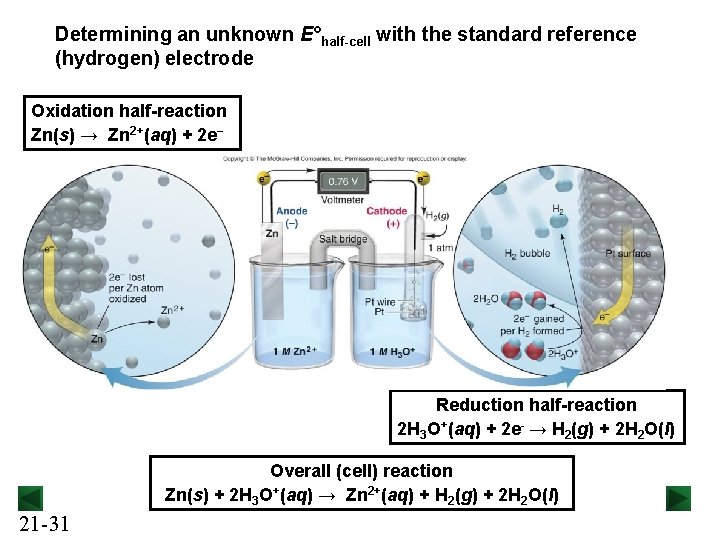
Determining an unknown E°half-cell with the standard reference (hydrogen) electrode Oxidation half-reaction Zn(s) → Zn 2+(aq) + 2 e− Reduction half-reaction 2 H 3 O+(aq) + 2 e- → H 2(g) + 2 H 2 O(l) Overall (cell) reaction Zn(s) + 2 H 3 O+(aq) → Zn 2+(aq) + H 2(g) + 2 H 2 O(l) 21 -31

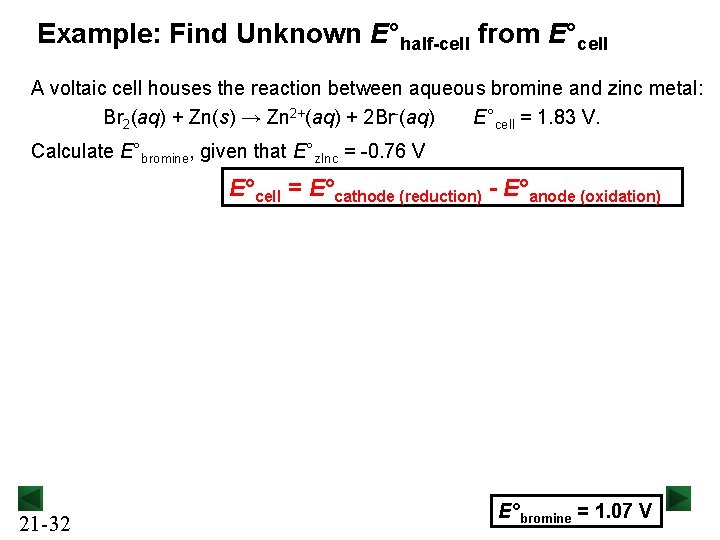
Example: Find Unknown E°half-cell from E°cell A voltaic cell houses the reaction between aqueous bromine and zinc metal: Br 2(aq) + Zn(s) → Zn 2+(aq) + 2 Br-(aq) E°cell = 1. 83 V. Calculate E°bromine, given that E°z. Inc = -0. 76 V E°cell = E°cathode (reduction) - E°anode (oxidation) 21 -32 E°bromine = 1. 07 V

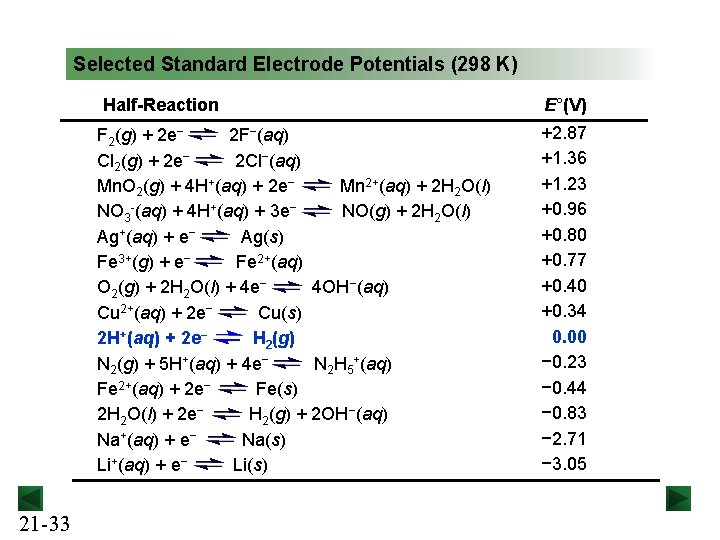
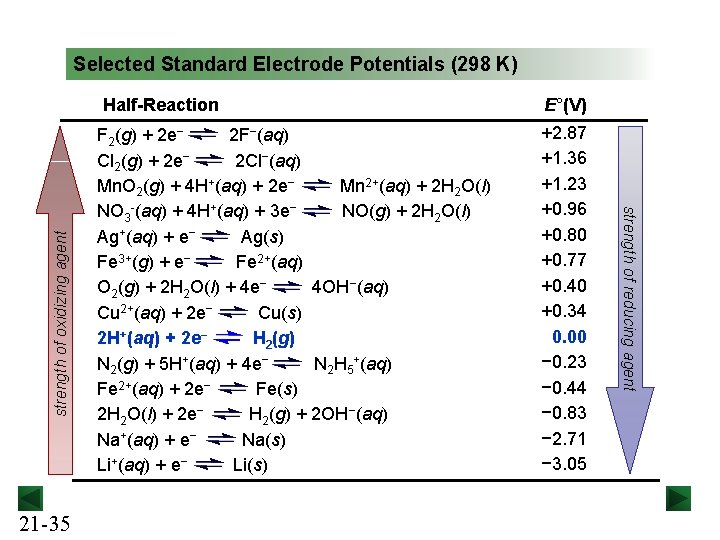
Selected Standard Electrode Potentials (298 K) Half-Reaction F 2(g) + 2 e− 2 F−(aq) Cl 2(g) + 2 e− 2 Cl−(aq) Mn. O 2(g) + 4 H+(aq) + 2 e− Mn 2+(aq) + 2 H 2 O(l) NO 3 -(aq) + 4 H+(aq) + 3 e− NO(g) + 2 H 2 O(l) Ag+(aq) + e− Ag(s) Fe 3+(g) + e− Fe 2+(aq) O 2(g) + 2 H 2 O(l) + 4 e− 4 OH−(aq) Cu 2+(aq) + 2 e− Cu(s) 2 H+(aq) + 2 e− H 2(g) N 2(g) + 5 H+(aq) + 4 e− N 2 H 5+(aq) Fe 2+(aq) + 2 e− Fe(s) 2 H 2 O(l) + 2 e− H 2(g) + 2 OH−(aq) Na+(aq) + e− Na(s) Li+(aq) + e− Li(s) 21 -33 E°(V) +2. 87 +1. 36 +1. 23 +0. 96 +0. 80 +0. 77 +0. 40 +0. 34 0. 00 − 0. 23 − 0. 44 − 0. 83 − 2. 71 − 3. 05

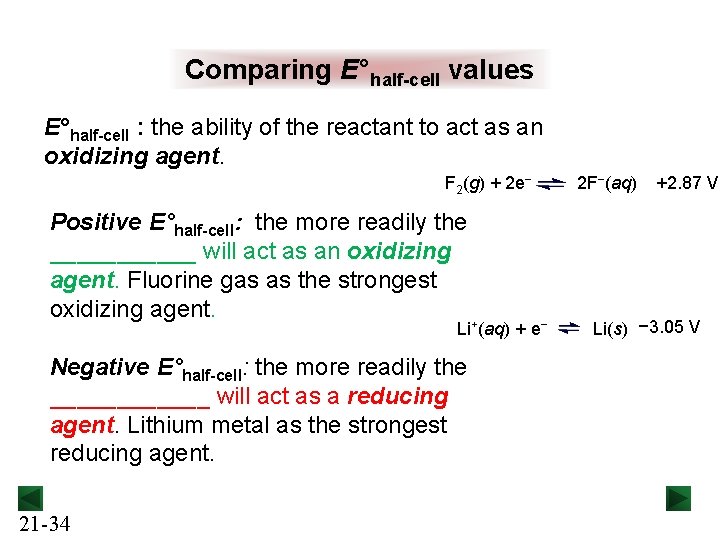
Comparing E°half-cell values E°half-cell : the ability of the reactant to act as an oxidizing agent. F 2(g) + 2 e− Positive E°half-cell: the more readily the ______ will act as an oxidizing agent. Fluorine gas as the strongest oxidizing agent. Li+(aq) + e− Negative E°half-cell: the more readily the ______ will act as a reducing agent. Lithium metal as the strongest reducing agent. 21 -34 2 F−(aq) +2. 87 V Li(s) − 3. 05 V

Selected Standard Electrode Potentials (298 K) 21 -35 F 2(g) + 2 e− 2 F−(aq) Cl 2(g) + 2 e− 2 Cl−(aq) Mn. O 2(g) + 4 H+(aq) + 2 e− Mn 2+(aq) + 2 H 2 O(l) NO 3 -(aq) + 4 H+(aq) + 3 e− NO(g) + 2 H 2 O(l) Ag+(aq) + e− Ag(s) Fe 3+(g) + e− Fe 2+(aq) O 2(g) + 2 H 2 O(l) + 4 e− 4 OH−(aq) Cu 2+(aq) + 2 e− Cu(s) 2 H+(aq) + 2 e− H 2(g) N 2(g) + 5 H+(aq) + 4 e− N 2 H 5+(aq) Fe 2+(aq) + 2 e− Fe(s) 2 H 2 O(l) + 2 e− H 2(g) + 2 OH−(aq) Na+(aq) + e− Na(s) Li+(aq) + e− Li(s) E°(V) +2. 87 +1. 36 +1. 23 +0. 96 +0. 80 +0. 77 +0. 40 +0. 34 0. 00 − 0. 23 − 0. 44 − 0. 83 − 2. 71 − 3. 05 strength of reducing agent strength of oxidizing agent Half-Reaction

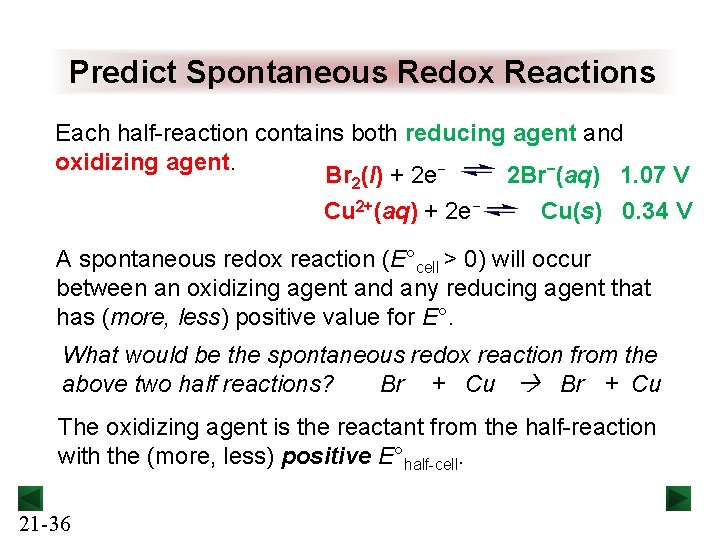
Predict Spontaneous Redox Reactions Each half-reaction contains both reducing agent and oxidizing agent. Br 2(l) + 2 e− 2 Br−(aq) 1. 07 V Cu 2+(aq) + 2 e− Cu(s) 0. 34 V A spontaneous redox reaction (E°cell > 0) will occur between an oxidizing agent and any reducing agent that has (more, less) positive value for E°. What would be the spontaneous redox reaction from the above two half reactions? Br + Cu The oxidizing agent is the reactant from the half-reaction with the (more, less) positive E°half-cell. 21 -36

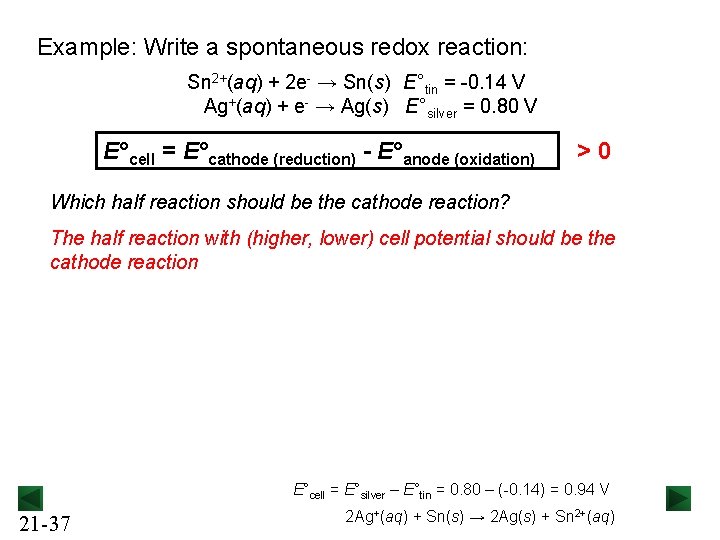
Example: Write a spontaneous redox reaction: Sn 2+(aq) + 2 e- → Sn(s) E°tin = -0. 14 V Ag+(aq) + e- → Ag(s) E°silver = 0. 80 V E°cell = E°cathode (reduction) - E°anode (oxidation) > 0 Which half reaction should be the cathode reaction? The half reaction with (higher, lower) cell potential should be the cathode reaction E°cell = E°silver – E°tin = 0. 80 – (-0. 14) = 0. 94 V 21 -37 2 Ag+(aq) + Sn(s) → 2 Ag(s) + Sn 2+(aq)

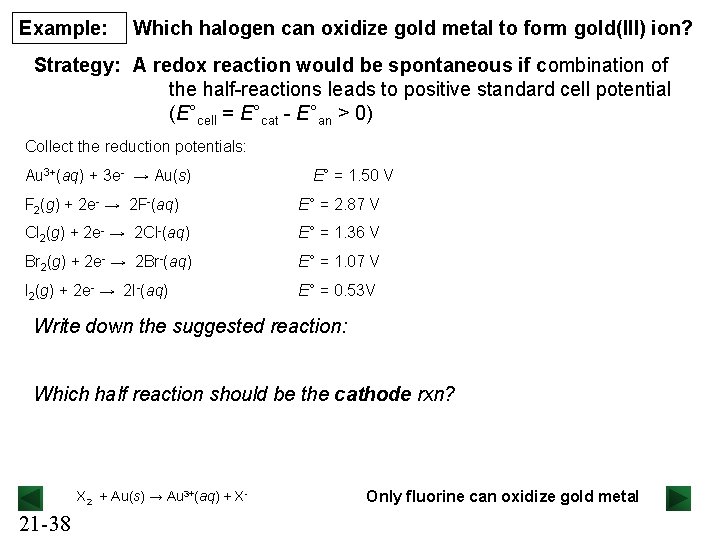
Example: Which halogen can oxidize gold metal to form gold(III) ion? Strategy: A redox reaction would be spontaneous if combination of the half-reactions leads to positive standard cell potential (E°cell = E°cat - E°an > 0) Collect the reduction potentials: Au 3+(aq) + 3 e- → Au(s) E° = 1. 50 V F 2(g) + 2 e- → 2 F-(aq) E° = 2. 87 V Cl 2(g) + 2 e- → 2 Cl-(aq) E° = 1. 36 V Br 2(g) + 2 e- → 2 Br-(aq) E° = 1. 07 V I 2(g) + 2 e- → 2 I-(aq) E° = 0. 53 V Write down the suggested reaction: Which half reaction should be the cathode rxn? X 2 + Au(s) → Au 3+(aq) + X- 21 -38 Only fluorine can oxidize gold metal

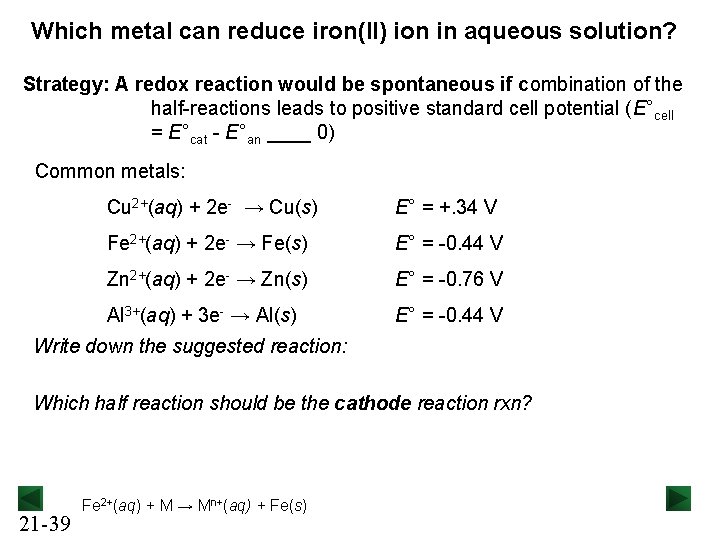
Which metal can reduce iron(II) ion in aqueous solution? Strategy: A redox reaction would be spontaneous if combination of the half-reactions leads to positive standard cell potential (E°cell = E°cat - E°an ____ 0) Common metals: Cu 2+(aq) + 2 e- → Cu(s) E° = +. 34 V Fe 2+(aq) + 2 e- → Fe(s) E° = -0. 44 V Zn 2+(aq) + 2 e- → Zn(s) E° = -0. 76 V Al 3+(aq) + 3 e- → Al(s) E° = -0. 44 V Write down the suggested reaction: Which half reaction should be the cathode reaction rxn? 21 -39 Fe 2+(aq) + M → Mn+(aq) + Fe(s)

Voltaic Cell in Mercury Dental Fillings Biting down with a filled tooth on a scrap of aluminum foil will cause pain. The foil acts as an active anode (E°aluminum = -1. 66 V), saliva as the electrolyte, and the filling as an inactive cathode as O 2 is reduced to H 2 O. 21 -40

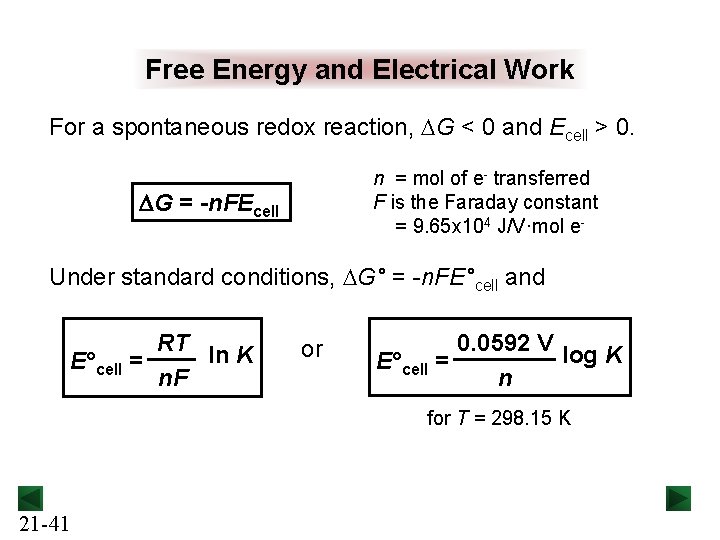
Free Energy and Electrical Work For a spontaneous redox reaction, DG < 0 and Ecell > 0. n = mol of e- transferred F is the Faraday constant = 9. 65 x 104 J/V·mol e- DG = -n. FEcell Under standard conditions, DG° = -n. FE°cell and RT ln K E°cell = n. F or 0. 0592 V log K E°cell = n for T = 298. 15 K 21 -41

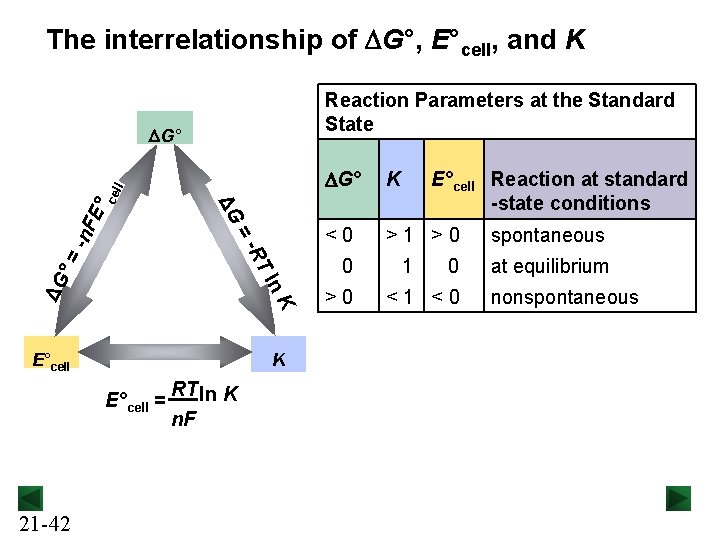
The interrelationship of DG°, E°cell, and K Reaction Parameters at the Standard State n K ΔG T l ° = -n -R = FE ΔG °c ell DG° E°cell K E°cell = 21 -42 RT ln K n. F DG° K <0 >1 >0 0 >0 E°cell Reaction at standard -state conditions 1 0 <1 <0 spontaneous at equilibrium nonspontaneous

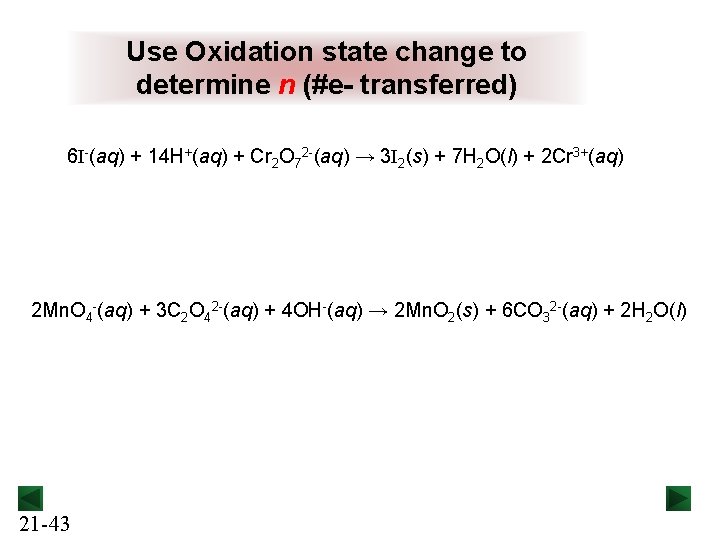
Use Oxidation state change to determine n (#e- transferred) 6 I-(aq) + 14 H+(aq) + Cr 2 O 72 -(aq) → 3 I 2(s) + 7 H 2 O(l) + 2 Cr 3+(aq) 2 Mn. O 4 -(aq) + 3 C 2 O 42 -(aq) + 4 OH-(aq) → 2 Mn. O 2(s) + 6 CO 32 -(aq) + 2 H 2 O(l) 21 -43

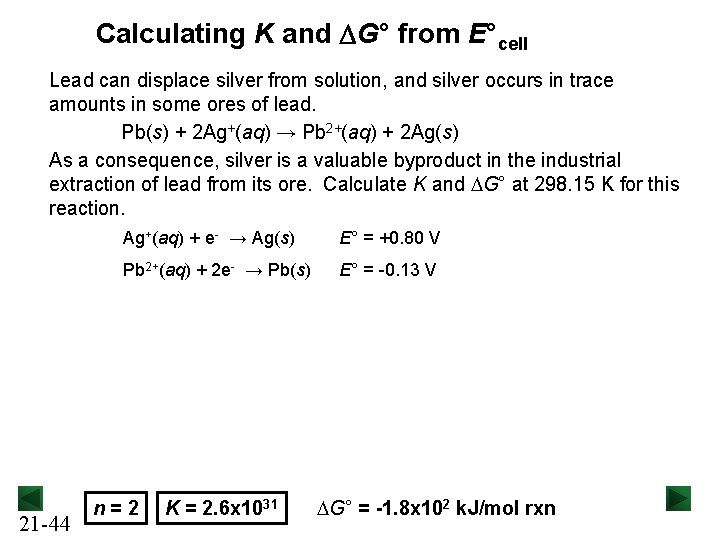
Calculating K and DG° from E°cell Lead can displace silver from solution, and silver occurs in trace amounts in some ores of lead. Pb(s) + 2 Ag+(aq) → Pb 2+(aq) + 2 Ag(s) As a consequence, silver is a valuable byproduct in the industrial extraction of lead from its ore. Calculate K and DG° at 298. 15 K for this reaction. 21 -44 Ag+(aq) + e- → Ag(s) E° = +0. 80 V Pb 2+(aq) + 2 e- → Pb(s) E° = -0. 13 V n = 2 K = 2. 6 x 1031 DG° = -1. 8 x 102 k. J/mol rxn

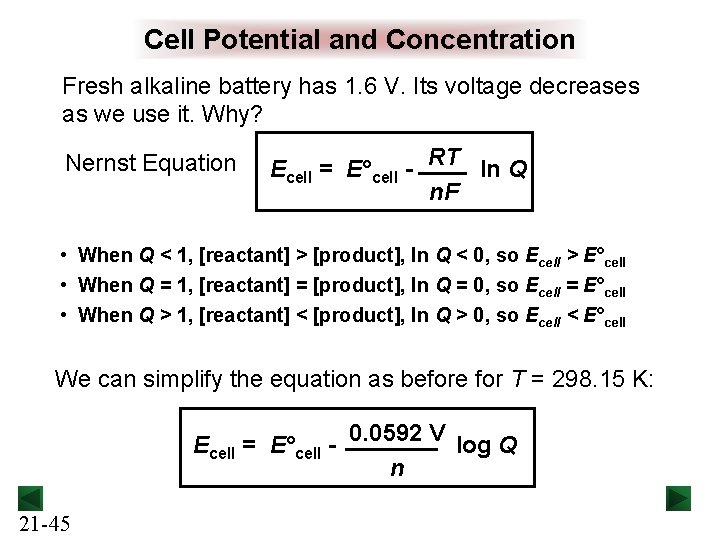
Cell Potential and Concentration Fresh alkaline battery has 1. 6 V. Its voltage decreases as we use it. Why? Nernst Equation Ecell = E°cell - RT ln Q n. F • When Q < 1, [reactant] > [product], ln Q < 0, so Ecell > E°cell • When Q = 1, [reactant] = [product], ln Q = 0, so Ecell = E°cell • When Q > 1, [reactant] < [product], ln Q > 0, so Ecell < E°cell We can simplify the equation as before for T = 298. 15 K: Ecell = E°cell - 0. 0592 V log Q n 21 -45

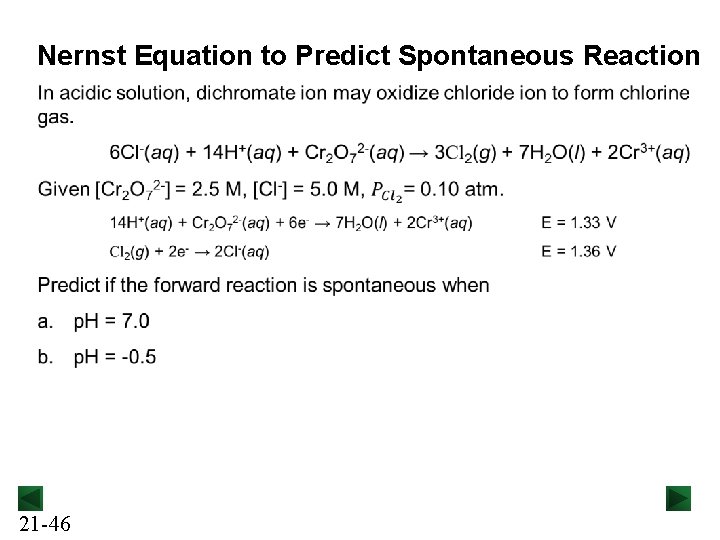
Nernst Equation to Predict Spontaneous Reaction 21 -46

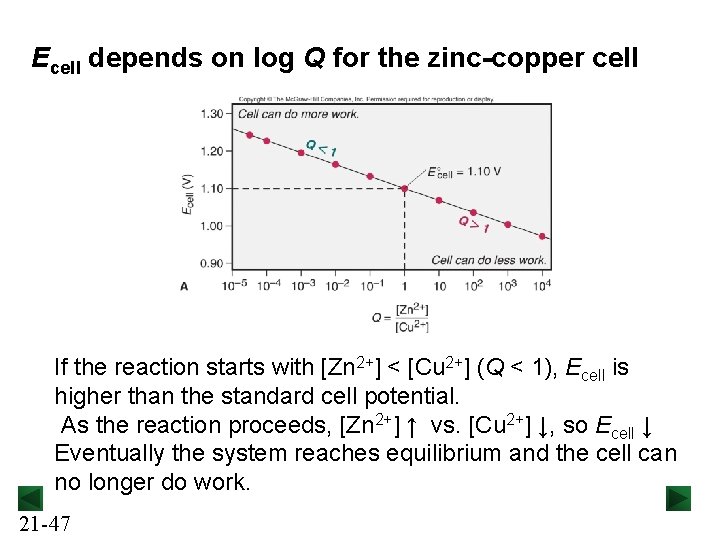
Ecell depends on log Q for the zinc-copper cell If the reaction starts with [Zn 2+] < [Cu 2+] (Q < 1), Ecell is higher than the standard cell potential. As the reaction proceeds, [Zn 2+] ↑ vs. [Cu 2+] ↓, so Ecell ↓ Eventually the system reaches equilibrium and the cell can no longer do work. 21 -47

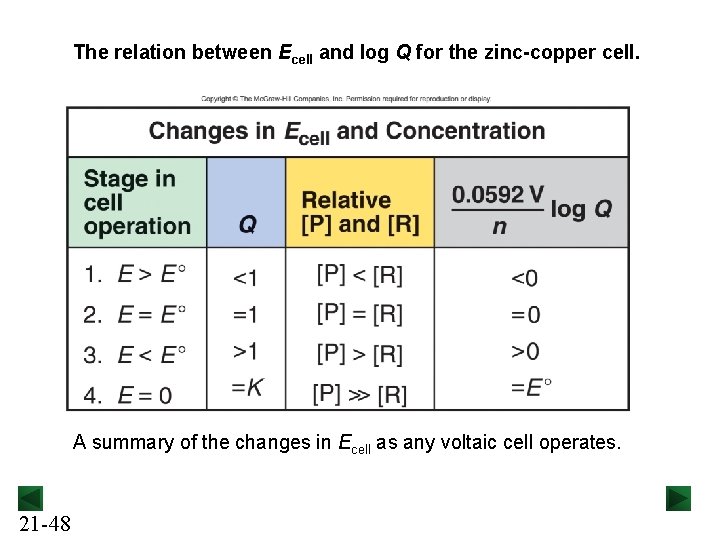
The relation between Ecell and log Q for the zinc-copper cell. A summary of the changes in Ecell as any voltaic cell operates. 21 -48

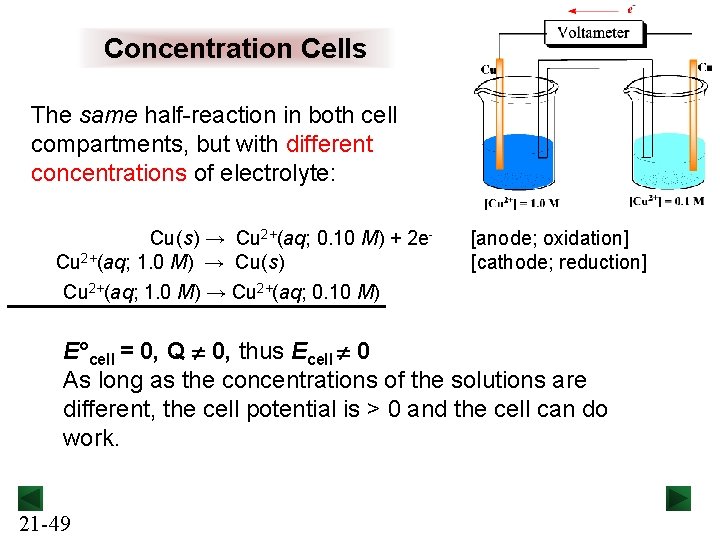
Concentration Cells The same half-reaction in both cell compartments, but with different concentrations of electrolyte: Cu(s) → Cu 2+(aq; 0. 10 M) + 2 e. Cu 2+(aq; 1. 0 M) → Cu(s) Cu 2+(aq; 1. 0 M) → Cu 2+(aq; 0. 10 M) [anode; oxidation] [cathode; reduction] E°cell = 0, Q 0, thus Ecell 0 As long as the concentrations of the solutions are different, the cell potential is > 0 and the cell can do work. 21 -49

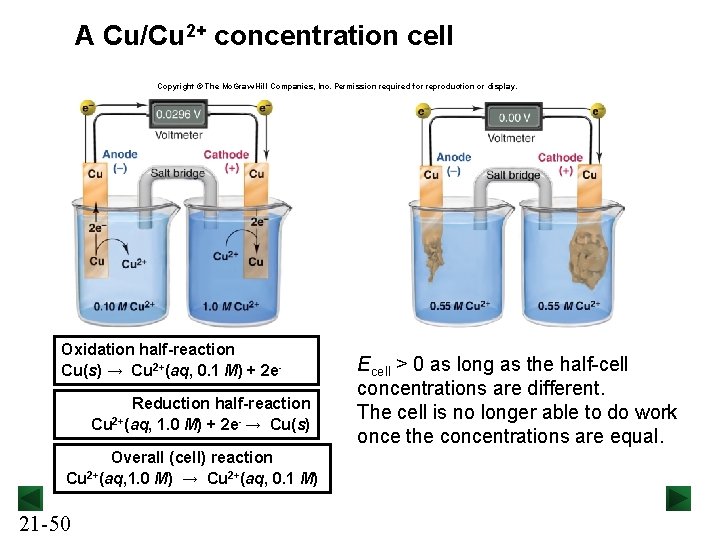
A Cu/Cu 2+ concentration cell Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Oxidation half-reaction Cu(s) → Cu 2+(aq, 0. 1 M) + 2 e. Reduction half-reaction Cu 2+(aq, 1. 0 M) + 2 e- → Cu(s) Overall (cell) reaction Cu 2+(aq, 1. 0 M) → Cu 2+(aq, 0. 1 M) 21 -50 Ecell > 0 as long as the half-cell concentrations are different. The cell is no longer able to do work once the concentrations are equal.
![Application of Concentration Cell p H meter The glass electrode monitors the H of Application of Concentration Cell: p. H meter The glass electrode monitors the [H+] of](https://slidetodoc.com/presentation_image_h/f998de9add2cf19829efb771db85852e/image-51.jpg)
Application of Concentration Cell: p. H meter The glass electrode monitors the [H+] of the solution relative to its own fixed internal [H+]. An older style of p. H meter includes two electrodes. 21 -51 Modern p. H meters use a combination electrode.

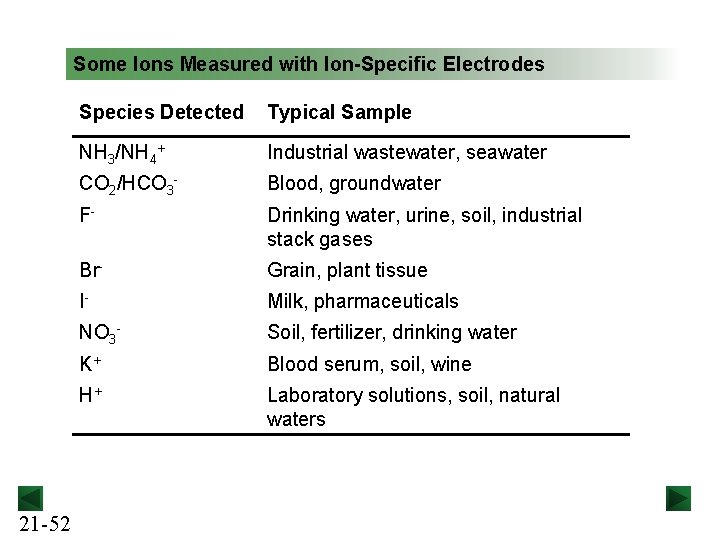
Some Ions Measured with Ion-Specific Electrodes 21 -52 Species Detected Typical Sample NH 3/NH 4+ Industrial wastewater, seawater CO 2/HCO 3 - Blood, groundwater F- Drinking water, urine, soil, industrial stack gases Br- Grain, plant tissue I- Milk, pharmaceuticals NO 3 - Soil, fertilizer, drinking water K+ Blood serum, soil, wine H+ Laboratory solutions, soil, natural waters

Electrochemical Processes in Batteries A battery consists of self-contained voltaic cells arranged in series, so their individual voltages are added. A primary battery cannot be recharged. The battery is “dead” when the cell reaction has reached equilibrium. A secondary battery is rechargeable. Once it has run down, electrical energy is supplied to reverse the cell reaction and form more reactant. 21 -53

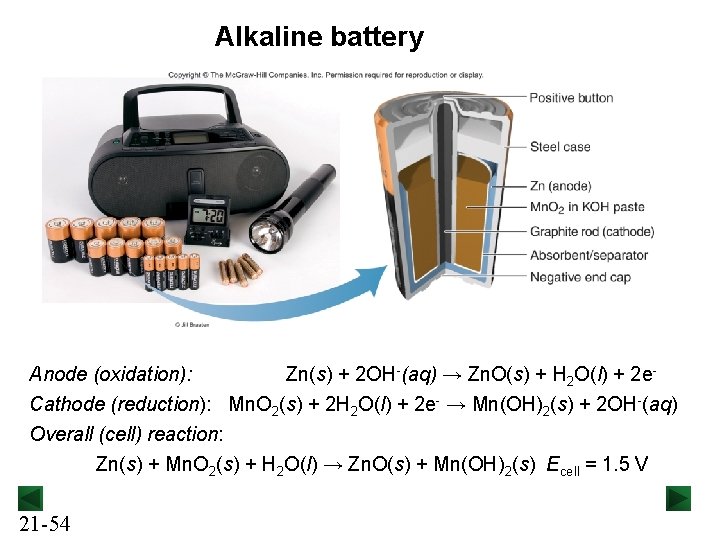
Alkaline battery Anode (oxidation): Zn(s) + 2 OH-(aq) → Zn. O(s) + H 2 O(l) + 2 e. Cathode (reduction): Mn. O 2(s) + 2 H 2 O(l) + 2 e- → Mn(OH)2(s) + 2 OH-(aq) Overall (cell) reaction: Zn(s) + Mn. O 2(s) + H 2 O(l) → Zn. O(s) + Mn(OH)2(s) Ecell = 1. 5 V 21 -54

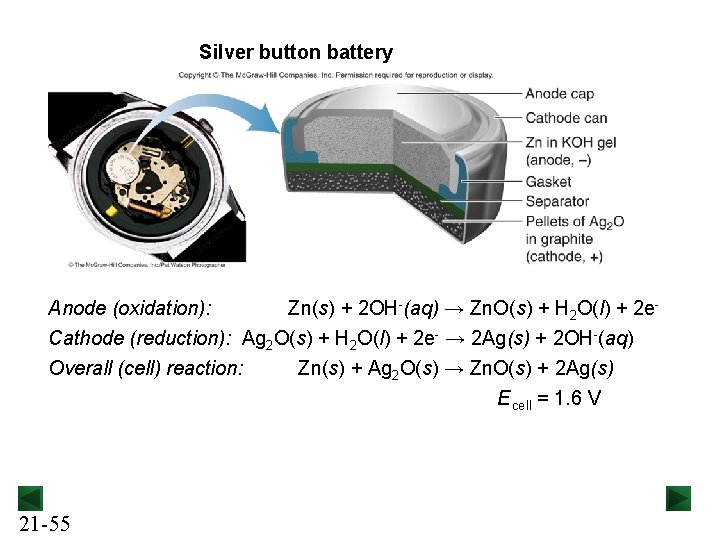
Silver button battery Anode (oxidation): Zn(s) + 2 OH-(aq) → Zn. O(s) + H 2 O(l) + 2 e. Cathode (reduction): Ag 2 O(s) + H 2 O(l) + 2 e- → 2 Ag(s) + 2 OH-(aq) Overall (cell) reaction: 21 -55 Zn(s) + Ag 2 O(s) → Zn. O(s) + 2 Ag(s) E cell = 1. 6 V

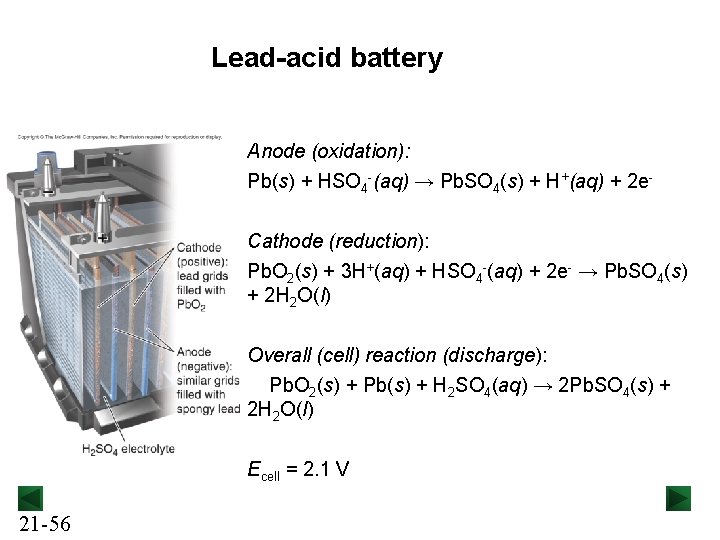
Lead-acid battery Anode (oxidation): Pb(s) + HSO 4 -(aq) → Pb. SO 4(s) + H+(aq) + 2 e. Cathode (reduction): Pb. O 2(s) + 3 H+(aq) + HSO 4 -(aq) + 2 e- → Pb. SO 4(s) + 2 H 2 O(l) Overall (cell) reaction (discharge): Pb. O 2(s) + Pb(s) + H 2 SO 4(aq) → 2 Pb. SO 4(s) + 2 H 2 O(l) Ecell = 2. 1 V 21 -56

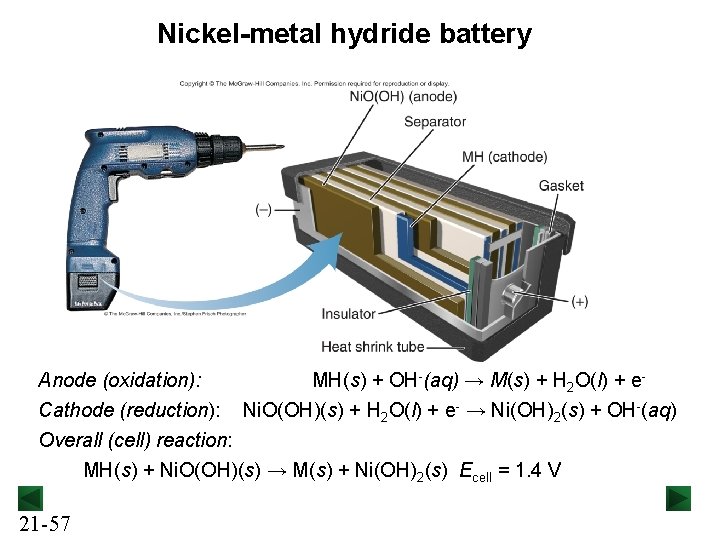
Nickel-metal hydride battery Anode (oxidation): Cathode (reduction): MH(s) + OH-(aq) → M(s) + H 2 O(l) + e. Ni. O(OH)(s) + H 2 O(l) + e- → Ni(OH)2(s) + OH-(aq) Overall (cell) reaction: MH(s) + Ni. O(OH)(s) → M(s) + Ni(OH)2(s) Ecell = 1. 4 V 21 -57

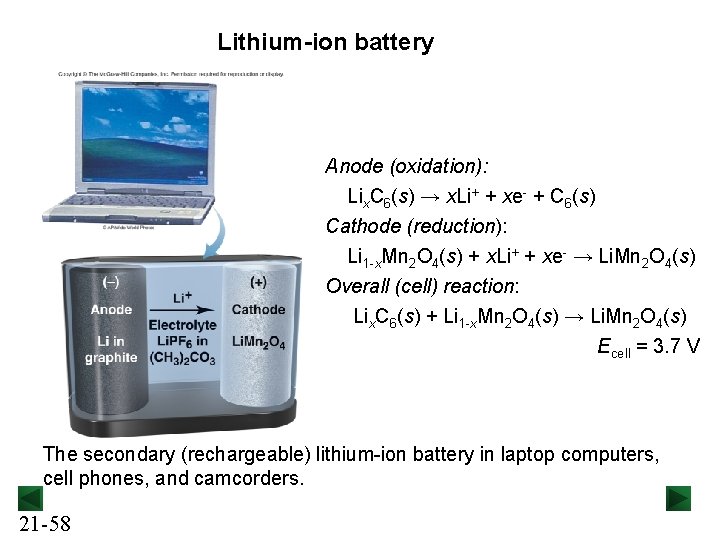
Lithium-ion battery Anode (oxidation): Lix. C 6(s) → x. Li+ + xe- + C 6(s) Cathode (reduction): Li 1 -x. Mn 2 O 4(s) + x. Li+ + xe- → Li. Mn 2 O 4(s) Overall (cell) reaction: Lix. C 6(s) + Li 1 -x. Mn 2 O 4(s) → Li. Mn 2 O 4(s) Ecell = 3. 7 V The secondary (rechargeable) lithium-ion battery in laptop computers, cell phones, and camcorders. 21 -58

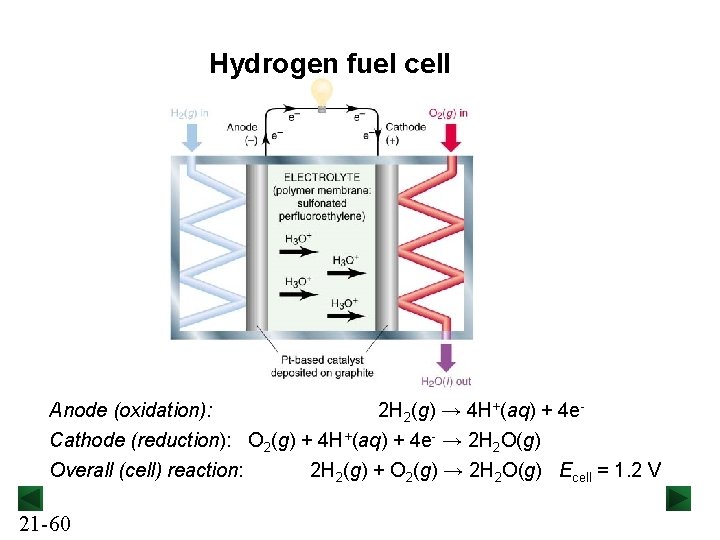
Fuel Cells In a fuel cell, also called a flow cell, reactants enter the cell and products leave, generating electricity through controlled combustion. Reaction rates are lower in fuel cells than in other batteries, so an electrocatalyst is used to decrease the activation energy. 21 -59

Hydrogen fuel cell Anode (oxidation): 2 H 2(g) → 4 H+(aq) + 4 e. Cathode (reduction): O 2(g) + 4 H+(aq) + 4 e- → 2 H 2 O(g) Overall (cell) reaction: 21 -60 2 H 2(g) + O 2(g) → 2 H 2 O(g) Ecell = 1. 2 V

Corrosion: an Environmental Voltaic Cell Corrosion : metals are oxidized to their oxides and sulfides. The rusting of iron is a common form of corrosion. - Rust is not a direct product of the reaction between Fe and O 2, but arises through a complex electrochemical process. - Rusting requires moisture, and occurs more quickly at low p. H, in ionic solutions, and when the iron is in contact with a less active metal. 21 -61

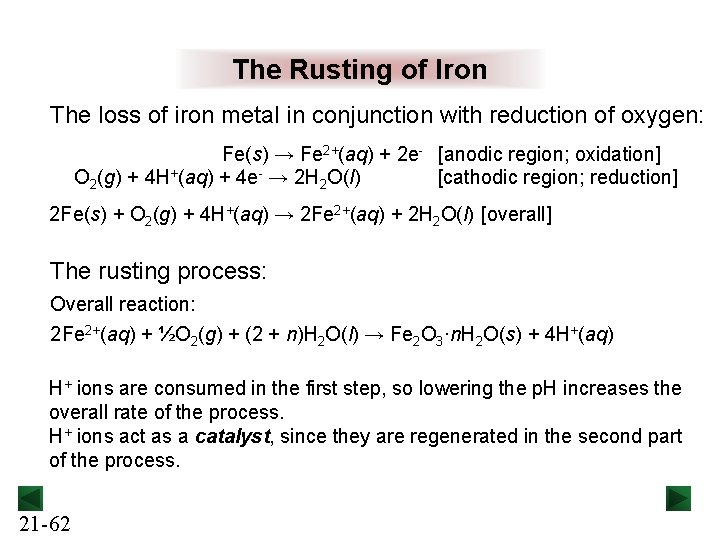
The Rusting of Iron The loss of iron metal in conjunction with reduction of oxygen: Fe(s) → Fe 2+(aq) + 2 e- [anodic region; oxidation] O 2(g) + 4 H+(aq) + 4 e- → 2 H 2 O(l) [cathodic region; reduction] 2 Fe(s) + O 2(g) + 4 H+(aq) → 2 Fe 2+(aq) + 2 H 2 O(l) [overall] The rusting process: Overall reaction: 2 Fe 2+(aq) + ½O 2(g) + (2 + n)H 2 O(l) → Fe 2 O 3·n. H 2 O(s) + 4 H+(aq) H+ ions are consumed in the first step, so lowering the p. H increases the overall rate of the process. H+ ions act as a catalyst, since they are regenerated in the second part of the process. 21 -62

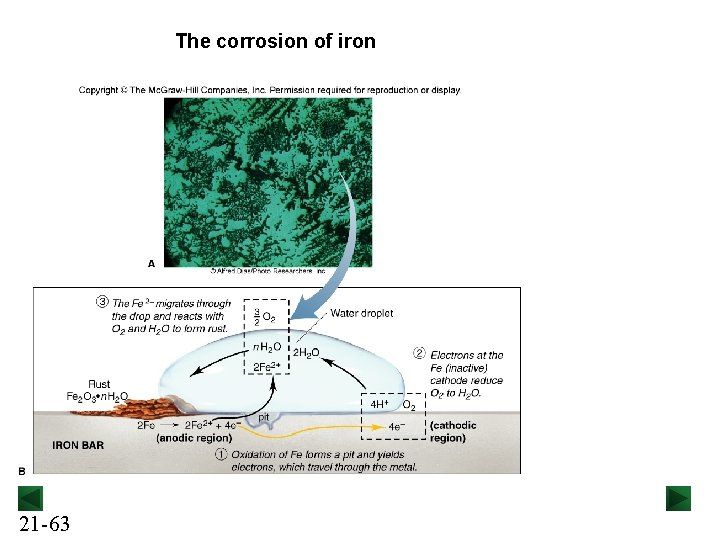
The corrosion of iron 21 -63

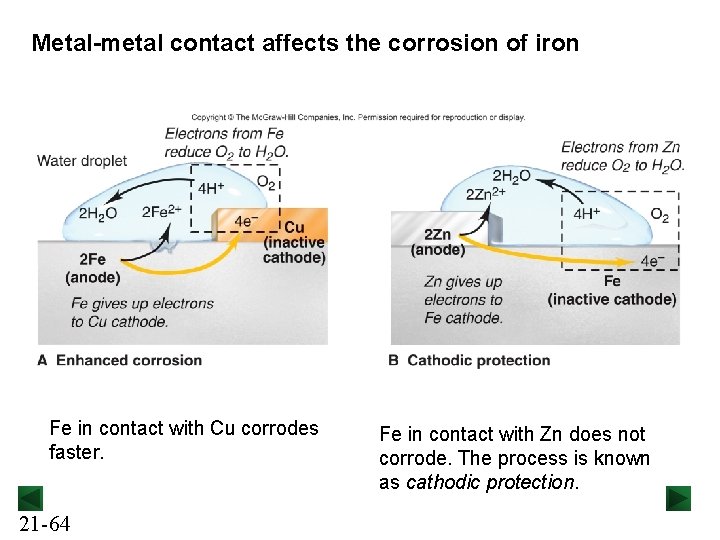
Metal-metal contact affects the corrosion of iron Fe in contact with Cu corrodes faster. 21 -64 Fe in contact with Zn does not corrode. The process is known as cathodic protection.

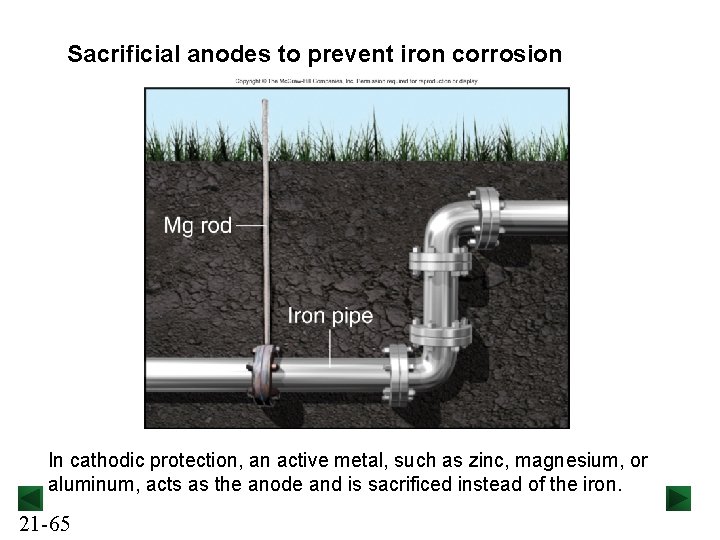
Sacrificial anodes to prevent iron corrosion In cathodic protection, an active metal, such as zinc, magnesium, or aluminum, acts as the anode and is sacrificed instead of the iron. 21 -65

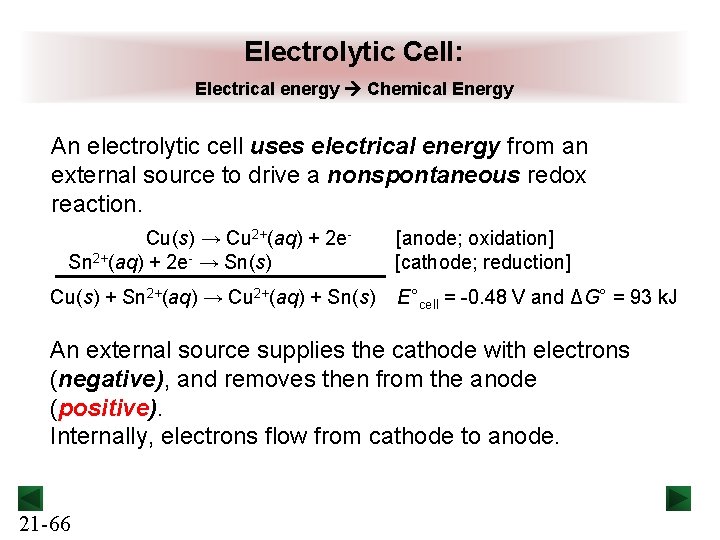
Electrolytic Cell: Electrical energy Chemical Energy An electrolytic cell uses electrical energy from an external source to drive a nonspontaneous redox reaction. Cu(s) → Cu 2+(aq) + 2 e. Sn 2+(aq) + 2 e- → Sn(s) Cu(s) + Sn 2+(aq) → Cu 2+(aq) + Sn(s) [anode; oxidation] [cathode; reduction] E°cell = -0. 48 V and ΔG° = 93 k. J An external source supplies the cathode with electrons (negative), and removes then from the anode (positive). Internally, electrons flow from cathode to anode. 21 -66

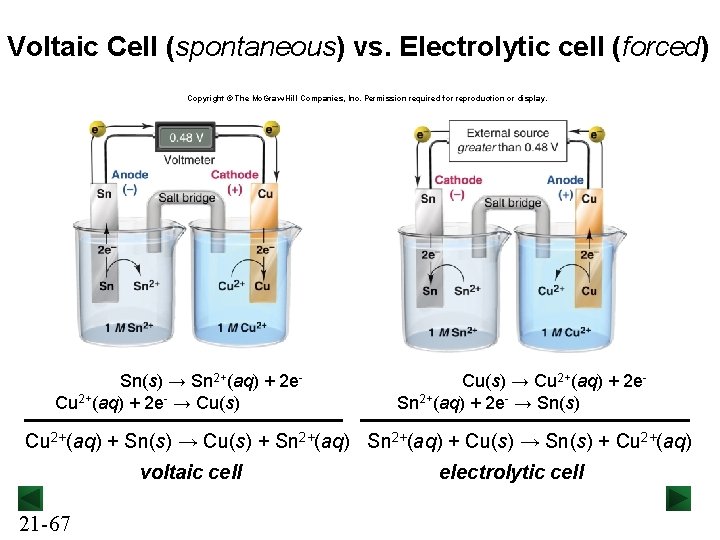
Voltaic Cell (spontaneous) vs. Electrolytic cell (forced) Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Sn(s) → Sn 2+(aq) + 2 e. Cu 2+(aq) + 2 e- → Cu(s) → Cu 2+(aq) + 2 e. Sn 2+(aq) + 2 e- → Sn(s) Cu 2+(aq) + Sn(s) → Cu(s) + Sn 2+(aq) + Cu(s) → Sn(s) + Cu 2+(aq) voltaic cell 21 -67 electrolytic cell

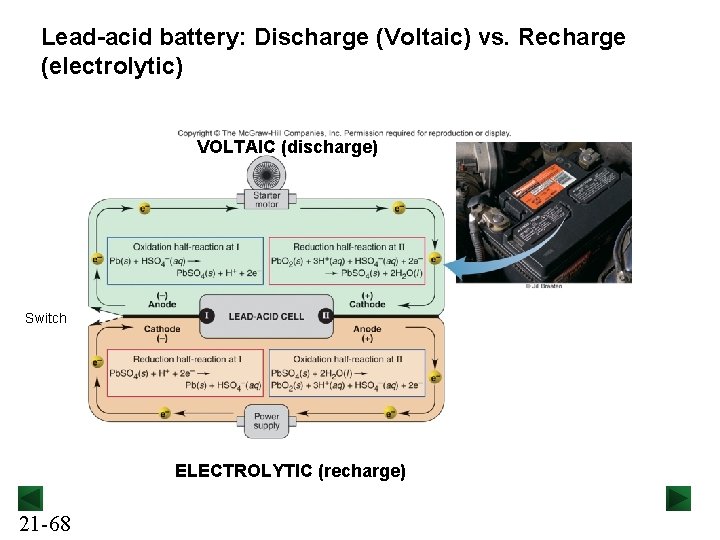
Lead-acid battery: Discharge (Voltaic) vs. Recharge (electrolytic) VOLTAIC (discharge) Switch ELECTROLYTIC (recharge) 21 -68

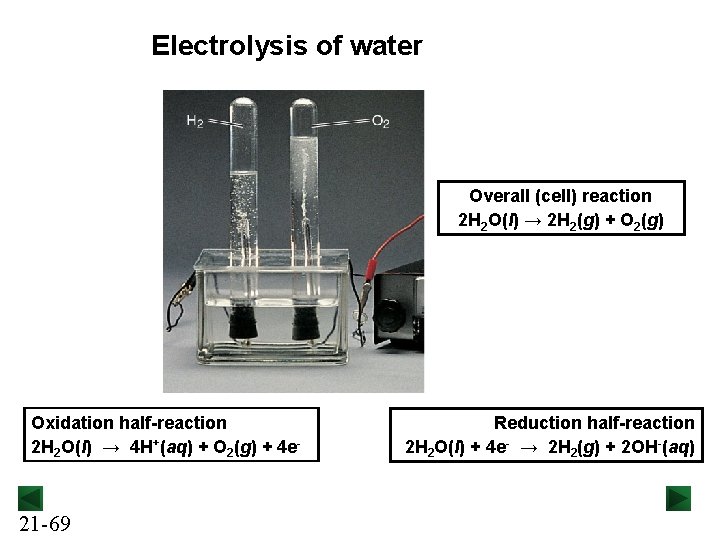
Electrolysis of water Overall (cell) reaction 2 H 2 O(l) → 2 H 2(g) + O 2(g) Oxidation half-reaction 2 H 2 O(l) → 4 H+(aq) + O 2(g) + 4 e- 21 -69 Reduction half-reaction 2 H 2 O(l) + 4 e- → 2 H 2(g) + 2 OH-(aq)

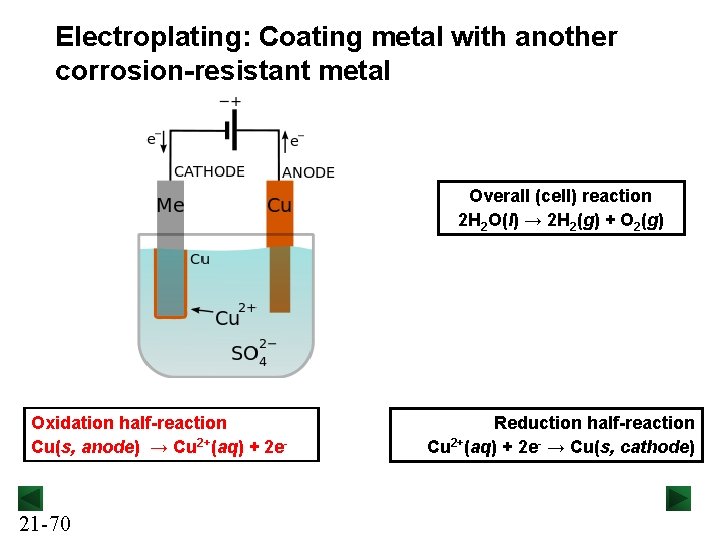
Electroplating: Coating metal with another corrosion-resistant metal Overall (cell) reaction 2 H 2 O(l) → 2 H 2(g) + O 2(g) Oxidation half-reaction Cu(s, anode) → Cu 2+(aq) + 2 e- 21 -70 Reduction half-reaction Cu 2+(aq) + 2 e- → Cu(s, cathode)

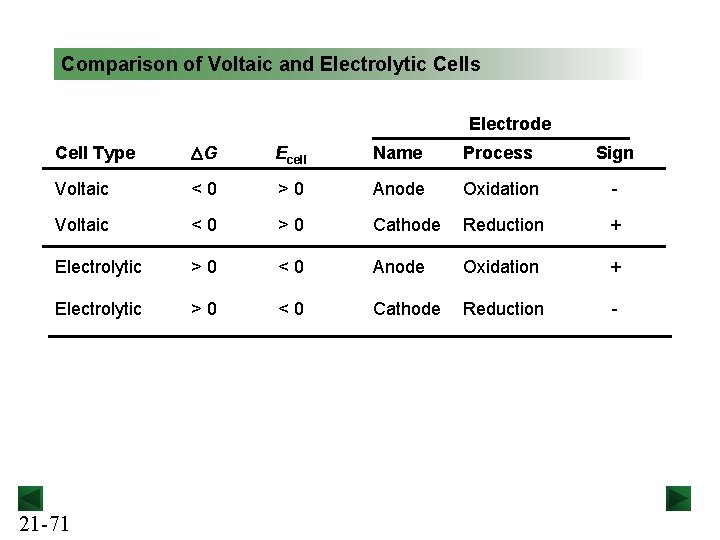
Comparison of Voltaic and Electrolytic Cells Electrode Cell Type DG Ecell Name Process Sign Voltaic <0 >0 Anode Oxidation - Voltaic <0 >0 Cathode Reduction + Electrolytic >0 <0 Anode Oxidation + Electrolytic >0 <0 Cathode Reduction - 21 -71

Products of Electrolysis is the splitting (lysing) of a substance by the input of electrical energy. During electrolysis of a pure, molten salt, the cation will be reduced and the anion will be oxidized. During electrolysis of a mixture of molten salts - the more easily oxidized species (stronger reducing agent) reacts at the anode, and - the more easily reduced species (stronger oxidizing agent) reacts at the cathode. 21 -72

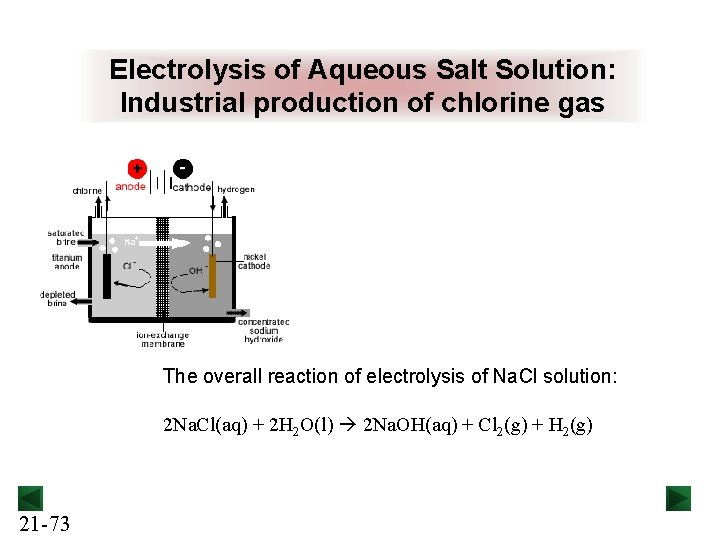
Electrolysis of Aqueous Salt Solution: Industrial production of chlorine gas The overall reaction of electrolysis of Na. Cl solution: 2 Na. Cl(aq) + 2 H 2 O(l) 2 Na. OH(aq) + Cl 2(g) + H 2(g) 21 -73

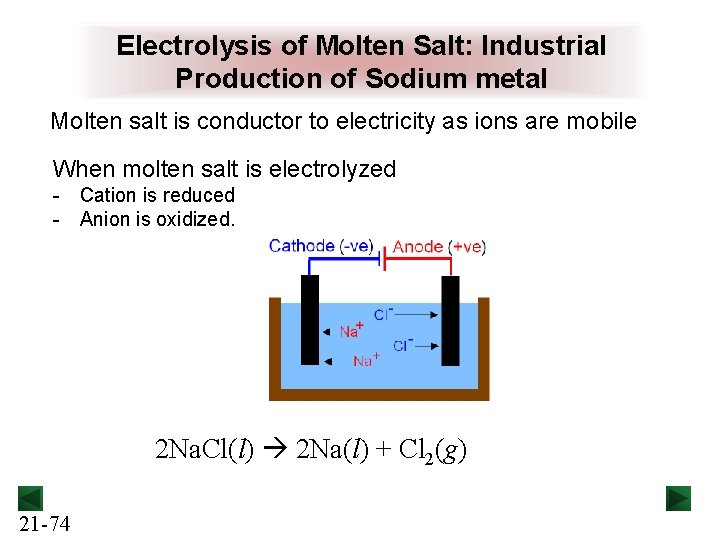
Electrolysis of Molten Salt: Industrial Production of Sodium metal Molten salt is conductor to electricity as ions are mobile When molten salt is electrolyzed - Cation is reduced - Anion is oxidized. 2 Na. Cl(l) 2 Na(l) + Cl 2(g) 21 -74

Stoichiometry in Electrolytic Cell Quantity of electrons in Coulombs (C): 1 mole = 96, 500 C Electric current (I) in Ampere (A): 1 A = 1 C/sec In an electrolytic cell, copper(II) ion is reduced to form copper metal. Cu 2+(aq) + 2 e- → Cu(s) If the current is 3. 00 A, how many grams of copper metal will form after 10. 0 minutes of electrolysis? 21 -75 1. 80 E+3 C, 9. 32 E-3 mol Cu, 0. 592 g

Writing Spontaneous Redox Reactions Each half-reaction contains both a reducing agent and an oxidizing agent. Br 2(l) + 2 e− 2 Br−(aq) The stronger oxidizing and reducing agents react spontaneously to form the weaker ones. A spontaneous redox reaction (E°cell > 0) will occur between an oxidizing agent and any reducing agent that lies below it in the emf series (i. e. , one that has a less positive value for E°). The oxidizing agent is the reactant from the half-reaction with the more positive E°half-cell. 21 -76

Summary of the Electrolysis of Aqueous Salt Solutions • Cations of less active metals (Au, Ag, Cu, Cr, Pt, Cd) are reduced to the metal. • Cations of more active metals are not reduced. H 2 O is reduced instead. • Anions that are oxidized, because of overvoltage from O 2 formation, include the halides, except for F-. • Anions that are not oxidized include F- and common oxoanions. H 2 O is oxidized instead. 21 -77
 Spontaneity of redox reactions
Spontaneity of redox reactions Example of redox reaction
Example of redox reaction Is the redox spontaneity rule empirical
Is the redox spontaneity rule empirical Balance redox
Balance redox Half reduction reaction
Half reduction reaction How to balance an equation step by step
How to balance an equation step by step Downs cell
Downs cell Concept map of oxidative phosphorylation
Concept map of oxidative phosphorylation Balancing redox reactions in acid
Balancing redox reactions in acid Reduction half equation
Reduction half equation Redox reactions ncea level 2
Redox reactions ncea level 2 Chapter 19 redox reactions answers
Chapter 19 redox reactions answers Nernst equation khan academy
Nernst equation khan academy Balancing redox reactions
Balancing redox reactions Balancing redox reactions in acidic solution
Balancing redox reactions in acidic solution Half equation worksheet
Half equation worksheet Redox reaction leo ger
Redox reaction leo ger 2 exp 16
2 exp 16 Balancing redox reactions calculator
Balancing redox reactions calculator Rules for balancing redox reactions
Rules for balancing redox reactions Anode half reaction
Anode half reaction How redox reactions work
How redox reactions work Balance redox reactions
Balance redox reactions Different types of redox reactions
Different types of redox reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Gibbs free energy of formation
Gibbs free energy of formation Predicting spontaneity
Predicting spontaneity Spontaneous reaction
Spontaneous reaction Ap chemistry spontaneity entropy and free energy
Ap chemistry spontaneity entropy and free energy Complete the following table on reaction spontaneity
Complete the following table on reaction spontaneity Predicting spontaneity
Predicting spontaneity Gibbs free energy practice problems
Gibbs free energy practice problems Predicting spontaneity
Predicting spontaneity Electrochemistry
Electrochemistry What is electrochemistry in chemistry
What is electrochemistry in chemistry Electrochemistry ap chemistry
Electrochemistry ap chemistry Chapter 20 review electrochemistry
Chapter 20 review electrochemistry Transport no. in electrochemistry
Transport no. in electrochemistry Analytical electrochemistry
Analytical electrochemistry Mass transport electrochemistry
Mass transport electrochemistry Electrochemistry lesson
Electrochemistry lesson Electrochemistry eds
Electrochemistry eds What is electrochemical
What is electrochemical Applications of electrolytic cell
Applications of electrolytic cell Diagonal rule electrochemistry
Diagonal rule electrochemistry Line notation galvanic cell
Line notation galvanic cell Chapter 21 electrochemistry
Chapter 21 electrochemistry Electrochemistry tutorial
Electrochemistry tutorial Red cat electrochemistry
Red cat electrochemistry Introduction of electrochemistry
Introduction of electrochemistry Slidesdgo
Slidesdgo Fundamentals of electrochemistry
Fundamentals of electrochemistry Electrochemistry balancing equations
Electrochemistry balancing equations Ir drop
Ir drop Chapter 20 electrochemistry
Chapter 20 electrochemistry Coupon technology
Coupon technology Diagonal rule electrochemistry
Diagonal rule electrochemistry E cell formula
E cell formula Ap chemistry chapter 18 electrochemistry test
Ap chemistry chapter 18 electrochemistry test Concentration cell definition
Concentration cell definition Fundamentals of electrochemistry
Fundamentals of electrochemistry What is electrochemistry
What is electrochemistry Electrochemistry stoichiometry
Electrochemistry stoichiometry Chapter 20 review electrochemistry
Chapter 20 review electrochemistry Faradays contant
Faradays contant Electrochemistry balancing equations
Electrochemistry balancing equations Polarization in electrochemistry
Polarization in electrochemistry Oxidation reduction memory tricks
Oxidation reduction memory tricks Interfacial method in electrochemistry
Interfacial method in electrochemistry Nernst equation simplified
Nernst equation simplified Electrochemistry
Electrochemistry Cellular respiration redox
Cellular respiration redox Example of redox reaction
Example of redox reaction Chapter 20 worksheet: redox answers
Chapter 20 worksheet: redox answers Cation joke
Cation joke