Chapter 18 Electrochemistry Khan Academy Galvanic Cell Review



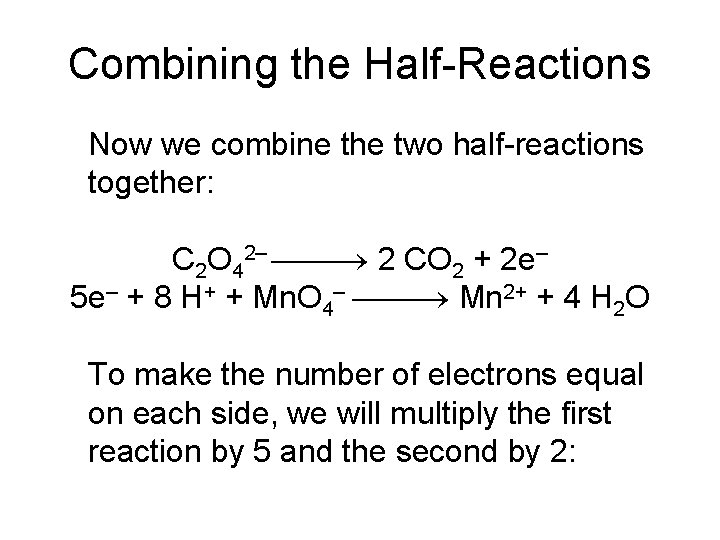
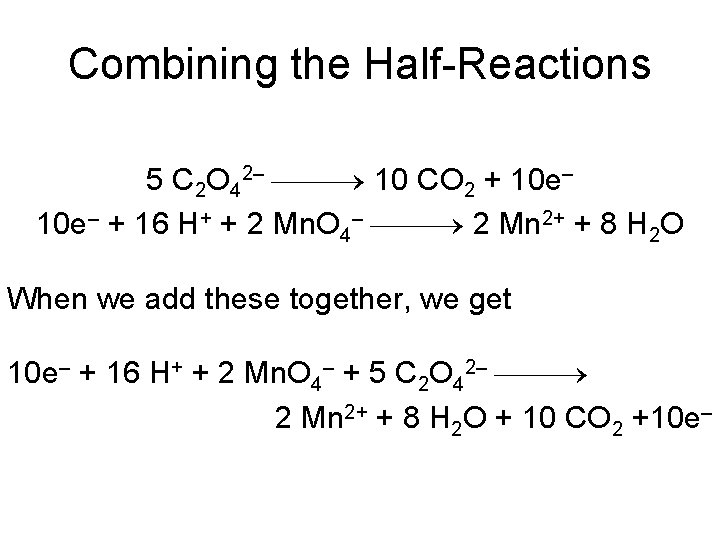
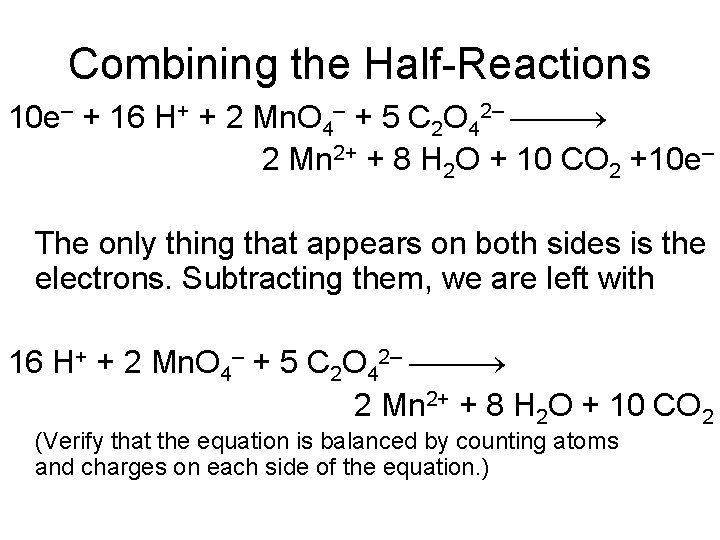















- Slides: 95

Chapter 18 Electrochemistry

• Khan Academy Galvanic Cell

Review of Terms • Electrochemistry – the study of the interchange of chemical and electrical energy • Oxidation–reduction (redox) reaction – involves a transfer of electrons from the reducing agent to the oxidizing agent • Oxidation – loss of electrons • Reduction – gain of electrons • Reducing agent – electron donor • Oxidizing agent – electron acceptor Copyright © Cengage Learning. All rights reserved 3

Electrochemistry is the study of the relationships between electricity and chemical reactions. It includes the study of both spontaneous and nonspontaneous processes.

Synopsis of Assigning Oxidation Numbers (as a Reminder) 1. 2. 3. 4. 5. 6. Elements = 0 Monatomic ion = charge F: – 1 O: – 2 (unless peroxide = – 1) H: +1 (unless a metal hydride = – 1) The sum of the oxidation numbers equals the overall charge (0 in a compound).

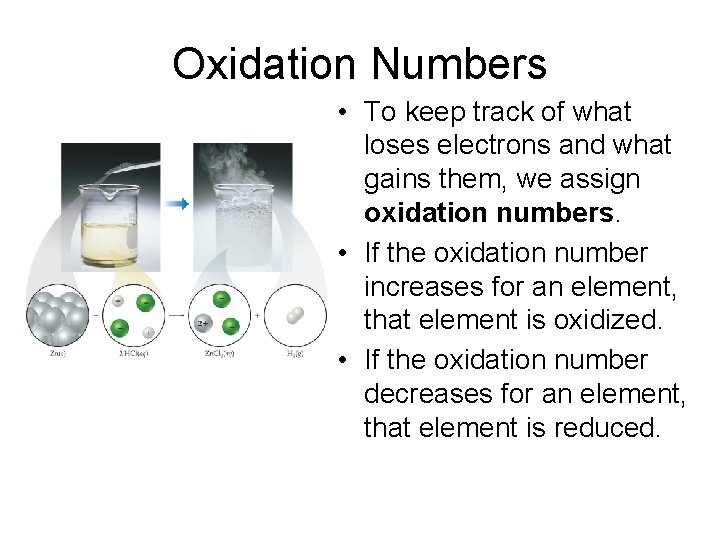
Oxidation Numbers • To keep track of what loses electrons and what gains them, we assign oxidation numbers. • If the oxidation number increases for an element, that element is oxidized. • If the oxidation number decreases for an element, that element is reduced.

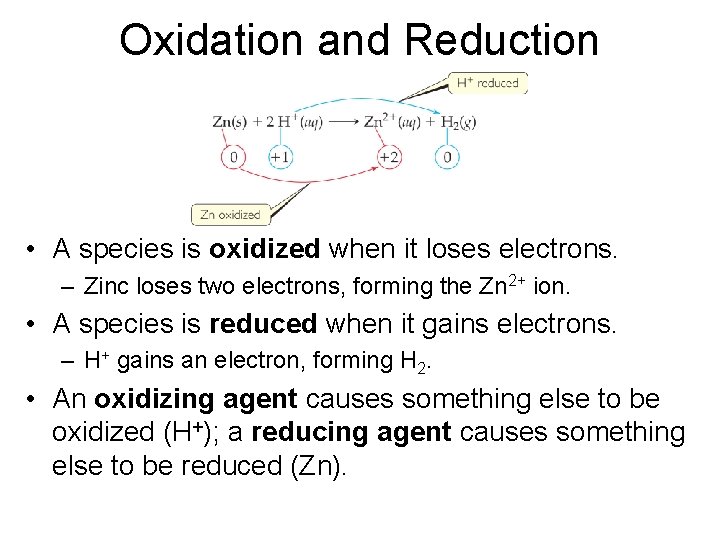
Oxidation and Reduction • A species is oxidized when it loses electrons. – Zinc loses two electrons, forming the Zn 2+ ion. • A species is reduced when it gains electrons. – H+ gains an electron, forming H 2. • An oxidizing agent causes something else to be oxidized (H+); a reducing agent causes something else to be reduced (Zn).

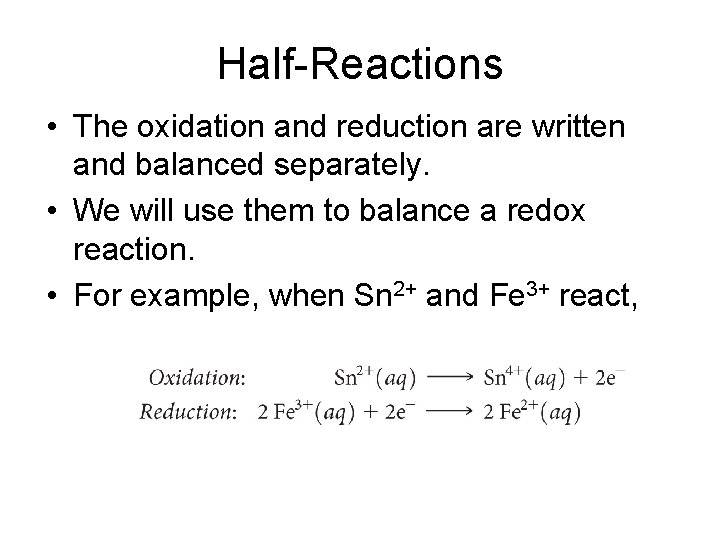
Half-Reactions • The oxidation and reduction are written and balanced separately. • We will use them to balance a redox reaction. • For example, when Sn 2+ and Fe 3+ react,

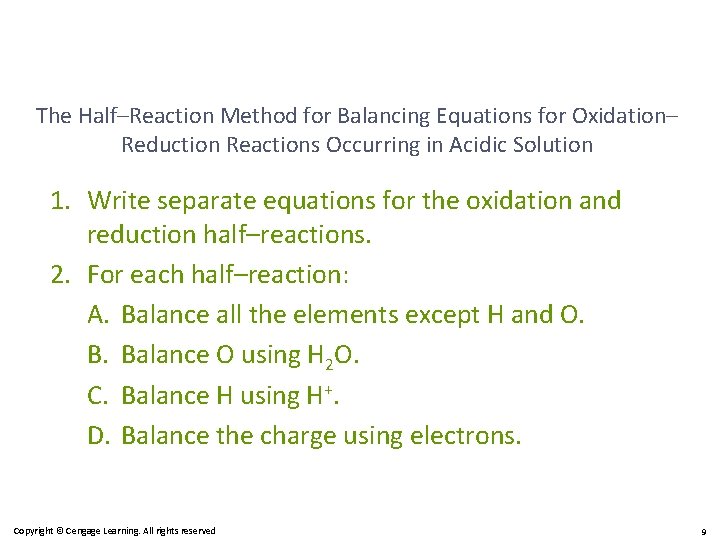
The Half–Reaction Method for Balancing Equations for Oxidation– Reduction Reactions Occurring in Acidic Solution 1. Write separate equations for the oxidation and reduction half–reactions. 2. For each half–reaction: A. Balance all the elements except H and O. B. Balance O using H 2 O. C. Balance H using H+. D. Balance the charge using electrons. Copyright © Cengage Learning. All rights reserved 9

The Half–Reaction Method for Balancing Equations for Oxidation– Reduction Reactions Occurring in Acidic Solution 3. If necessary, multiply one or both balanced half–reactions by an integer to equalize the number of electrons transferred in the two half–reactions. 4. Add the half–reactions, and cancel identical species. 5. Check that the elements and charges are balanced. Copyright © Cengage Learning. All rights reserved 10

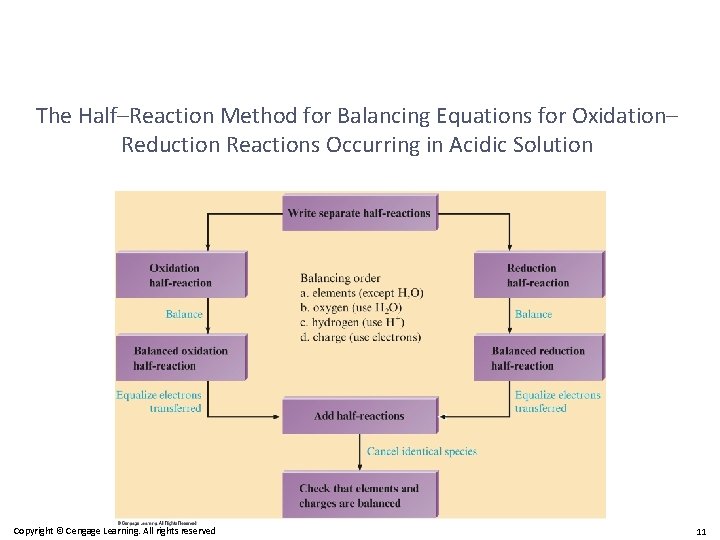
The Half–Reaction Method for Balancing Equations for Oxidation– Reduction Reactions Occurring in Acidic Solution Copyright © Cengage Learning. All rights reserved 11

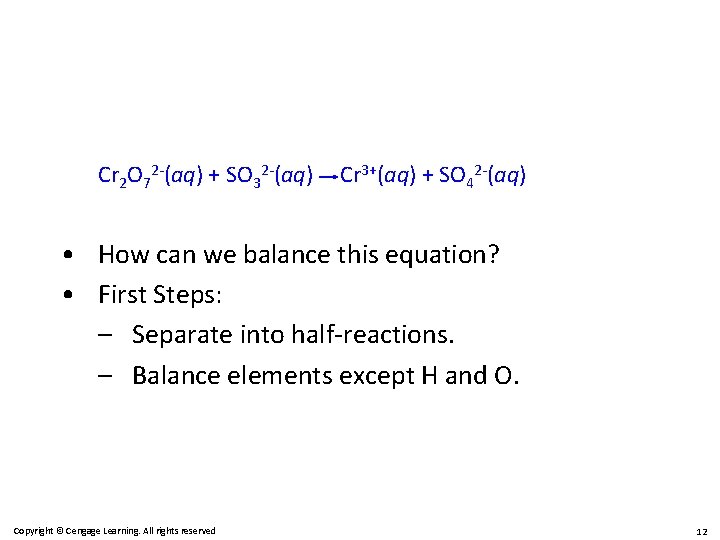
Cr 2 O 72 -(aq) + SO 32 -(aq) Cr 3+(aq) + SO 42 -(aq) • How can we balance this equation? • First Steps: – Separate into half-reactions. – Balance elements except H and O. Copyright © Cengage Learning. All rights reserved 12

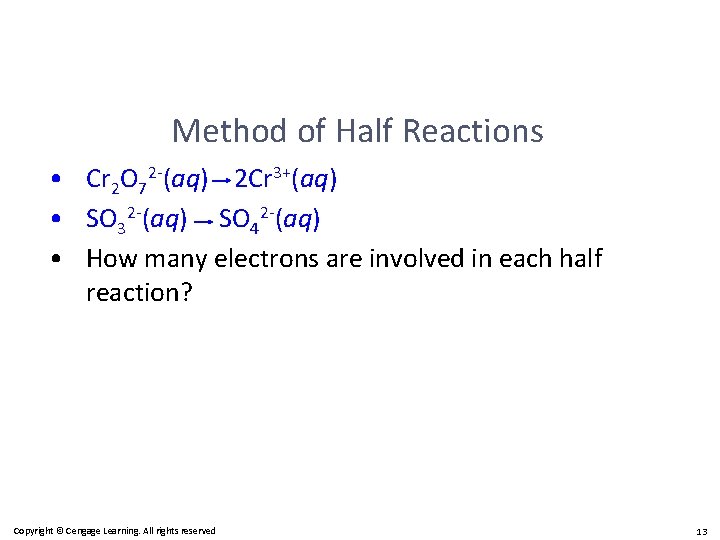
Method of Half Reactions • Cr 2 O 72 -(aq) 2 Cr 3+(aq) • SO 32 -(aq) SO 42 -(aq) • How many electrons are involved in each half reaction? Copyright © Cengage Learning. All rights reserved 13

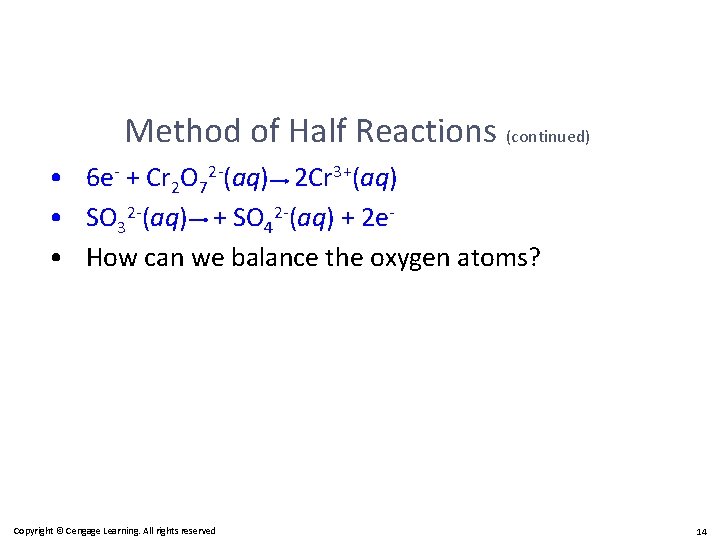
Method of Half Reactions (continued) • 6 e- + Cr 2 O 72 -(aq) 2 Cr 3+(aq) • SO 32 -(aq) + SO 42 -(aq) + 2 e • How can we balance the oxygen atoms? Copyright © Cengage Learning. All rights reserved 14

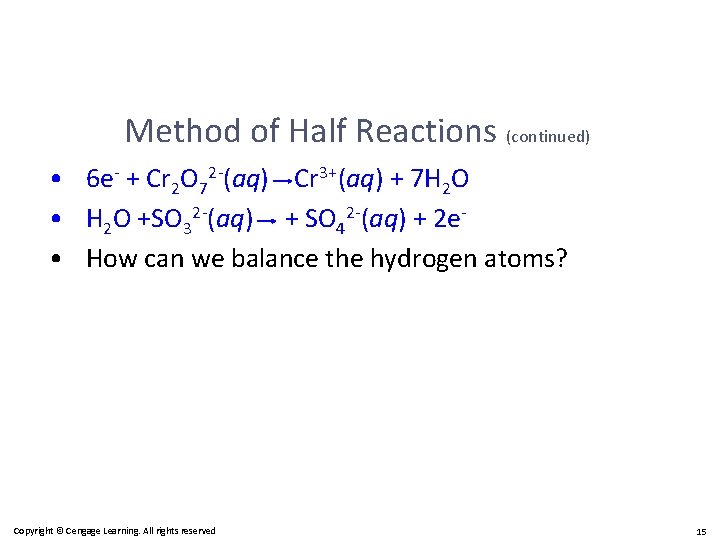
Method of Half Reactions (continued) • 6 e- + Cr 2 O 72 -(aq) Cr 3+(aq) + 7 H 2 O • H 2 O +SO 32 -(aq) + SO 42 -(aq) + 2 e • How can we balance the hydrogen atoms? Copyright © Cengage Learning. All rights reserved 15

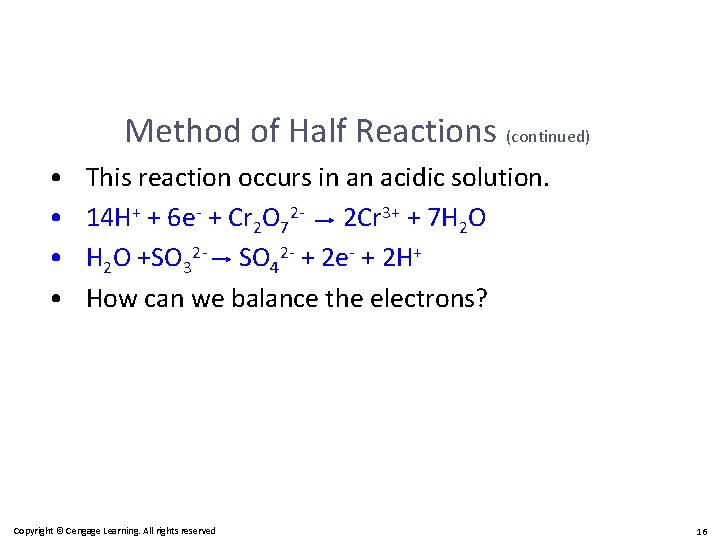
Method of Half Reactions (continued) • • This reaction occurs in an acidic solution. 14 H+ + 6 e- + Cr 2 O 72 - 2 Cr 3+ + 7 H 2 O +SO 32 - SO 42 - + 2 e- + 2 H+ How can we balance the electrons? Copyright © Cengage Learning. All rights reserved 16

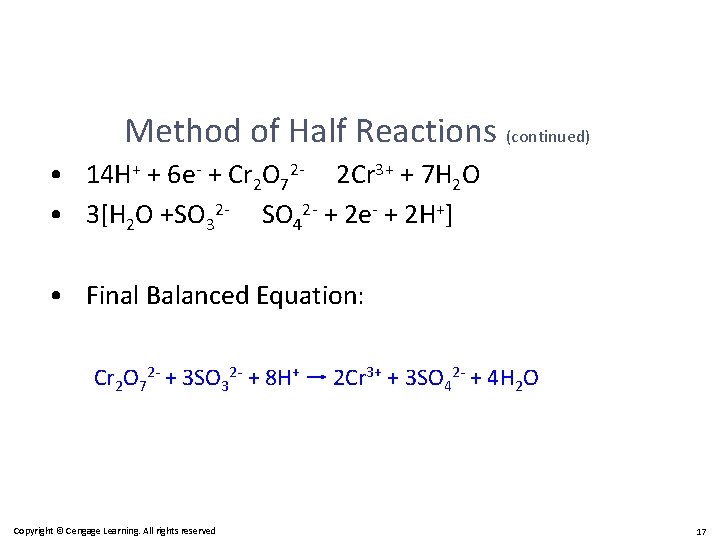
Method of Half Reactions (continued) • 14 H+ + 6 e- + Cr 2 O 72 - 2 Cr 3+ + 7 H 2 O • 3[H 2 O +SO 32 - SO 42 - + 2 e- + 2 H+] • Final Balanced Equation: Cr 2 O 72 - + 3 SO 32 - + 8 H+ 2 Cr 3+ + 3 SO 42 - + 4 H 2 O Copyright © Cengage Learning. All rights reserved 17

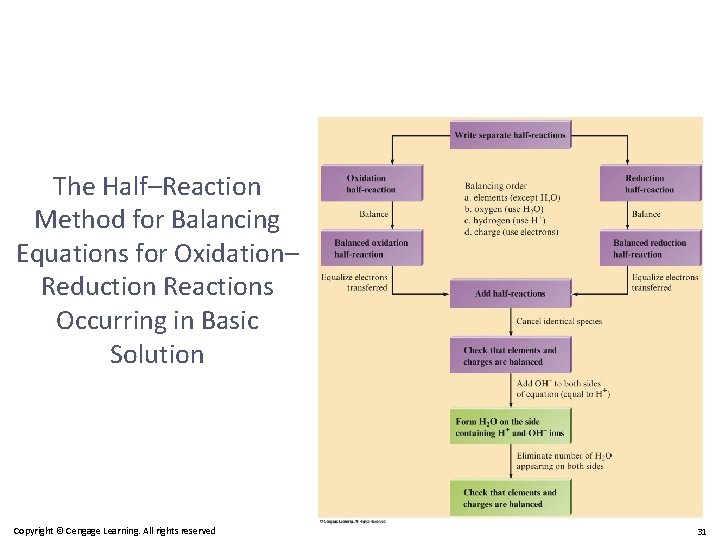
Balancing Redox Equations: The Half-Reactions Method (a Synopsis) 1) Make two half-reactions (oxidation and reduction). 2) Balance atoms other than O and H. Then, balance O and H using H 2 O/H+. 3) Add electrons to balance charges. 4) Multiply by common factor to make electrons in halfreactions equal. 5) Add the half-reactions. 6) Simplify by dividing by common factor or converting H+ to OH– if basic. 7) Double-check atoms and charges balance!

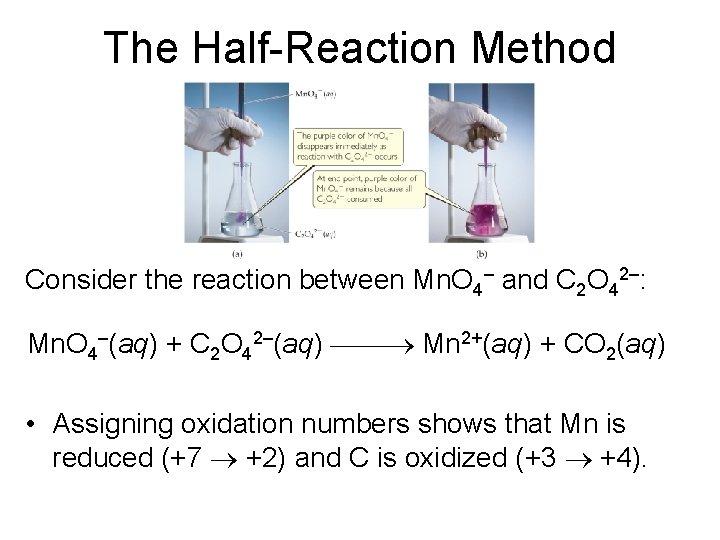
The Half-Reaction Method Consider the reaction between Mn. O 4– and C 2 O 42–: Mn. O 4–(aq) + C 2 O 42–(aq) Mn 2+(aq) + CO 2(aq) • Assigning oxidation numbers shows that Mn is reduced (+7 +2) and C is oxidized (+3 +4).

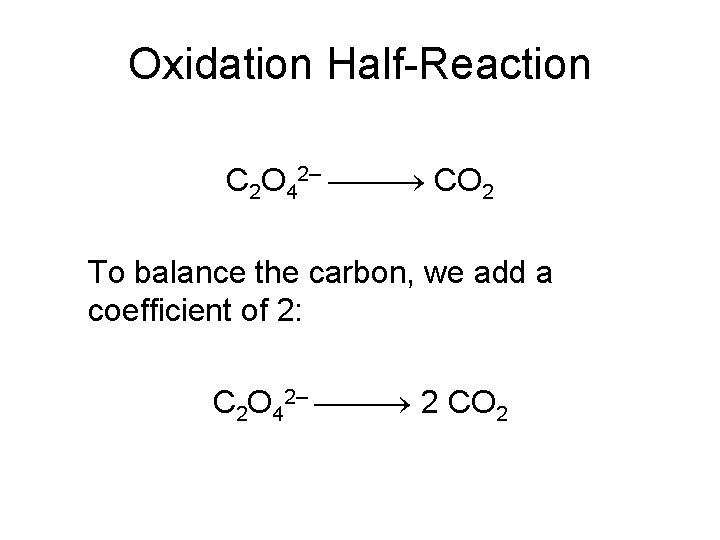
Oxidation Half-Reaction C 2 O 42– CO 2 To balance the carbon, we add a coefficient of 2: C 2 O 42– 2 CO 2

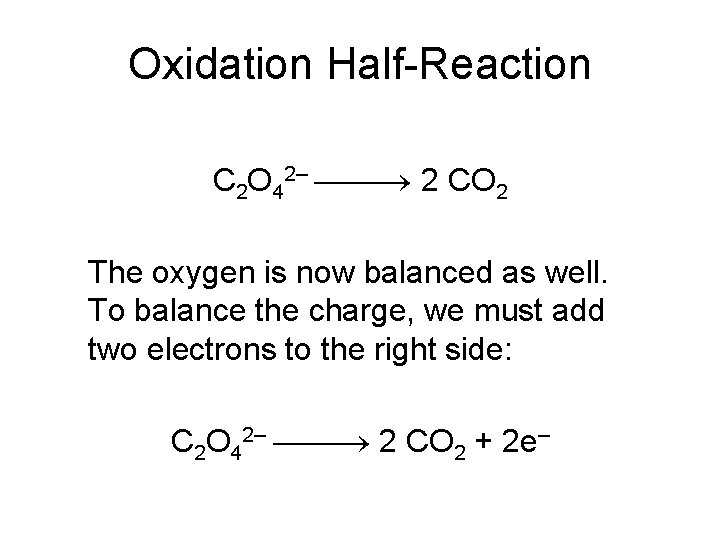
Oxidation Half-Reaction C 2 O 42– 2 CO 2 The oxygen is now balanced as well. To balance the charge, we must add two electrons to the right side: C 2 O 42– 2 CO 2 + 2 e–

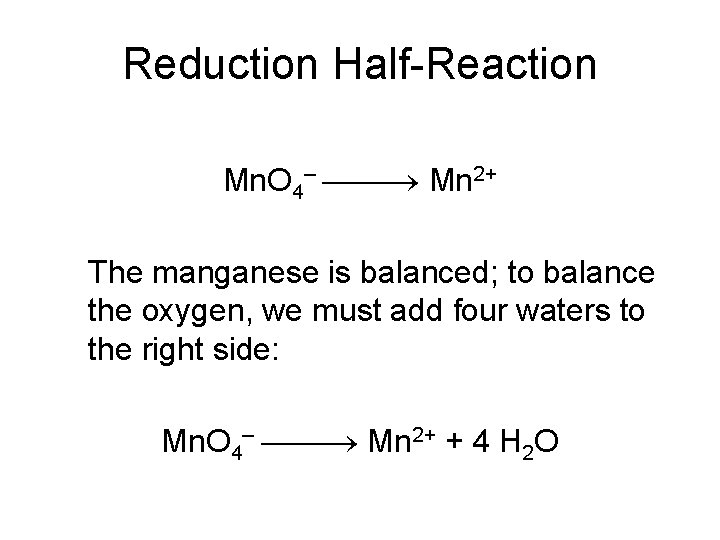
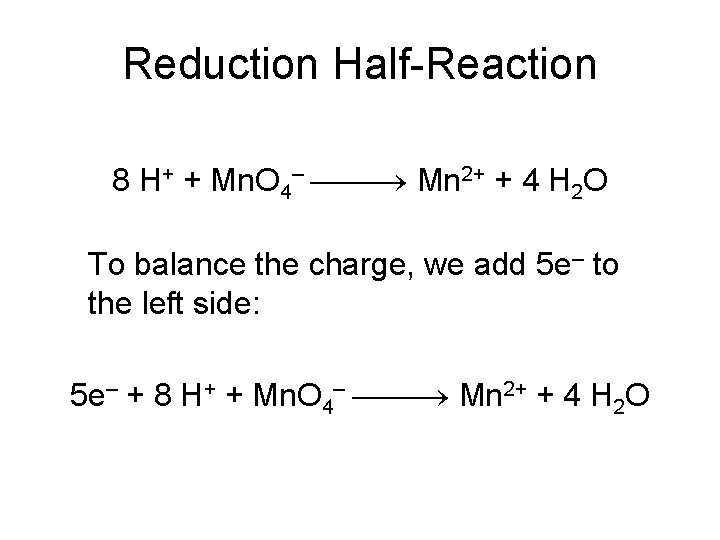
Reduction Half-Reaction Mn. O 4– Mn 2+ The manganese is balanced; to balance the oxygen, we must add four waters to the right side: Mn. O 4– Mn 2+ + 4 H 2 O

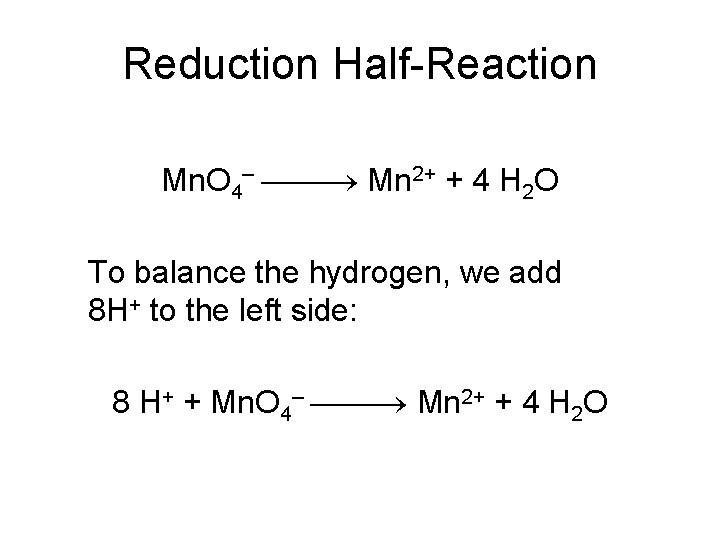
Reduction Half-Reaction Mn. O 4– Mn 2+ + 4 H 2 O To balance the hydrogen, we add 8 H+ to the left side: 8 H+ + Mn. O 4– Mn 2+ + 4 H 2 O

Reduction Half-Reaction 8 H+ + Mn. O 4– Mn 2+ + 4 H 2 O To balance the charge, we add 5 e– to the left side: 5 e– + 8 H+ + Mn. O 4– Mn 2+ + 4 H 2 O
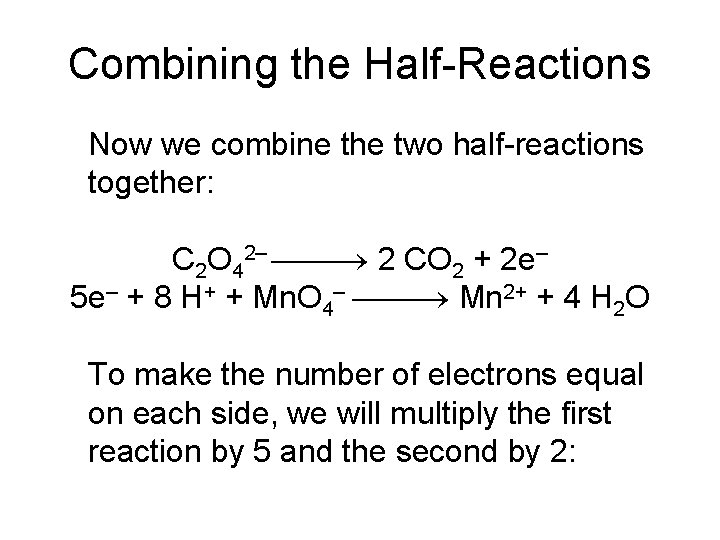
Combining the Half-Reactions Now we combine the two half-reactions together: C 2 O 42– 2 CO 2 + 2 e– 5 e– + 8 H+ + Mn. O 4– Mn 2+ + 4 H 2 O To make the number of electrons equal on each side, we will multiply the first reaction by 5 and the second by 2:
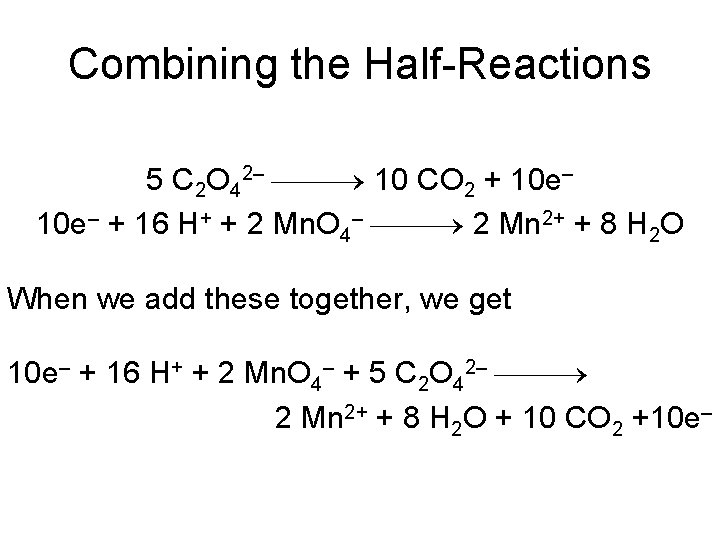
Combining the Half-Reactions 5 C 2 O 42– 10 CO 2 + 10 e– + 16 H+ + 2 Mn. O 4– 2 Mn 2+ + 8 H 2 O When we add these together, we get 10 e– + 16 H+ + 2 Mn. O 4– + 5 C 2 O 42– 2 Mn 2+ + 8 H 2 O + 10 CO 2 +10 e–
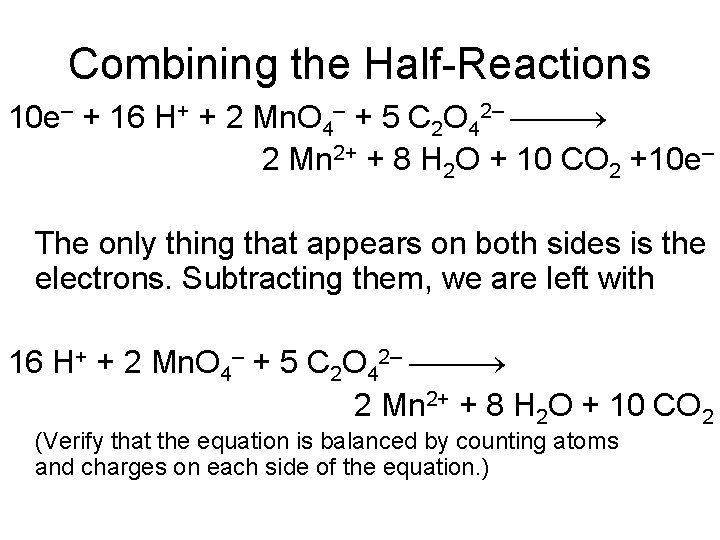
Combining the Half-Reactions 10 e– + 16 H+ + 2 Mn. O 4– + 5 C 2 O 42– 2 Mn 2+ + 8 H 2 O + 10 CO 2 +10 e– The only thing that appears on both sides is the electrons. Subtracting them, we are left with 16 H+ + 2 Mn. O 4– + 5 C 2 O 42– 2 Mn 2+ + 8 H 2 O + 10 CO 2 (Verify that the equation is balanced by counting atoms and charges on each side of the equation. )

EXERCISE! Balance the following oxidation–reduction reaction that occurs in acidic solution. Br–(aq) + Mn. O 4–(aq) Br 2(l)+ Mn 2+(aq) 10 Br–(aq) + 16 H+(aq) + 2 Mn. O 4–(aq) 5 Br 2(l)+ 2 Mn 2+(aq) + 8 H 2 O(l) Copyright © Cengage Learning. All rights reserved 28

The Half–Reaction Method for Balancing Equations for Oxidation–Reduction Reactions Occurring in Basic Solution 1. Use the half–reaction method as specified for acidic solutions to obtain the final balanced equation as if H+ ions were present. 2. To both sides of the equation obtained above, add a number of OH– ions that is equal to the number of H+ ions. (We want to eliminate H+ by forming H 2 O. ) Copyright © Cengage Learning. All rights reserved 29

The Half–Reaction Method for Balancing Equations for Oxidation– Reduction Reactions Occurring in Basic Solution 3. Form H 2 O on the side containing both H+ and OH– ions, and eliminate the number of H 2 O molecules that appear on both sides of the equation. 4. Check that elements and charges are balanced. Copyright © Cengage Learning. All rights reserved 30

The Half–Reaction Method for Balancing Equations for Oxidation– Reduction Reactions Occurring in Basic Solution Copyright © Cengage Learning. All rights reserved 31

Galvanic Cell • Device in which chemical energy is changed to electrical energy. • Uses a spontaneous redox reaction to produce a current that can be used to do work. Copyright © Cengage Learning. All rights reserved 32

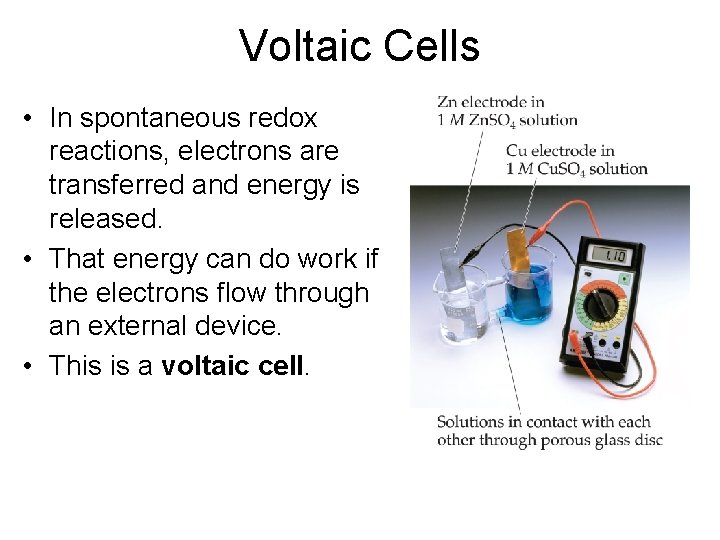
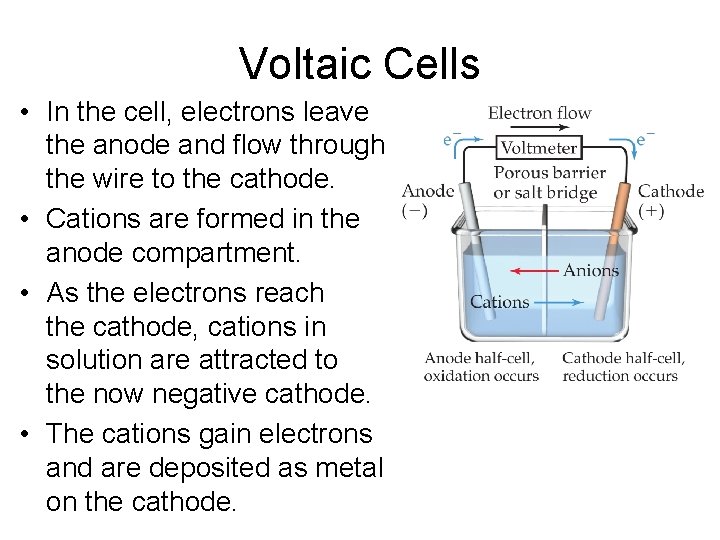
Voltaic Cells • In spontaneous redox reactions, electrons are transferred and energy is released. • That energy can do work if the electrons flow through an external device. • This is a voltaic cell.

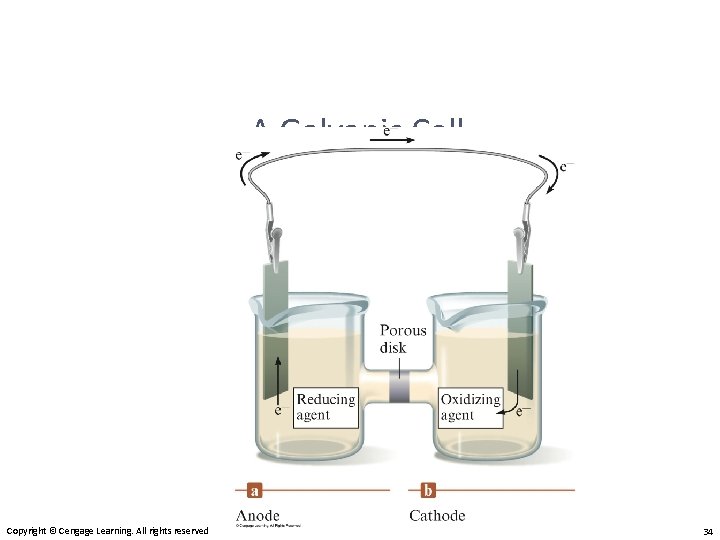
A Galvanic Cell Copyright © Cengage Learning. All rights reserved 34

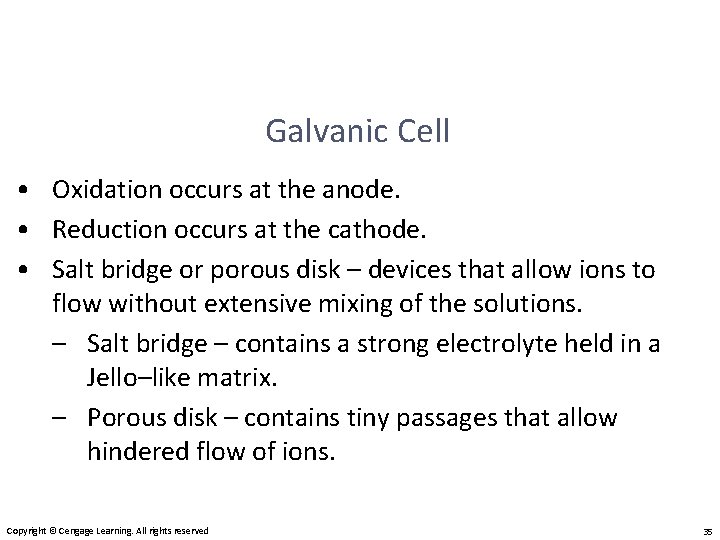
Galvanic Cell • Oxidation occurs at the anode. • Reduction occurs at the cathode. • Salt bridge or porous disk – devices that allow ions to flow without extensive mixing of the solutions. – Salt bridge – contains a strong electrolyte held in a Jello–like matrix. – Porous disk – contains tiny passages that allow hindered flow of ions. Copyright © Cengage Learning. All rights reserved 35

Voltaic Cells • In the cell, electrons leave the anode and flow through the wire to the cathode. • Cations are formed in the anode compartment. • As the electrons reach the cathode, cations in solution are attracted to the now negative cathode. • The cations gain electrons and are deposited as metal on the cathode.

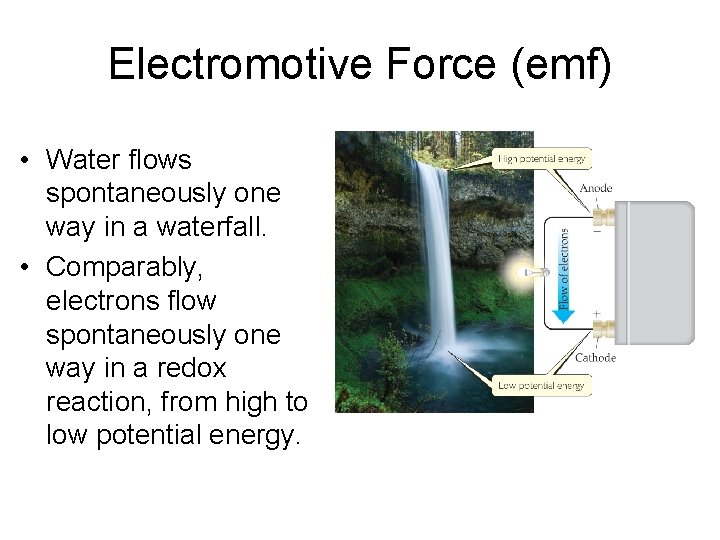
Electromotive Force (emf) • Water flows spontaneously one way in a waterfall. • Comparably, electrons flow spontaneously one way in a redox reaction, from high to low potential energy.

Electromotive Force (emf) • The potential difference between the anode and cathode in a cell is called the electromotive force (emf). • It is also called the cell potential and is designated Ecell. • It is measured in volts (V). One volt is one joule per coulomb (1 V = 1 J/C).

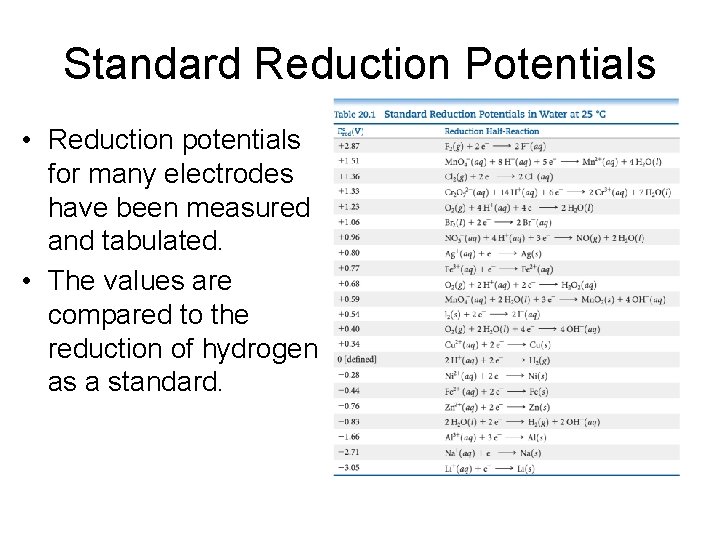
Standard Reduction Potentials • Reduction potentials for many electrodes have been measured and tabulated. • The values are compared to the reduction of hydrogen as a standard.

CONCEPT CHECK! Order the following from strongest to weakest oxidizing agent and justify. Of those you cannot order, explain why. Fe Na F- Na+ Cl 2

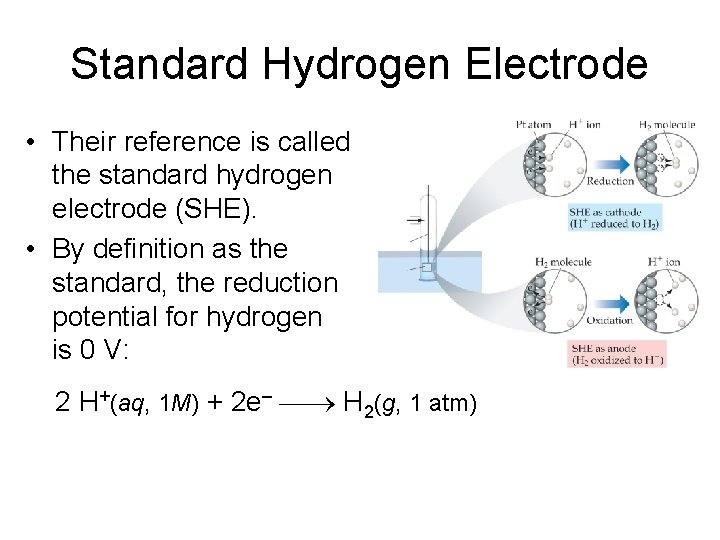
Standard Hydrogen Electrode • Their reference is called the standard hydrogen electrode (SHE). • By definition as the standard, the reduction potential for hydrogen is 0 V: 2 H+(aq, 1 M) + 2 e– H 2(g, 1 atm)

Standard Cell Potentials The cell potential at standard conditions can be found through this equation: ° = Ered ° (cathode) – Ered ° (anode) Ecell Because cell potential is based on the potential energy per unit of charge, it is an intensive property.

Voltaic Cell: Cathode Reaction To play movie you must be in Slide Show Mode PC Users: Please wait for content to load, then click to play Mac Users: CLICK HERE Copyright © Cengage Learning. All rights reserved 43

Voltaic Cell: Anode Reaction To play movie you must be in Slide Show Mode PC Users: Please wait for content to load, then click to play Mac Users: CLICK HERE Copyright © Cengage Learning. All rights reserved 44

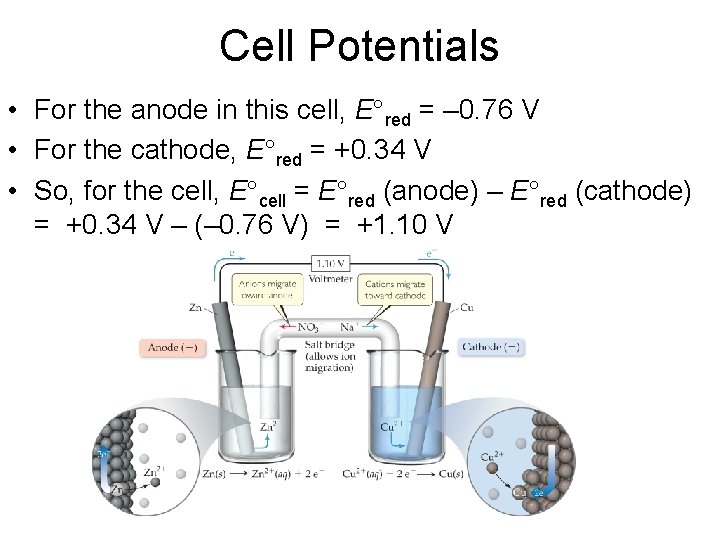
Cell Potentials • For the anode in this cell, E°red = – 0. 76 V • For the cathode, E°red = +0. 34 V • So, for the cell, E°cell = E°red (anode) – E°red (cathode) = +0. 34 V – (– 0. 76 V) = +1. 10 V

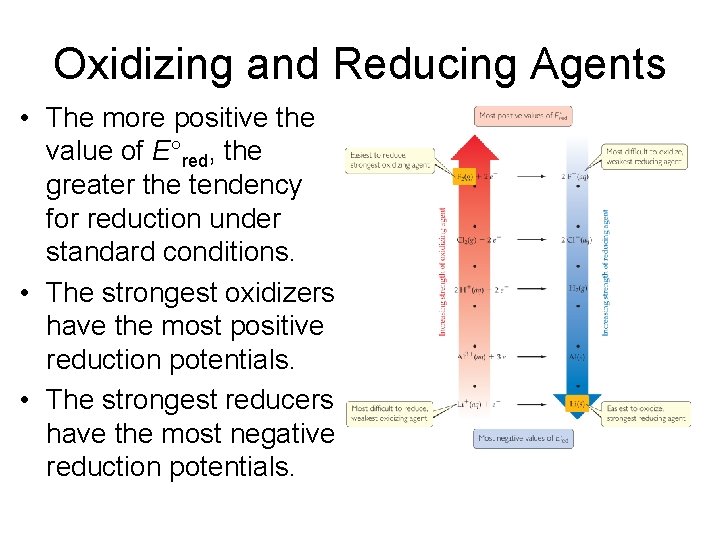
Oxidizing and Reducing Agents • The more positive the value of E°red, the greater the tendency for reduction under standard conditions. • The strongest oxidizers have the most positive reduction potentials. • The strongest reducers have the most negative reduction potentials.

Cell Potential • A galvanic cell consists of an oxidizing agent in one compartment that pulls electrons through a wire from a reducing agent in the other compartment. • The “pull”, or driving force, on the electrons is called the cell potential ( ), or the electromotive force (emf) of the cell. – Unit of electrical potential is the volt (V). Ø 1 joule of work per coulomb of charge transferred. Copyright © Cengage Learning. All rights reserved 47

Galvanic Cell • All half-reactions are given as reduction processes in standard tables. – Table 18. 1 – 1 M, 1 atm, 25°C • When a half-reaction is reversed, the sign of E° is reversed. • When a half-reaction is multiplied by an integer, E° remains the same. • A galvanic cell runs spontaneously in the direction that gives a positive value for E°cell. Copyright © Cengage Learning. All rights reserved 48

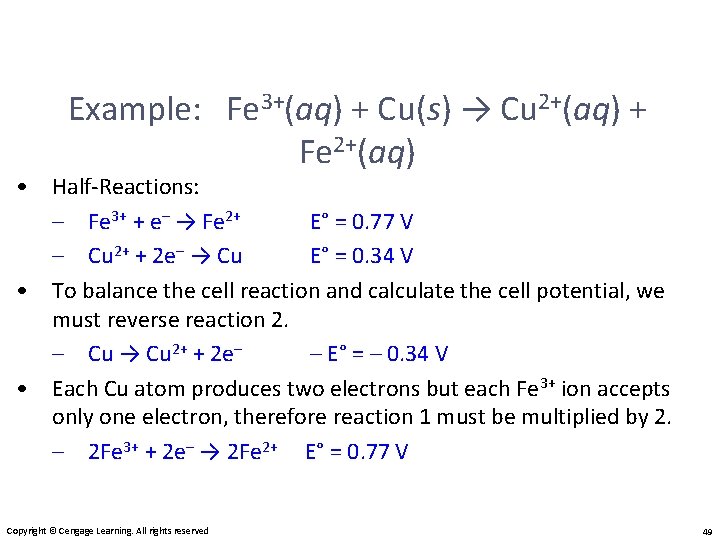
Example: Fe 3+(aq) + Cu(s) → Cu 2+(aq) + Fe 2+(aq) • Half-Reactions: – Fe 3+ + e– → Fe 2+ E° = 0. 77 V – Cu 2+ + 2 e– → Cu E° = 0. 34 V • To balance the cell reaction and calculate the cell potential, we must reverse reaction 2. – Cu → Cu 2+ + 2 e– – E° = – 0. 34 V • Each Cu atom produces two electrons but each Fe 3+ ion accepts only one electron, therefore reaction 1 must be multiplied by 2. – 2 Fe 3+ + 2 e– → 2 Fe 2+ E° = 0. 77 V Copyright © Cengage Learning. All rights reserved 49

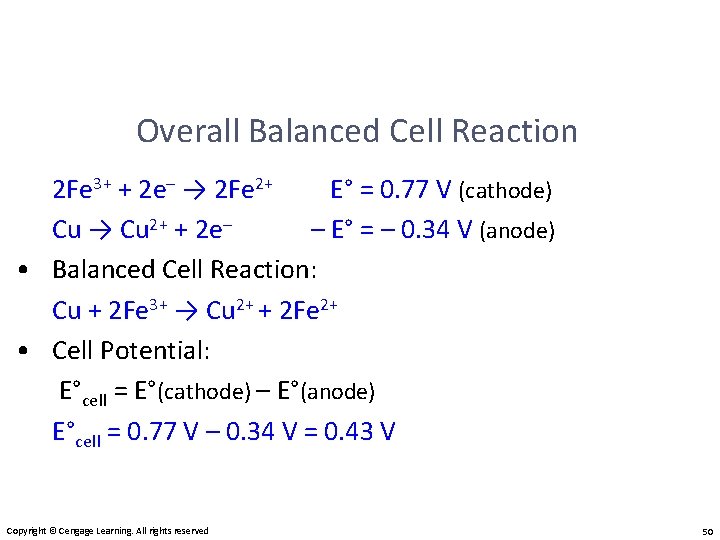
Overall Balanced Cell Reaction 2 Fe 3+ + 2 e– → 2 Fe 2+ E° = 0. 77 V (cathode) Cu → Cu 2+ + 2 e– – E° = – 0. 34 V (anode) • Balanced Cell Reaction: Cu + 2 Fe 3+ → Cu 2+ + 2 Fe 2+ • Cell Potential: E°cell = E°(cathode) – E°(anode) E°cell = 0. 77 V – 0. 34 V = 0. 43 V Copyright © Cengage Learning. All rights reserved 50

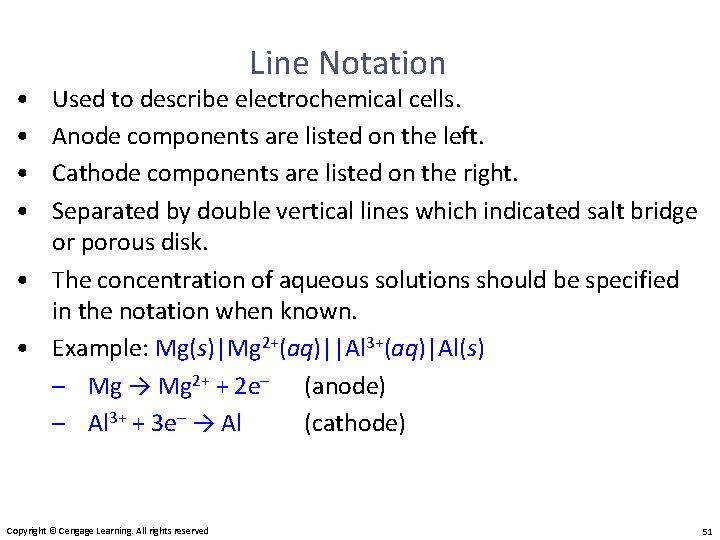
• • Line Notation Used to describe electrochemical cells. Anode components are listed on the left. Cathode components are listed on the right. Separated by double vertical lines which indicated salt bridge or porous disk. • The concentration of aqueous solutions should be specified in the notation when known. • Example: Mg(s)|Mg 2+(aq)||Al 3+(aq)|Al(s) – Mg → Mg 2+ + 2 e– (anode) – Al 3+ + 3 e– → Al (cathode) Copyright © Cengage Learning. All rights reserved 51

Description of a Galvanic Cell • The cell potential (always positive for a galvanic cell where E°cell = E°(cathode) – E°(anode)) and the balanced cell reaction. • The direction of electron flow, obtained by inspecting the half–reactions and using the direction that gives a positive E°cell. Copyright © Cengage Learning. All rights reserved 52

Description of a Galvanic Cell • Designation of the anode and cathode. • The nature of each electrode and the ions present in each compartment. A chemically inert conductor is required if none of the substances participating in the half–reaction is a conducting solid. Copyright © Cengage Learning. All rights reserved 53

CONCEPT CHECK! Sketch a cell using the following solutions and electrodes. Include: – The potential of the cell – The direction of electron flow – Labels on the anode and the cathode a) Ag electrode in 1. 0 M Ag+(aq) and Cu electrode in 1. 0 M Cu 2+(aq) Copyright © Cengage Learning. All rights reserved 54

CONCEPT CHECK! Sketch a cell using the following solutions and electrodes. Include: – The potential of the cell – The direction of electron flow – Labels on the anode and the cathode b) Zn electrode in 1. 0 M Zn 2+(aq) and Cu electrode in 1. 0 M Cu 2+(aq) Copyright © Cengage Learning. All rights reserved 55

CONCEPT CHECK! Consider the cell from part b. What would happen to the potential if you increase the [Cu 2+]? Explain. The cell potential should increase. Copyright © Cengage Learning. All rights reserved 56

Work • Work is never the maximum possible if any current is flowing. • In any real, spontaneous process some energy is always wasted – the actual work realized is always less than the calculated maximum. Copyright © Cengage Learning. All rights reserved 57

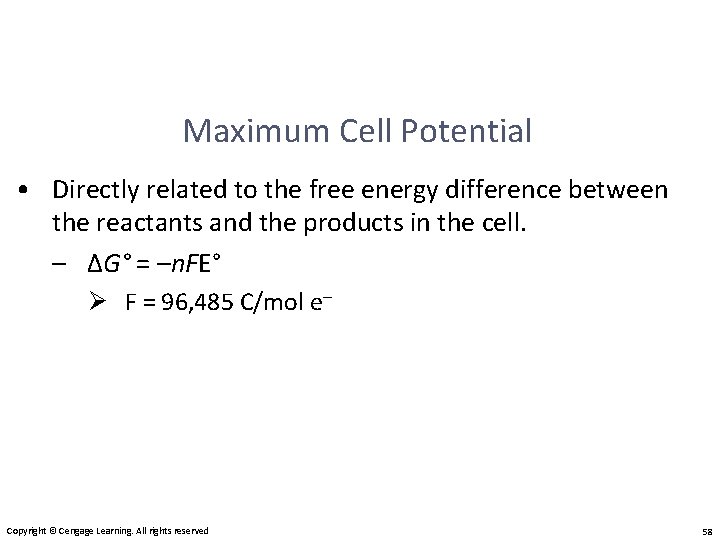
Maximum Cell Potential • Directly related to the free energy difference between the reactants and the products in the cell. – ΔG° = –n. FE° Ø F = 96, 485 C/mol e– Copyright © Cengage Learning. All rights reserved 58

Free Energy and Redox • Spontaneous redox reactions produce a positive cell potential, or emf. • E° = E°red (reduction) – E°red (oxidation) • Note that this is true for ALL redox reactions, not only for voltaic cells. • Since Gibbs free energy is the measure of spontaneity, positive emf corresponds to negative ΔG. • How do they relate? ΔG = –n. FE (F is the Faraday constant, 96, 485 C/mol. )

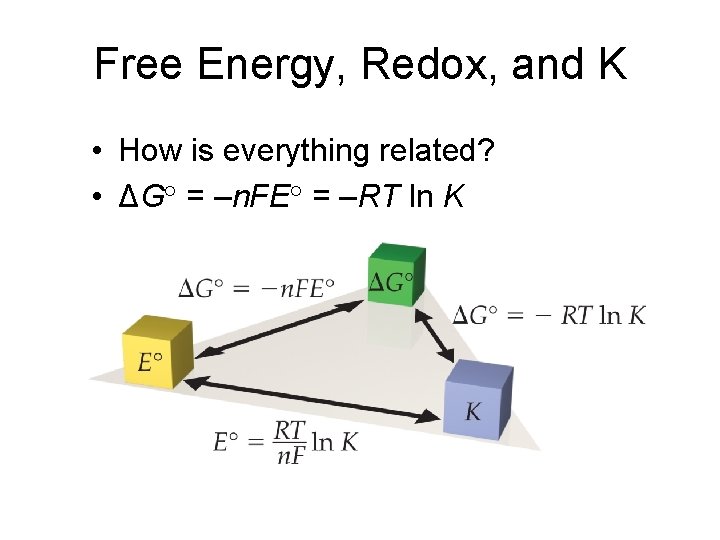
Free Energy, Redox, and K • How is everything related? • ΔG° = –n. FE° = –RT ln K

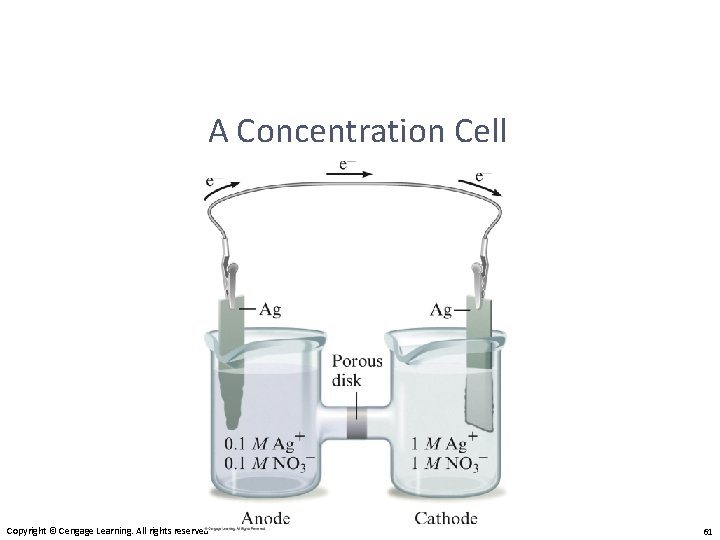
A Concentration Cell Copyright © Cengage Learning. All rights reserved 61

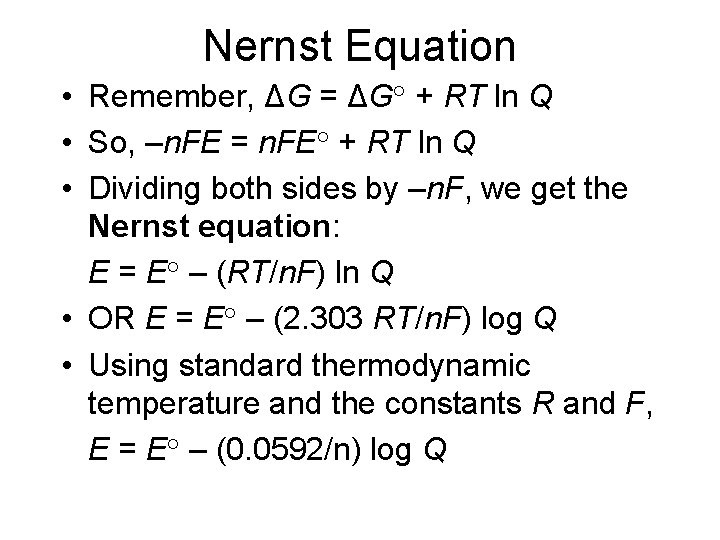
Nernst Equation • Remember, ΔG = ΔG° + RT ln Q • So, –n. FE = n. FE° + RT ln Q • Dividing both sides by –n. F, we get the Nernst equation: E = E° – (RT/n. F) ln Q • OR E = E° – (2. 303 RT/n. F) log Q • Using standard thermodynamic temperature and the constants R and F, E = E° – (0. 0592/n) log Q

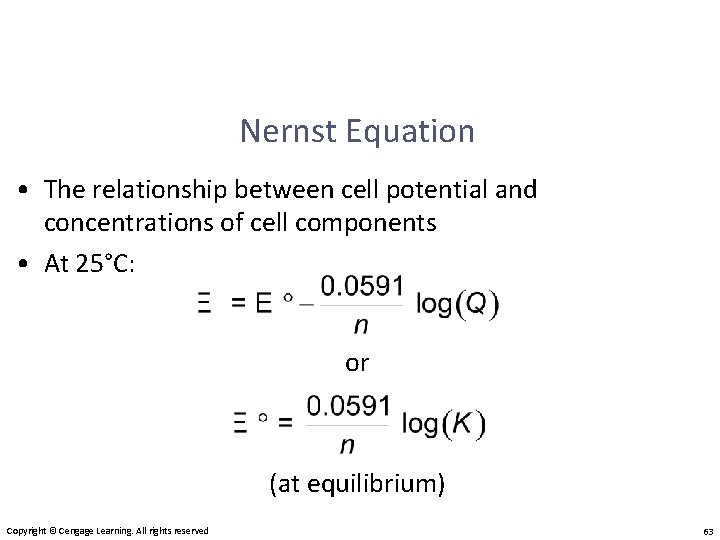
Nernst Equation • The relationship between cell potential and concentrations of cell components • At 25°C: or (at equilibrium) Copyright © Cengage Learning. All rights reserved 63

CONCEPT CHECK! Explain the difference between E and E°. When is E equal to zero? When the cell is in equilibrium ("dead" battery). When is E° equal to zero? E is equal to zero for a concentration cell. Copyright © Cengage Learning. All rights reserved 64

EXERCISE! A concentration cell is constructed using two nickel electrodes with Ni 2+ concentrations of 1. 0 M and 1. 00 × 10 -4 M in the two half-cells. Calculate the potential of this cell at 25°C. 0. 118 V Copyright © Cengage Learning. All rights reserved 65

CONCEPT CHECK! You make a galvanic cell at 25°C containing: – A nickel electrode in 1. 0 M Ni 2+(aq) – A silver electrode in 1. 0 M Ag+(aq) Sketch this cell, labeling the anode and cathode, showing the direction of the electron flow, and calculate the cell potential. 1. 03 V Copyright © Cengage Learning. All rights reserved 66

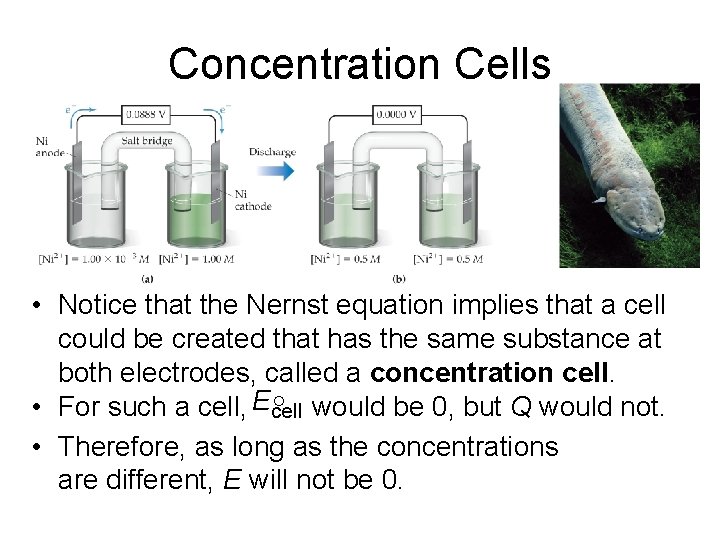
Concentration Cells • Notice that the Nernst equation implies that a cell could be created that has the same substance at both electrodes, called a concentration cell. • For such a cell, Ecell ° would be 0, but Q would not. • Therefore, as long as the concentrations are different, E will not be 0.

Some Applications of Cells • Electrochemistry can be applied as follows: v Batteries: a portable, self-contained electrochemical power source that consists of one or more voltaic cells. Ø Batteries can be primary cells (cannot be recharged when “dead”—the reaction is complete) or secondary cells (can be recharged). v Prevention of corrosion (“rust-proofing”) v Electrolysis

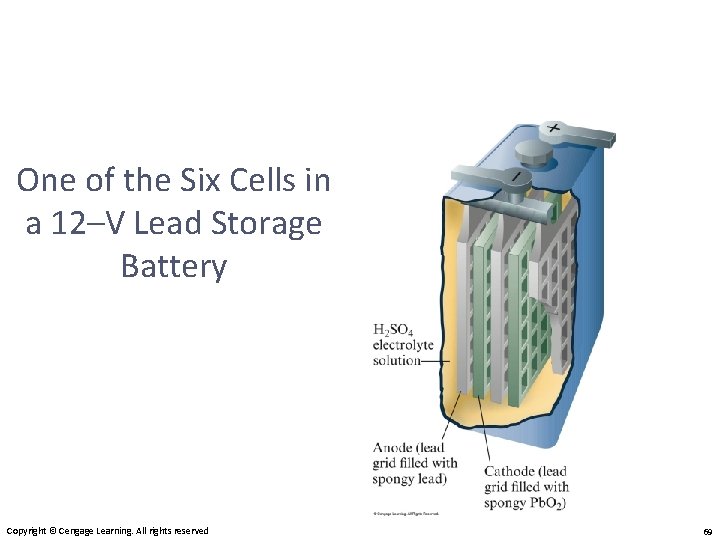
One of the Six Cells in a 12–V Lead Storage Battery Copyright © Cengage Learning. All rights reserved 69

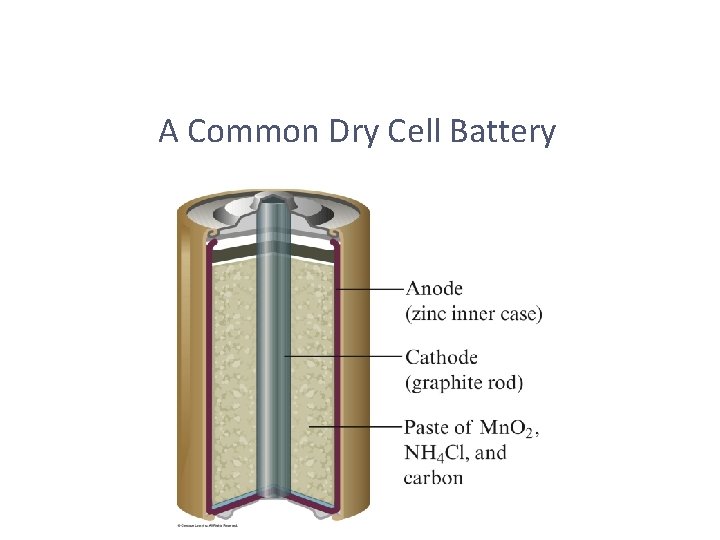
A Common Dry Cell Battery

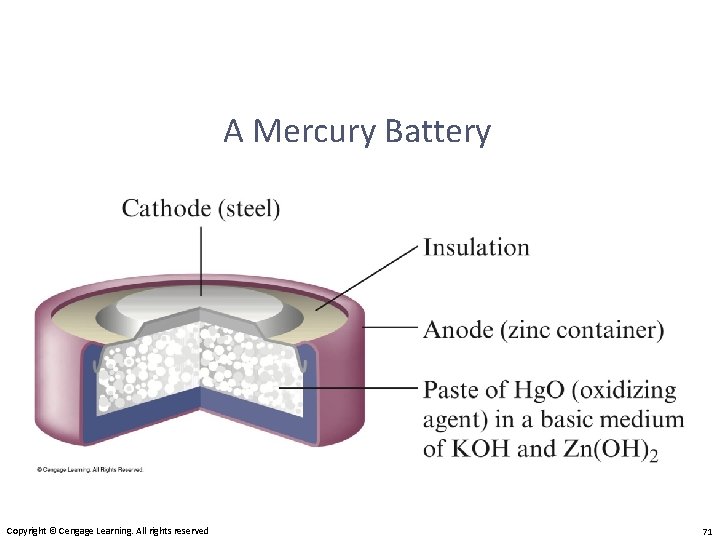
A Mercury Battery Copyright © Cengage Learning. All rights reserved 71

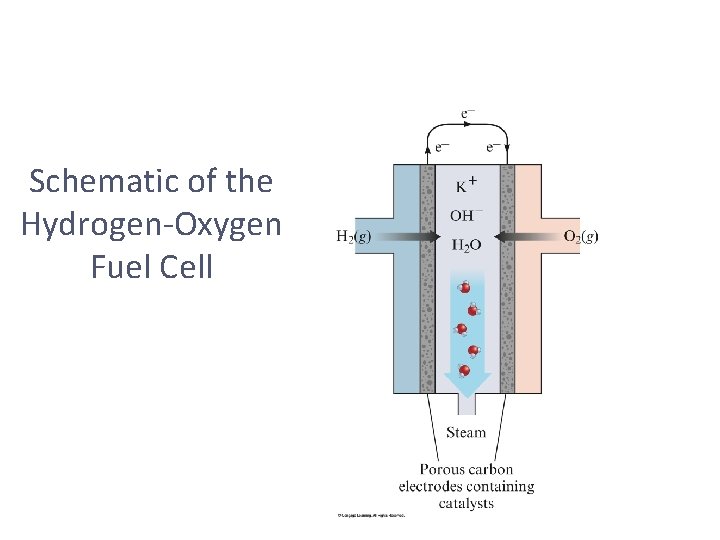
Schematic of the Hydrogen-Oxygen Fuel Cell

• Process of returning metals to their natural state – the ores from which they were originally obtained. • Involves oxidation of the metal. Copyright © Cengage Learning. All rights reserved 73

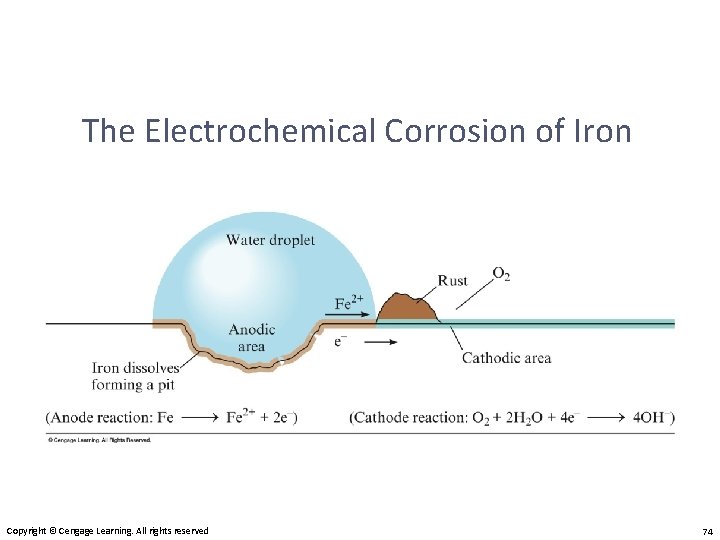
The Electrochemical Corrosion of Iron Copyright © Cengage Learning. All rights reserved 74

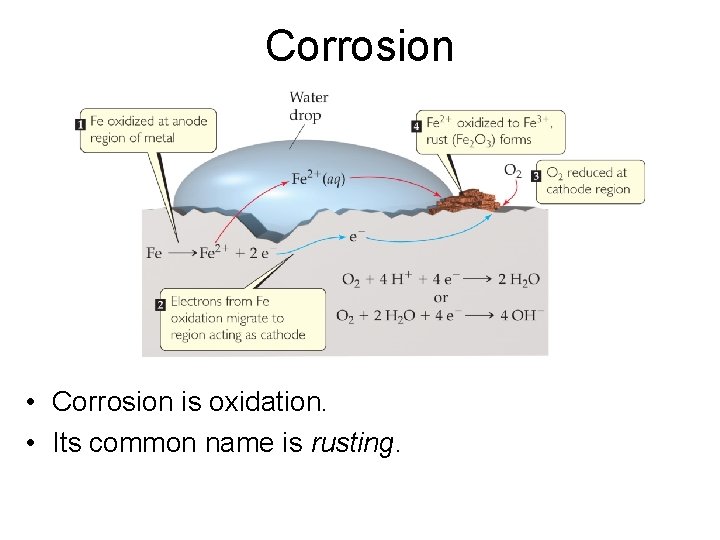
Corrosion • Corrosion is oxidation. • Its common name is rusting.

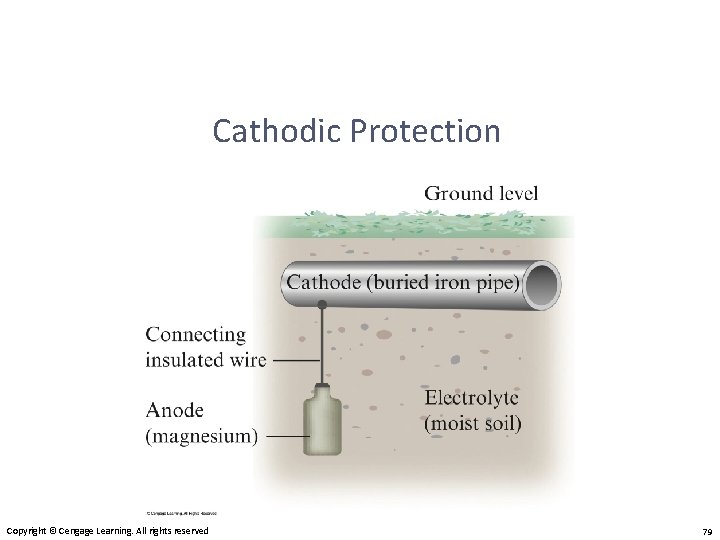
Corrosion Prevention • Application of a coating (like paint or metal plating) – Galvanizing • Alloying • Cathodic Protection – Protects steel in buried fuel tanks and pipelines. Copyright © Cengage Learning. All rights reserved 76

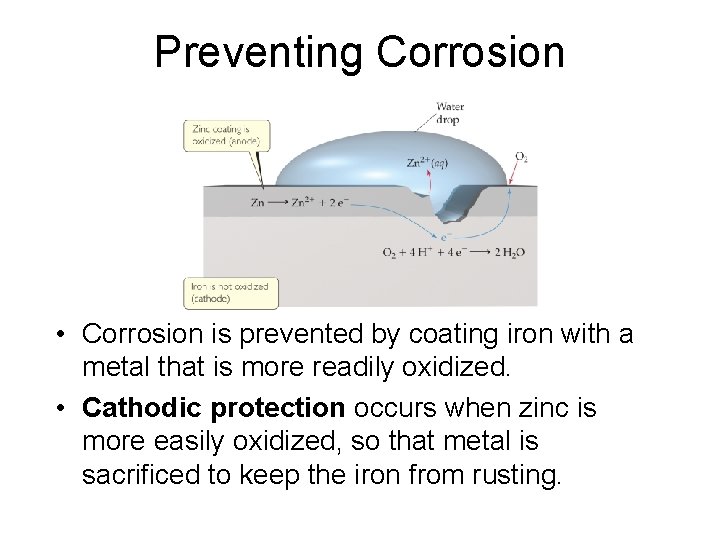
Preventing Corrosion • Corrosion is prevented by coating iron with a metal that is more readily oxidized. • Cathodic protection occurs when zinc is more easily oxidized, so that metal is sacrificed to keep the iron from rusting.

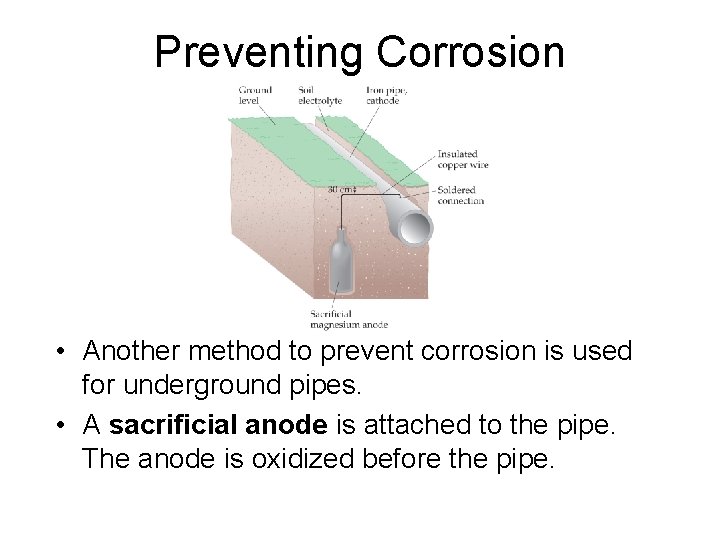
Preventing Corrosion • Another method to prevent corrosion is used for underground pipes. • A sacrificial anode is attached to the pipe. The anode is oxidized before the pipe.

Cathodic Protection Copyright © Cengage Learning. All rights reserved 79

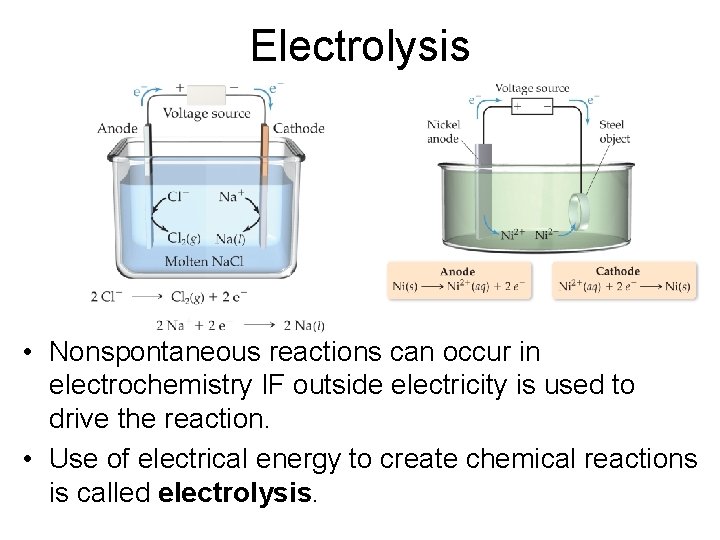
• Forcing a current through a cell to produce a chemical change for which the cell potential is negative. Copyright © Cengage Learning. All rights reserved 80

Electrolysis • Nonspontaneous reactions can occur in electrochemistry IF outside electricity is used to drive the reaction. • Use of electrical energy to create chemical reactions is called electrolysis.

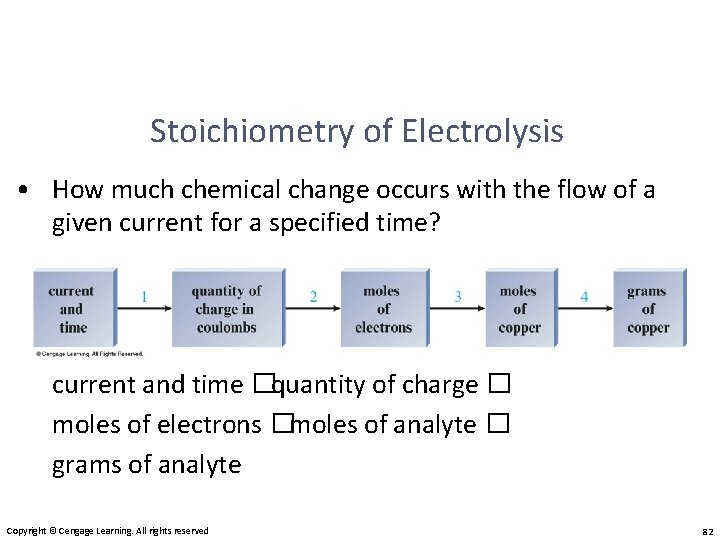
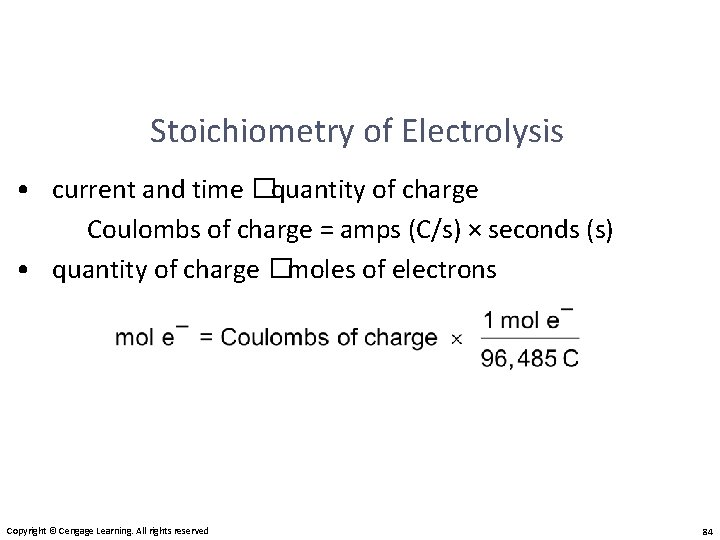
Stoichiometry of Electrolysis • How much chemical change occurs with the flow of a given current for a specified time? current and time � quantity of charge � moles of electrons � moles of analyte � grams of analyte Copyright © Cengage Learning. All rights reserved 82

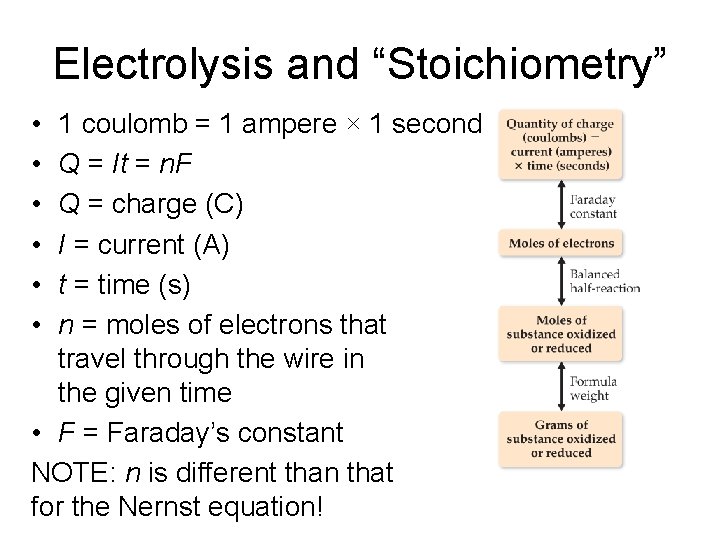
Electrolysis and “Stoichiometry” • • • 1 coulomb = 1 ampere × 1 second Q = It = n. F Q = charge (C) I = current (A) t = time (s) n = moles of electrons that travel through the wire in the given time • F = Faraday’s constant NOTE: n is different than that for the Nernst equation!

Stoichiometry of Electrolysis • current and time � quantity of charge Coulombs of charge = amps (C/s) × seconds (s) • quantity of charge � moles of electrons Copyright © Cengage Learning. All rights reserved 84

CONCEPT CHECK! An unknown metal (M) is electrolyzed. It took 52. 8 sec for a current of 2. 00 amp to plate 0. 0719 g of the metal from a solution containing M(NO 3)3. What is the metal? gold (Au) Copyright © Cengage Learning. All rights reserved 85

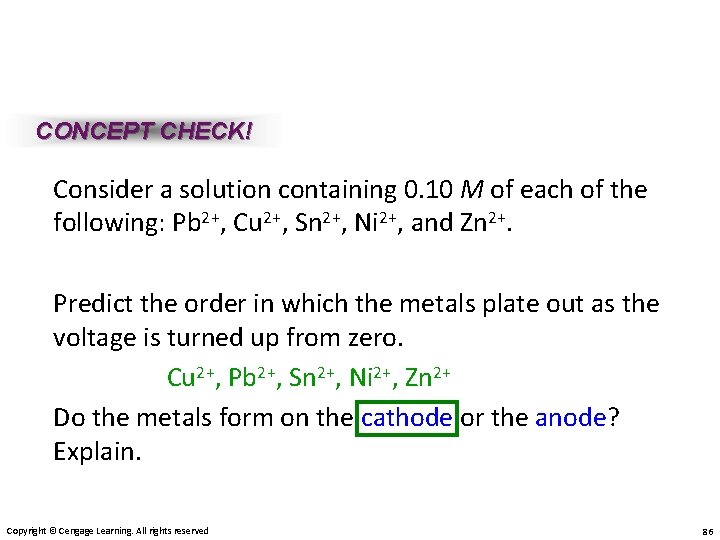
CONCEPT CHECK! Consider a solution containing 0. 10 M of each of the following: Pb 2+, Cu 2+, Sn 2+, Ni 2+, and Zn 2+. Predict the order in which the metals plate out as the voltage is turned up from zero. Cu 2+, Pb 2+, Sn 2+, Ni 2+, Zn 2+ Do the metals form on the cathode or the anode? Explain. Copyright © Cengage Learning. All rights reserved 86

• • • Production of aluminum Purification of metals Metal plating Electrolysis of sodium chloride Production of chlorine and sodium hydroxide Copyright © Cengage Learning. All rights reserved 87

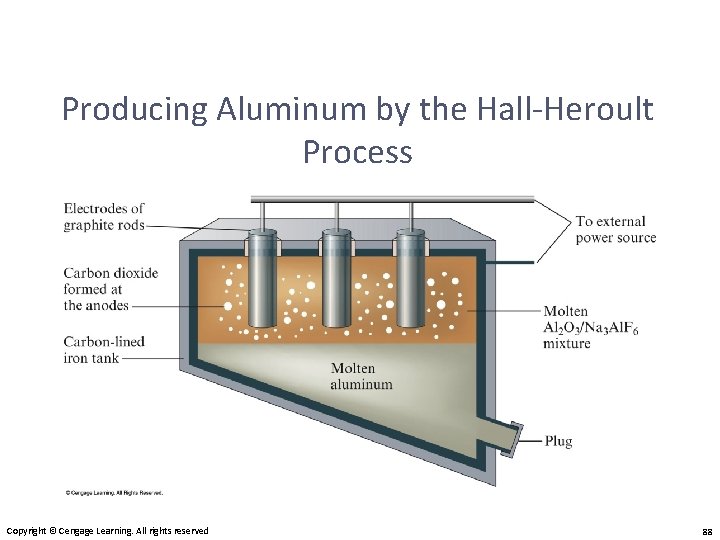
Producing Aluminum by the Hall-Heroult Process Copyright © Cengage Learning. All rights reserved 88

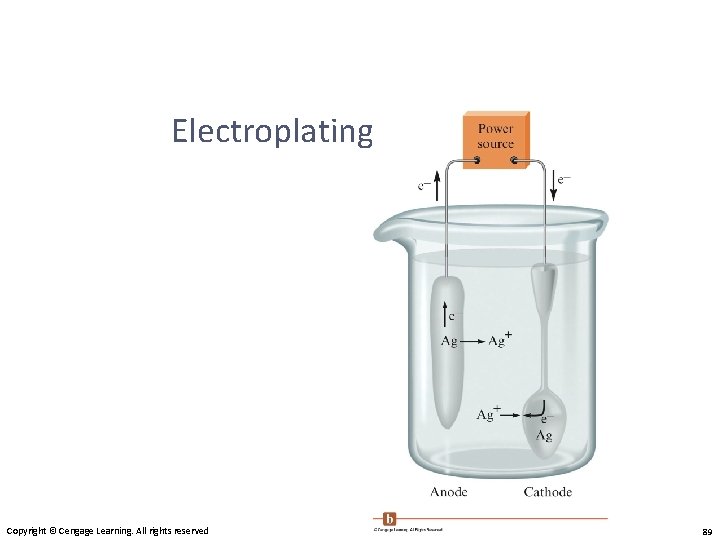
Electroplatinging a Spoon Copyright © Cengage Learning. All rights reserved 89

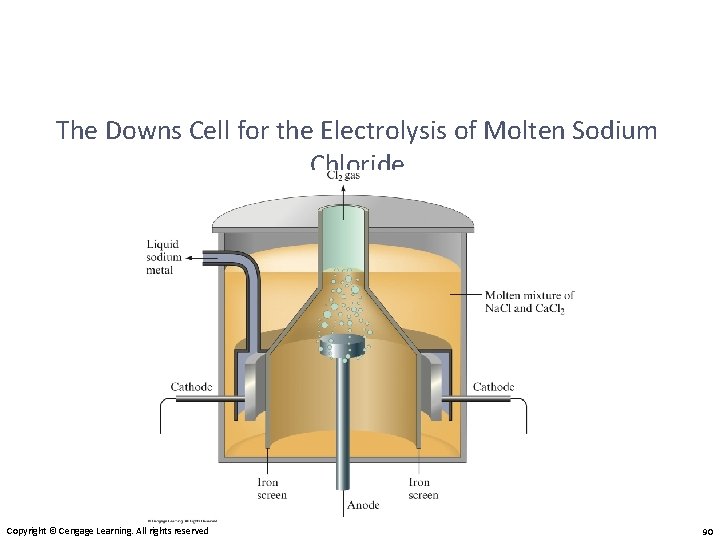
The Downs Cell for the Electrolysis of Molten Sodium Chloride Copyright © Cengage Learning. All rights reserved 90

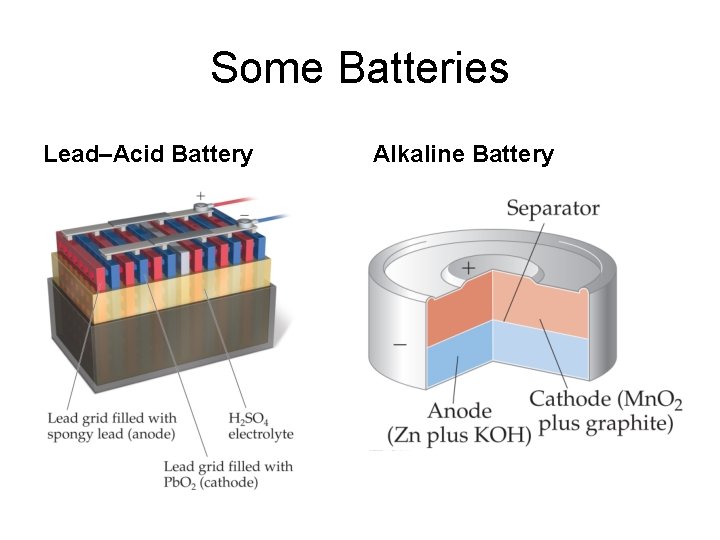
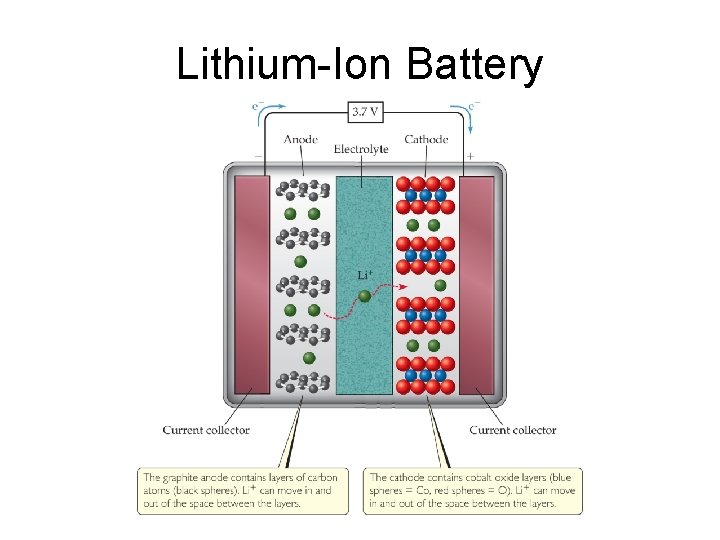
Some Examples of Batteries • Lead–acid battery: reactants and products are solids, so Q is 1 and the potential is independent of concentrations; however, made with lead and sulfuric acid (hazards). • Alkaline battery: most common primary battery. • Ni–Cd and Ni–metal hydride batteries: lightweight, rechargeable; Cd is toxic and heavy, so hydrides are replacing it. • Lithium-ion batteries: rechargeable, light; produce more voltage than Ni-based batteries.

Some Batteries Lead–Acid Battery Alkaline Battery

Lithium-Ion Battery

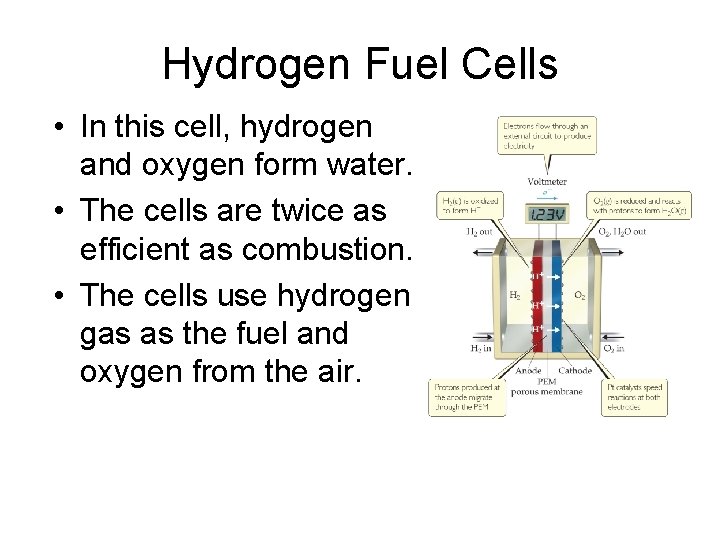
Fuel Cells • When a fuel is burned, the energy created can be converted to electrical energy. • Usually, this conversion is only 40% efficient, with the remainder lost as heat. • The direct conversion of chemical to electrical energy is expected to be more efficient and is the basis for fuel cells. • Fuel cells are NOT batteries; the source of energy must be continuously provided.

Hydrogen Fuel Cells • In this cell, hydrogen and oxygen form water. • The cells are twice as efficient as combustion. • The cells use hydrogen gas as the fuel and oxygen from the air.