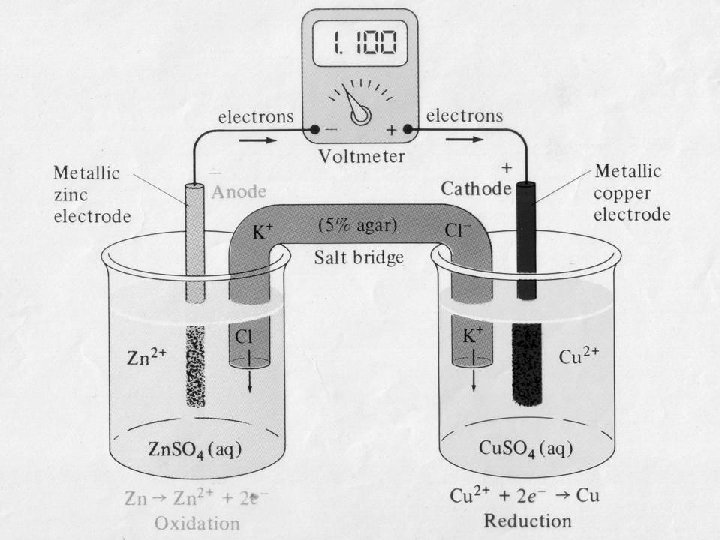
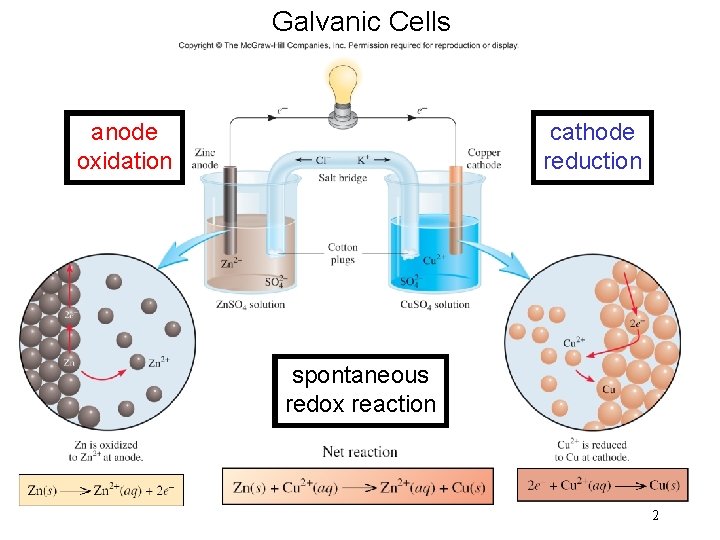
1 Galvanic Cells anode oxidation cathode reduction spontaneous



![Now try: [Fe 2+] = 1. 0 M and [Cd 2+] = 0. 01 Now try: [Fe 2+] = 1. 0 M and [Cd 2+] = 0. 01](https://slidetodoc.com/presentation_image/375cd27d6dd48e938de62fa236f43990/image-14.jpg)
- Slides: 23

1

Galvanic Cells anode oxidation cathode reduction spontaneous redox reaction 2

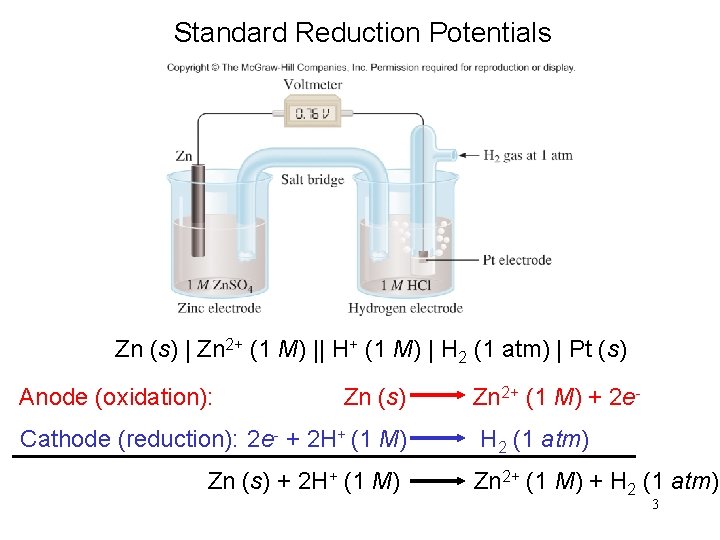
Standard Reduction Potentials Zn (s) | Zn 2+ (1 M) || H+ (1 M) | H 2 (1 atm) | Pt (s) Anode (oxidation): Zn (s) Cathode (reduction): 2 e- + 2 H+ (1 M) Zn (s) + 2 H+ (1 M) Zn 2+ (1 M) + 2 e. H 2 (1 atm) Zn 2+ (1 M) + H 2 (1 atm) 3

Zn Cu 4

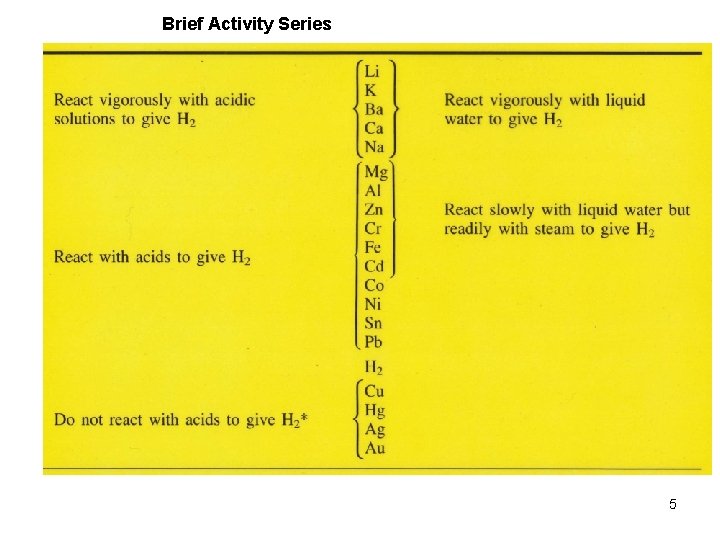
Brief Activity Series 5

6

7

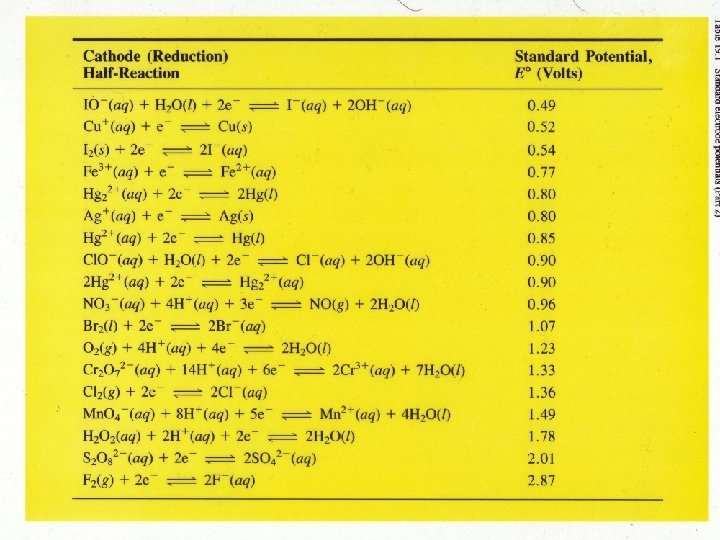
• E 0 is for the reaction as written • The more positive E 0 the greater the tendency for the substance to be reduced • The half-cell reactions are reversible • The sign of E 0 changes when the reaction is reversed • Changing the stoichiometric coefficients of a half-cell reaction does not change the value of E 0 8

9

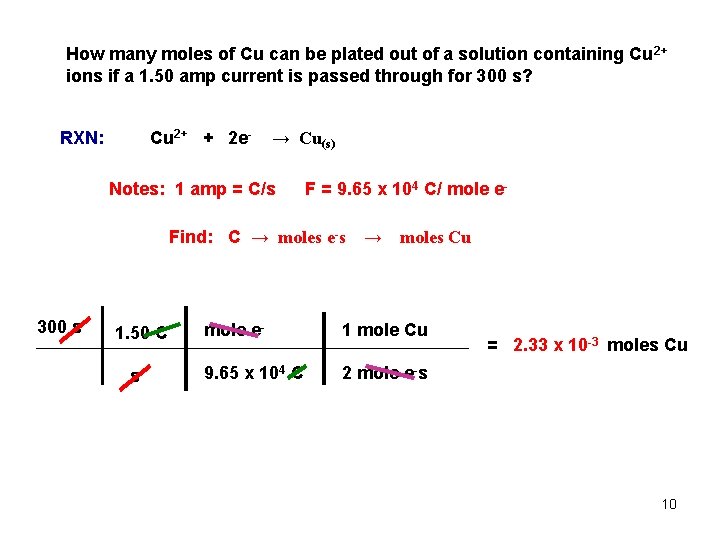
How many moles of Cu can be plated out of a solution containing Cu 2+ ions if a 1. 50 amp current is passed through for 300 s? Cu 2+ + 2 e- RXN: → Cu(s) Notes: 1 amp = C/s F = 9. 65 x 104 C/ mole e- Find: C → moles e-s 300 s 1. 50 C s → moles Cu mole e- 1 mole Cu 9. 65 x 104 C 2 mole e-s = 2. 33 x 10 -3 moles Cu 10

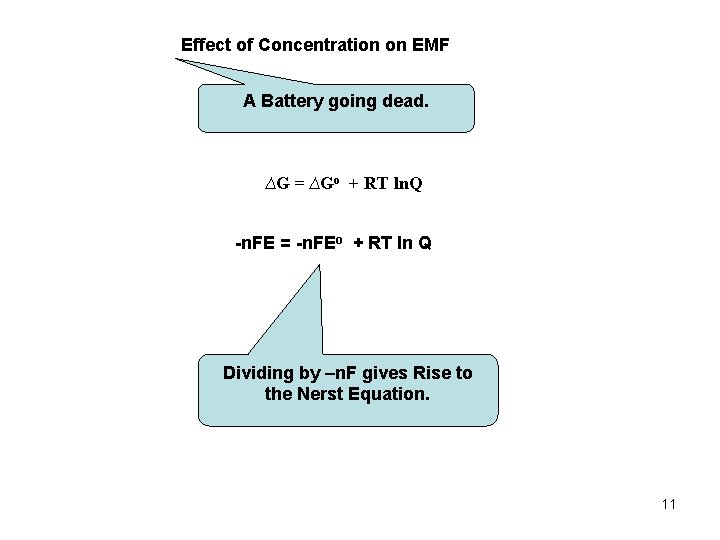
Effect of Concentration on EMF A Battery going dead. ∆G = ∆Go + RT ln. Q -n. FE = -n. FEo + RT ln Q Dividing by –n. F gives Rise to the Nerst Equation. 11

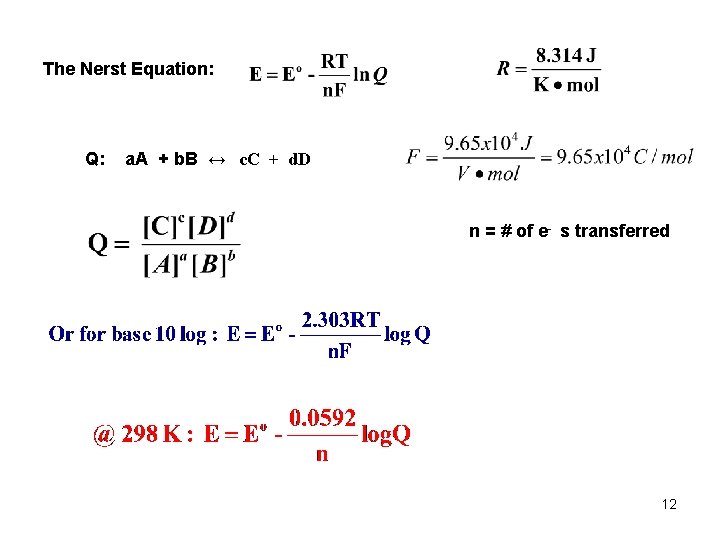
The Nerst Equation: Q: a. A + b. B ↔ c. C + d. D n = # of e- s transferred 12

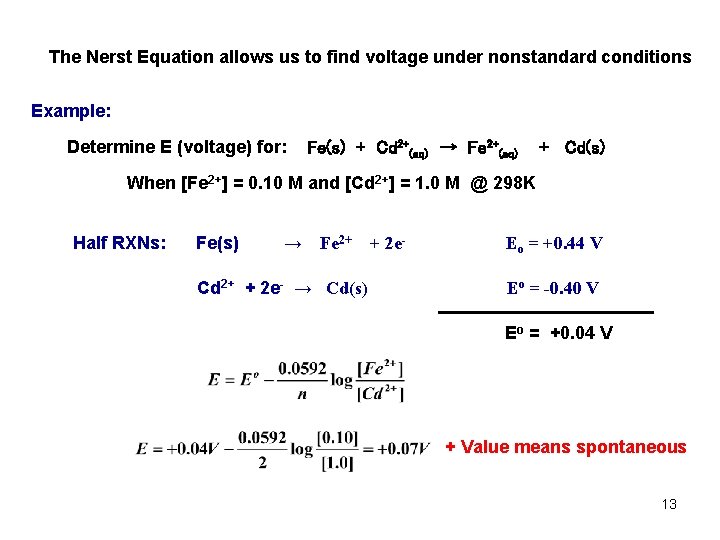
The Nerst Equation allows us to find voltage under nonstandard conditions Example: Determine E (voltage) for: Fe(s) + Cd 2+(aq) → Fe 2+(aq) + Cd(s) When [Fe 2+] = 0. 10 M and [Cd 2+] = 1. 0 M @ 298 K Half RXNs: Fe(s) → Fe 2+ Cd 2+ + 2 e- → Cd(s) + 2 e- Eo = +0. 44 V Eo = -0. 40 V Eo = +0. 04 V + Value means spontaneous 13
![Now try Fe 2 1 0 M and Cd 2 0 01 Now try: [Fe 2+] = 1. 0 M and [Cd 2+] = 0. 01](https://slidetodoc.com/presentation_image/375cd27d6dd48e938de62fa236f43990/image-14.jpg)
Now try: [Fe 2+] = 1. 0 M and [Cd 2+] = 0. 01 M @ 298 K - Value means non spontaneous and will go in opposite direction 14

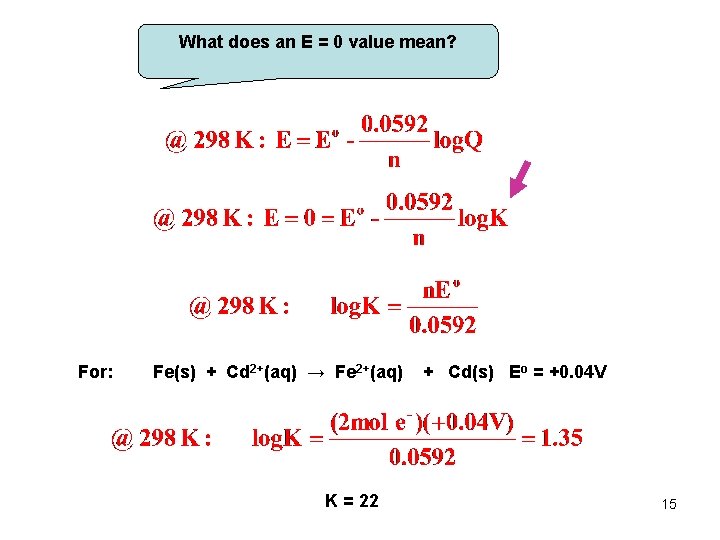
What does an E = 0 value mean? For: Fe(s) + Cd 2+(aq) → Fe 2+(aq) K = 22 + Cd(s) Eo = +0. 04 V 15

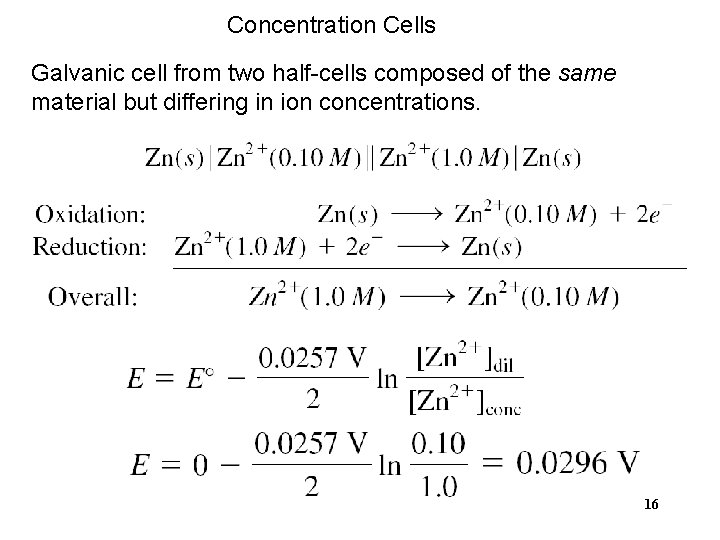
Concentration Cells Galvanic cell from two half-cells composed of the same material but differing in ion concentrations. 16

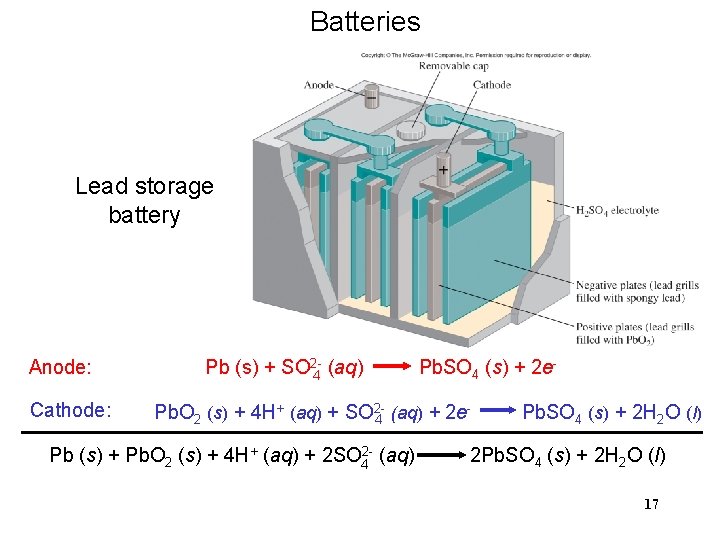
Batteries Lead storage battery Anode: Cathode: Pb (s) + SO 2 -4 (aq) Pb. SO 4 (s) + 2 e- Pb. O 2 (s) + 4 H+ (aq) + SO 24 (aq) + 2 e Pb (s) + Pb. O 2 (s) + 4 H+ (aq) + 2 SO 42 - (aq) Pb. SO 4 (s) + 2 H 2 O (l) 2 Pb. SO 4 (s) + 2 H 2 O (l) 17

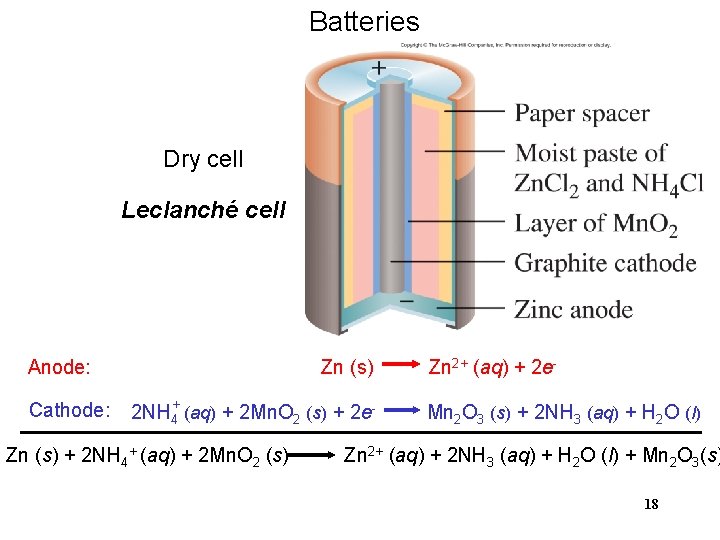
Batteries Dry cell Leclanché cell Anode: Cathode: Zn (s) 2 NH 4+ (aq) + 2 Mn. O 2 (s) + 2 e- Zn (s) + 2 NH 4+ (aq) + 2 Mn. O 2 (s) Zn 2+ (aq) + 2 e. Mn 2 O 3 (s) + 2 NH 3 (aq) + H 2 O (l) Zn 2+ (aq) + 2 NH 3 (aq) + H 2 O (l) + Mn 2 O 3(s) 18

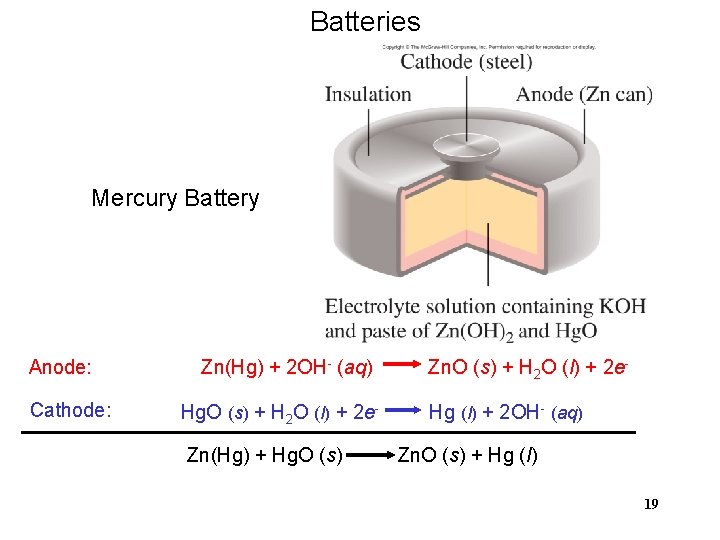
Batteries Mercury Battery Anode: Cathode: Zn(Hg) + 2 OH- (aq) Hg. O (s) + H 2 O (l) + 2 e. Zn(Hg) + Hg. O (s) Zn. O (s) + H 2 O (l) + 2 e. Hg (l) + 2 OH- (aq) Zn. O (s) + Hg (l) 19

Batteries Solid State Lithium Battery 20

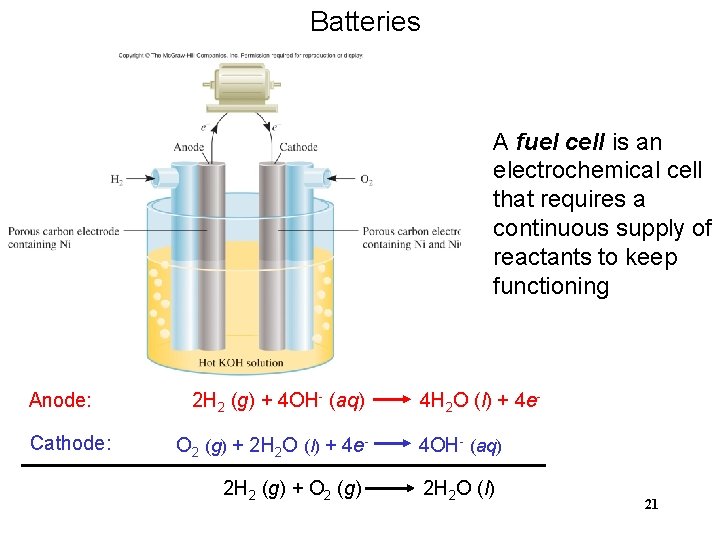
Batteries A fuel cell is an electrochemical cell that requires a continuous supply of reactants to keep functioning Anode: Cathode: 2 H 2 (g) + 4 OH- (aq) O 2 (g) + 2 H 2 O (l) + 4 e 2 H 2 (g) + O 2 (g) 4 H 2 O (l) + 4 e 4 OH- (aq) 2 H 2 O (l) 21

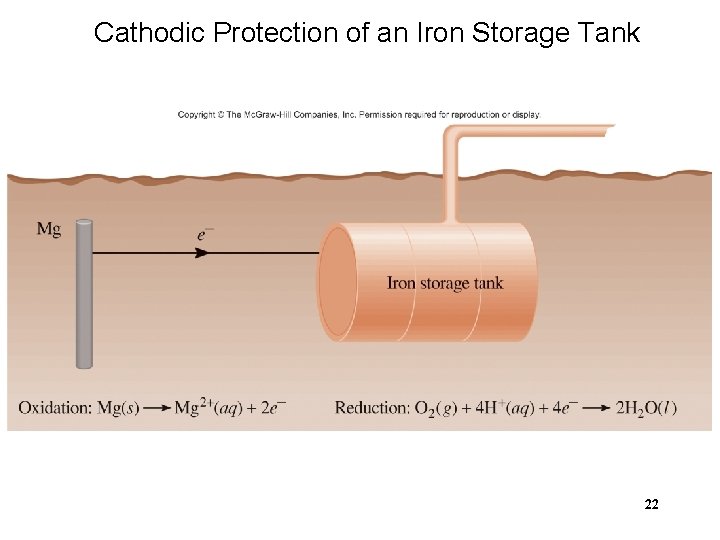
Cathodic Protection of an Iron Storage Tank 22

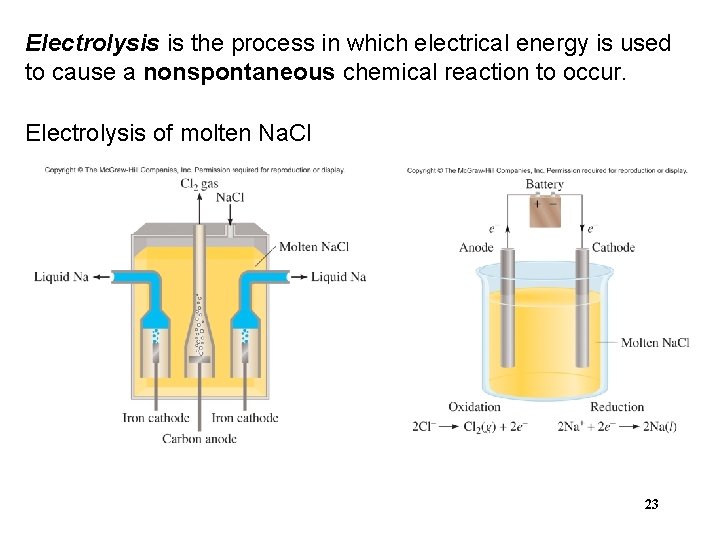
Electrolysis is the process in which electrical energy is used to cause a nonspontaneous chemical reaction to occur. Electrolysis of molten Na. Cl 23