Electrochemistry Redox Oxidation States Voltaic Cells Balancing Equations
Electrochemistry Redox Oxidation States Voltaic Cells Balancing Equations Cell EMF Concentration Cells Nernst Equation Equilibrium Corrosion Batteries Electrolysis 9/26/2020
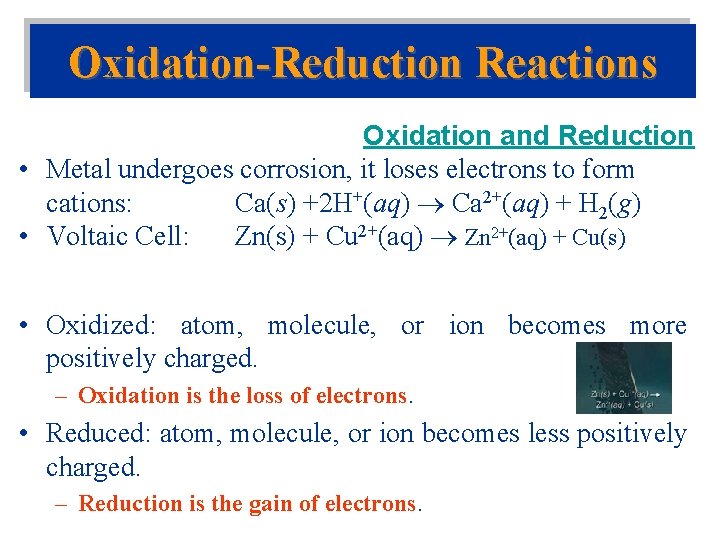
Oxidation-Reduction Reactions Oxidation and Reduction • Metal undergoes corrosion, it loses electrons to form cations: Ca(s) +2 H+(aq) Ca 2+(aq) + H 2(g) • Voltaic Cell: Zn(s) + Cu 2+(aq) Zn 2+(aq) + Cu(s) • Oxidized: atom, molecule, or ion becomes more positively charged. – Oxidation is the loss of electrons. • Reduced: atom, molecule, or ion becomes less positively charged. – Reduction is the gain of electrons.
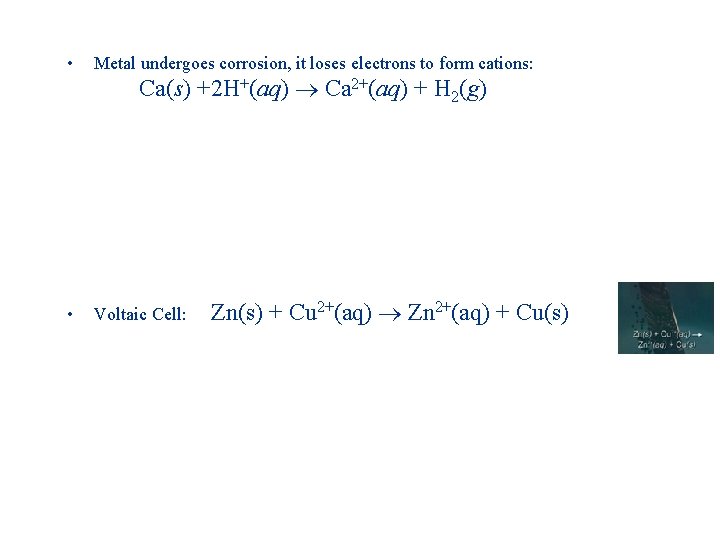
• Metal undergoes corrosion, it loses electrons to form cations: • Voltaic Cell: Ca(s) +2 H+(aq) Ca 2+(aq) + H 2(g) Zn(s) + Cu 2+(aq) Zn 2+(aq) + Cu(s)
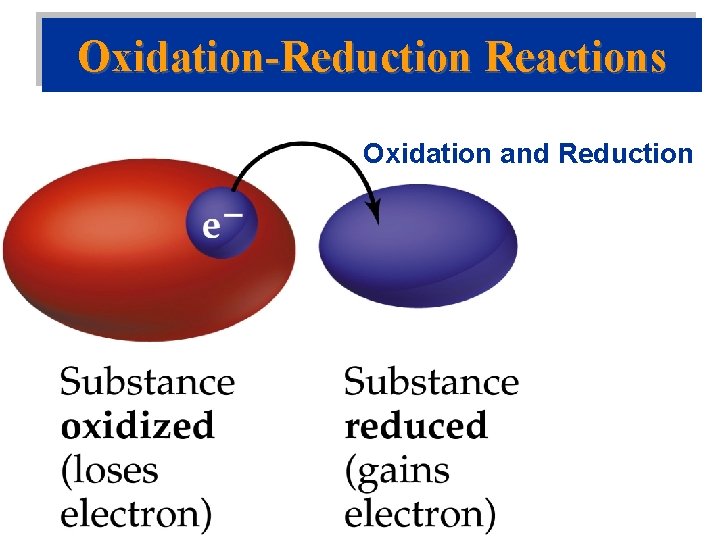
Oxidation-Reduction Reactions Oxidation and Reduction
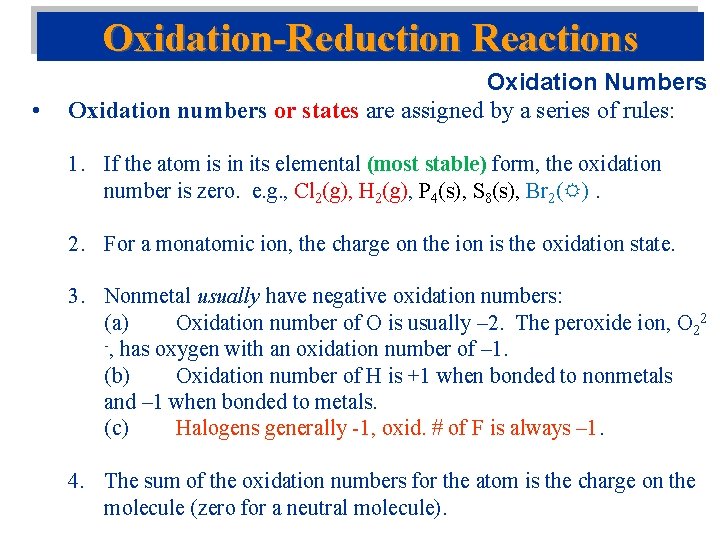
Oxidation-Reduction Reactions • Oxidation Numbers Oxidation numbers or states are assigned by a series of rules: 1. If the atom is in its elemental (most stable) form, the oxidation number is zero. e. g. , Cl 2(g), H 2(g), P 4(s), S 8(s), Br 2( ). 2. For a monatomic ion, the charge on the ion is the oxidation state. 3. Nonmetal usually have negative oxidation numbers: (a) Oxidation number of O is usually – 2. The peroxide ion, O 22 -, has oxygen with an oxidation number of – 1. (b) Oxidation number of H is +1 when bonded to nonmetals and – 1 when bonded to metals. (c) Halogens generally -1, oxid. # of F is always – 1. 4. The sum of the oxidation numbers for the atom is the charge on the molecule (zero for a neutral molecule).
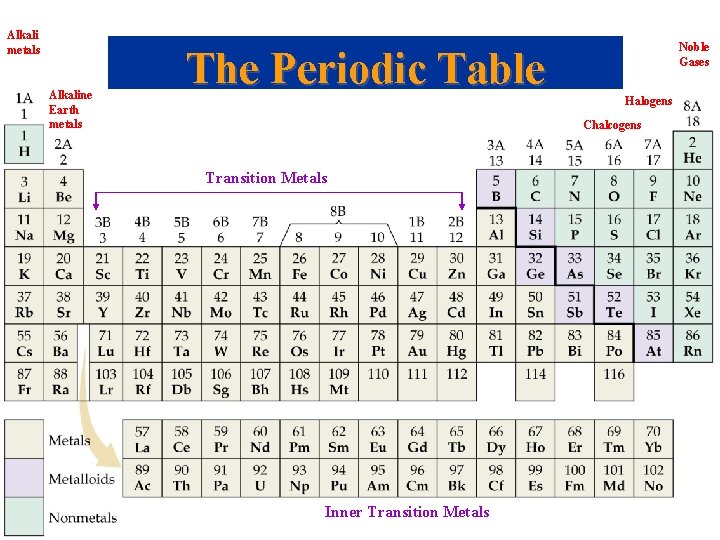
Alkali metals Alkaline Earth metals Noble Gases The Periodic Table Halogens Chalcogens Transition Metals Inner Transition Metals
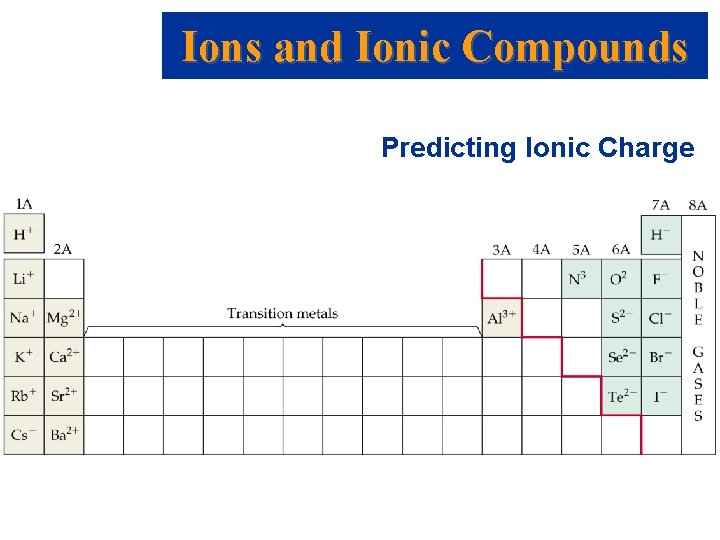
Ions and Ionic Compounds Predicting Ionic Charge
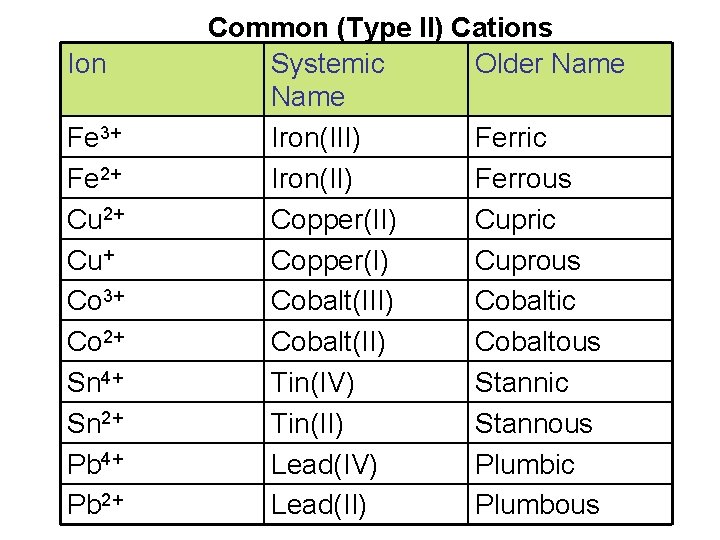
Ion Fe 3+ Fe 2+ Cu+ Co 3+ Co 2+ Sn 4+ Sn 2+ Pb 4+ Pb 2+ Common (Type II) Cations Systemic Older Name Iron(III) Ferric Iron(II) Ferrous Copper(II) Cupric Copper(I) Cuprous Cobalt(III) Cobaltic Cobalt(II) Cobaltous Tin(IV) Stannic Tin(II) Stannous Lead(IV) Plumbic Lead(II) Plumbous
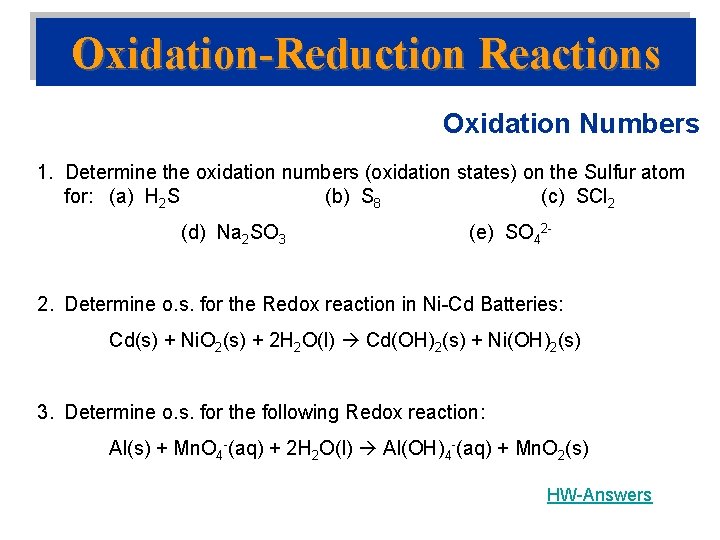
Oxidation-Reduction Reactions Oxidation Numbers 1. Determine the oxidation numbers (oxidation states) on the Sulfur atom for: (a) H 2 S (b) S 8 (c) SCl 2 (d) Na 2 SO 3 (e) SO 42 - 2. Determine o. s. for the Redox reaction in Ni-Cd Batteries: Cd(s) + Ni. O 2(s) + 2 H 2 O(l) Cd(OH)2(s) + Ni(OH)2(s) 3. Determine o. s. for the following Redox reaction: Al(s) + Mn. O 4 -(aq) + 2 H 2 O(l) Al(OH)4 -(aq) + Mn. O 2(s) HW-Answers

Balancing Redox Reactions • Law of conservation of mass: the amount of each element present at the beginning of the reaction must be present at the end. • Conservation of charge: electrons are not lost in a chemical reaction. Half Reactions • Half-reactions are a convenient way of separating oxidation and reduction reactions.
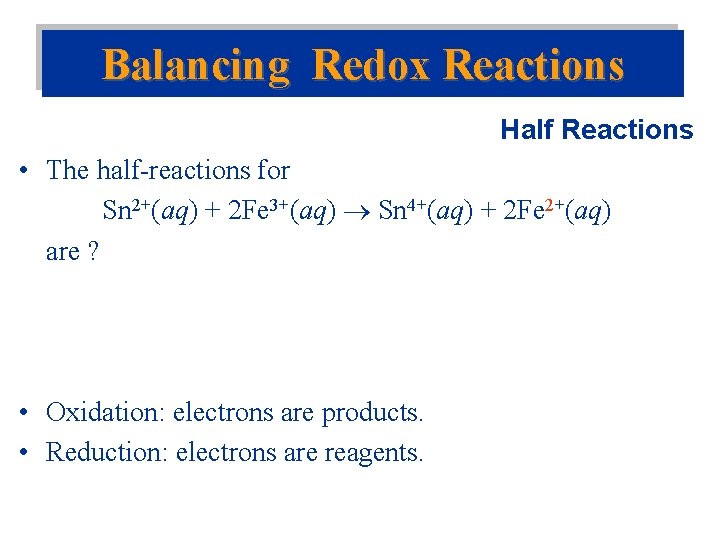
Balancing Redox Reactions Half Reactions • The half-reactions for Sn 2+(aq) + 2 Fe 3+(aq) Sn 4+(aq) + 2 Fe 2+(aq) are ? • Oxidation: electrons are products. • Reduction: electrons are reagents.
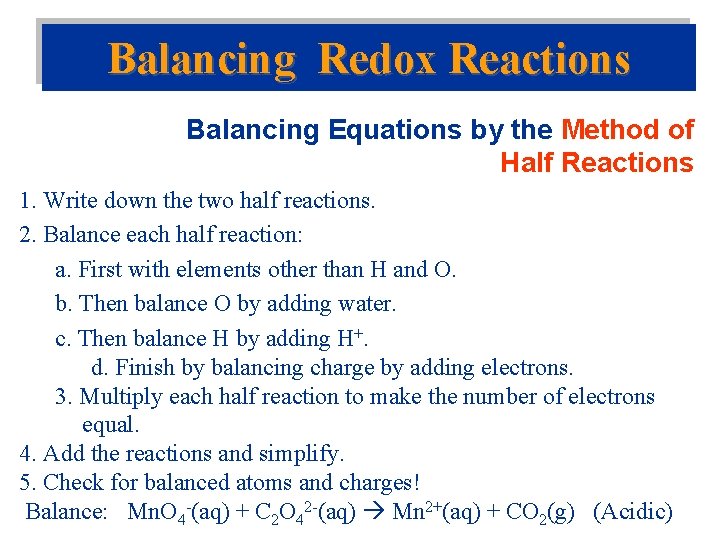
Balancing Redox Reactions Balancing Equations by the Method of Half Reactions 1. Write down the two half reactions. 2. Balance each half reaction: a. First with elements other than H and O. b. Then balance O by adding water. c. Then balance H by adding H+. d. Finish by balancing charge by adding electrons. 3. Multiply each half reaction to make the number of electrons equal. 4. Add the reactions and simplify. 5. Check for balanced atoms and charges! Balance: Mn. O 4 -(aq) + C 2 O 42 -(aq) Mn 2+(aq) + CO 2(g) (Acidic)

Balance: Mn. O 4 - (aq) + C 2 O 42 -(aq) Mn 2+(aq) + CO 2(g) (Acidic)

16 H+(aq) + 2 Mn. O 4 - (aq) + 5 C 2 O 42 -(aq) 2 Mn 2+(aq) + 10 CO 2(g) + 8 H 2 O(l) (Acidic)
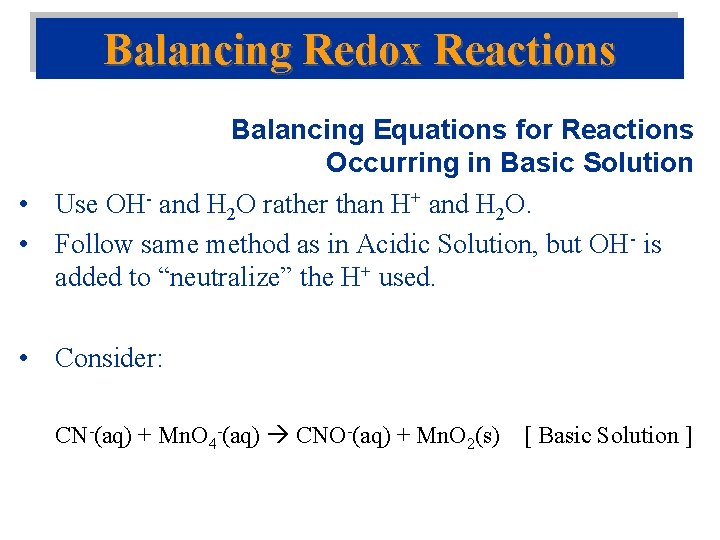
Balancing Redox Reactions Balancing Equations for Reactions Occurring in Basic Solution • Use OH- and H 2 O rather than H+ and H 2 O. • Follow same method as in Acidic Solution, but OH- is added to “neutralize” the H+ used. • Consider: CN-(aq) + Mn. O 4 -(aq) CNO-(aq) + Mn. O 2(s) [ Basic Solution ]

CN-(aq) + Mn. O 4 -(aq) CNO-(aq) + Mn. O 2(s) [ Basic Solution from Acidic ] Solution Key
Voltaic Cells • Energy released in a spontaneous redox reaction is used to perform electrical work. • Voltaic or galvanic cells are devices in which electron transfer occurs via an external circuit. • Voltaic cells are spontaneous. • If a strip of Zn is placed in a solution of Cu. SO 4, Cu is deposited on the Zn and the Zn dissolves by forming Zn 2+. Zn(s) + Cu 2+(aq) Zn 2+(aq) + Cu(s)
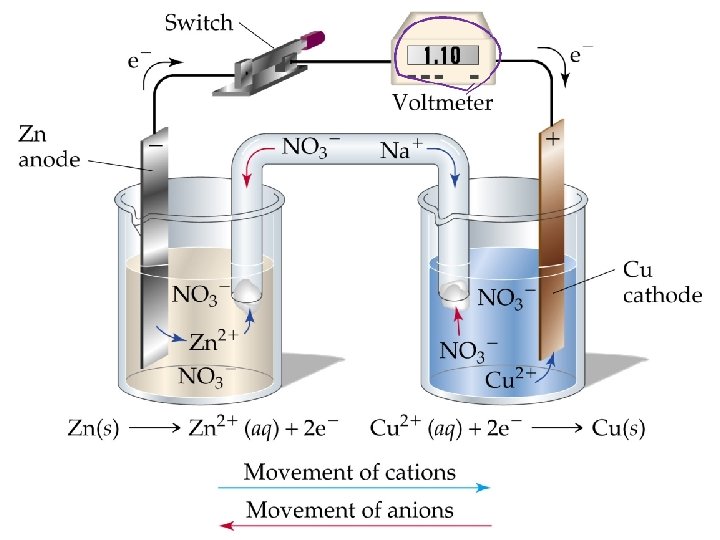
Cell EMF • Flow of electrons from anode to cathode is spontaneous. • Electrons flow from anode to cathode because the cathode has a lower electrical potential energy than the anode. • Potential difference: difference in electrical potential. Measured in volts. • One volt is the potential difference required to impart one joule of energy to a charge of one coulomb:
Cell EMF • Electromotive force (emf) is the force required to push electrons through the external circuit. • Cell potential: Ecell is the emf of a cell. • For 1 M solutions at 25 C (standard conditions), the standard emf (standard cell potential) is called E cell.
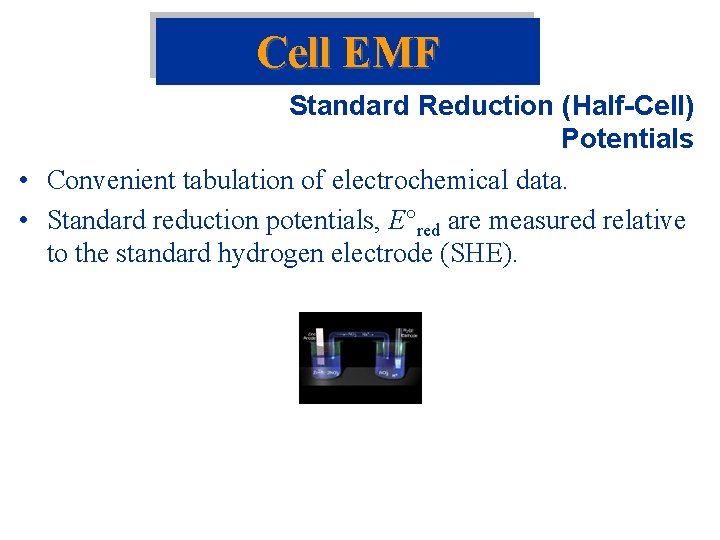
Cell EMF Standard Reduction (Half-Cell) Potentials • Convenient tabulation of electrochemical data. • Standard reduction potentials, E red are measured relative to the standard hydrogen electrode (SHE).
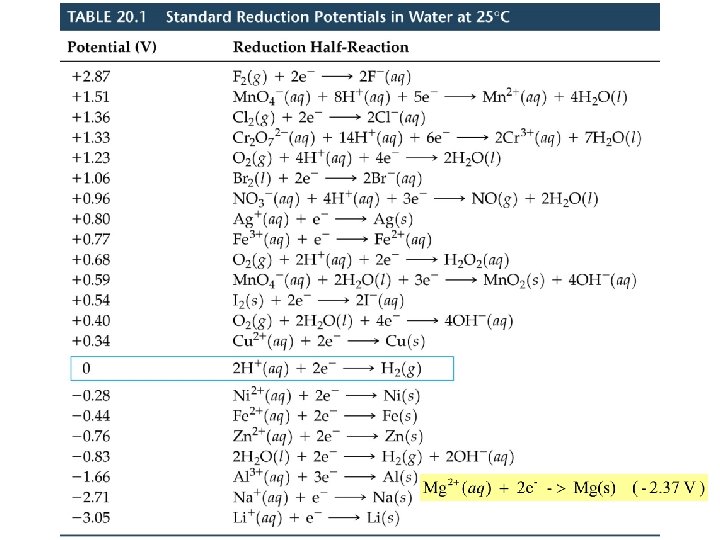
Cell EMF Standard Reduction (Half-Cell) Potentials • Reactions with E red < 0 are spontaneous oxidations relative to the SHE. • The larger the difference between E red values, the larger E cell. • In a voltaic (galvanic) cell (spontaneous) E red(cathode) is more positive than E red(anode). • Calculate Eocell for: 2 Al(s) + 3 I 2(s) 2 Al 3+(aq) + 6 I-(aq)

Calculate Eocell for: 2 Al(s) + 3 I 2(s) 2 Al 3+(aq) + 6 I-(aq)

Calculate Eocell for: 2 Al(s) + 3 I 2(s) 2 Al 3+(aq) + 6 I-(aq)
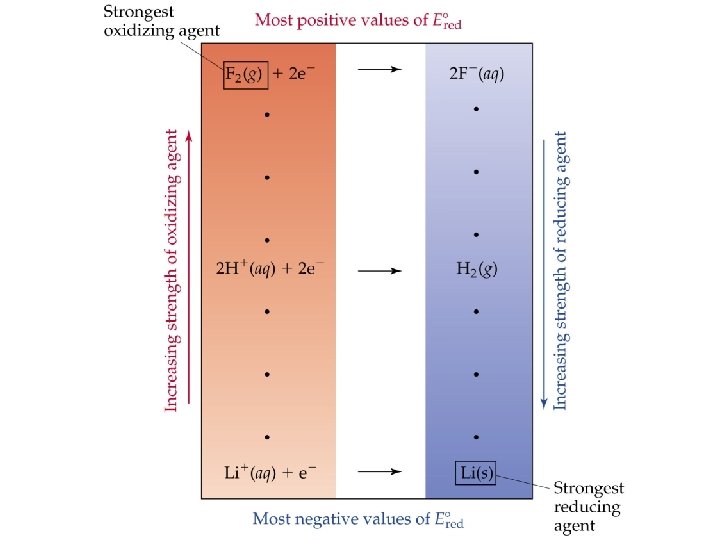
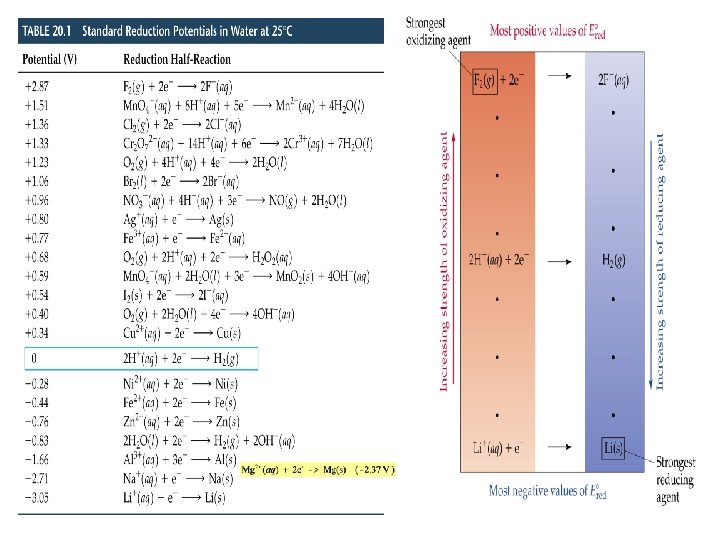
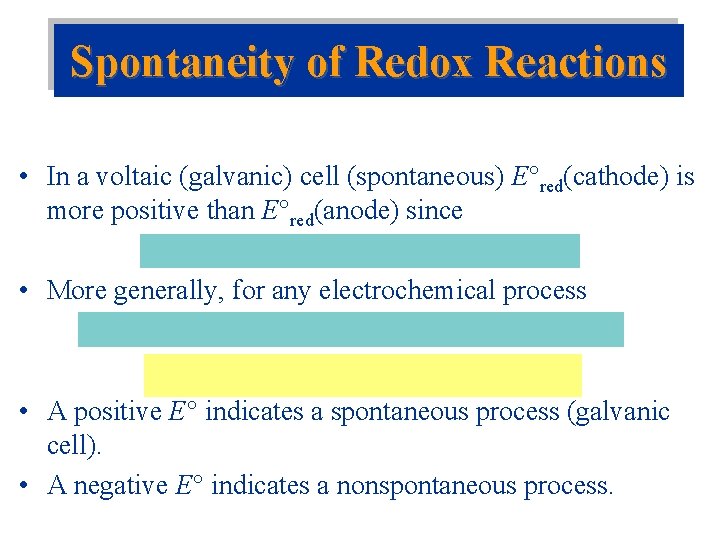
Spontaneity of Redox Reactions • In a voltaic (galvanic) cell (spontaneous) E red(cathode) is more positive than E red(anode) since • More generally, for any electrochemical process • A positive E indicates a spontaneous process (galvanic cell). • A negative E indicates a nonspontaneous process.
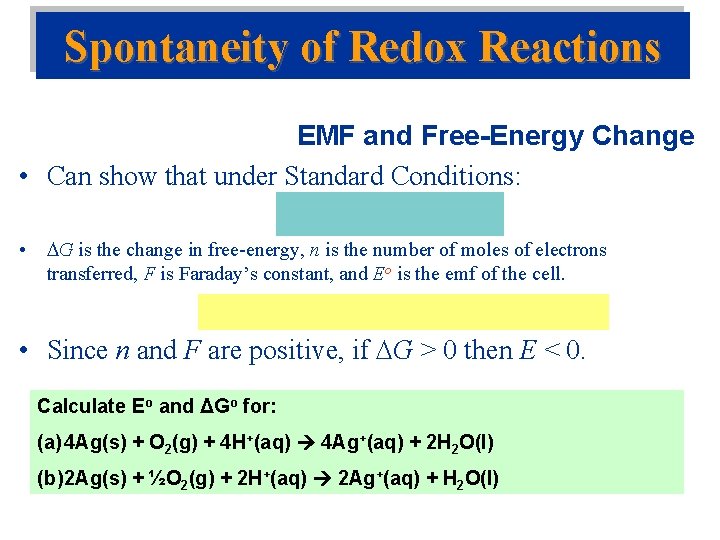
Spontaneity of Redox Reactions EMF and Free-Energy Change • Can show that under Standard Conditions: • G is the change in free-energy, n is the number of moles of electrons transferred, F is Faraday’s constant, and Eo is the emf of the cell. • Since n and F are positive, if G > 0 then E < 0. Calculate Eo and ΔGo for: (a) 4 Ag(s) + O 2(g) + 4 H+(aq) 4 Ag+(aq) + 2 H 2 O(l) (b) 2 Ag(s) + ½O 2(g) + 2 H+(aq) 2 Ag+(aq) + H 2 O(l)

Calculate Eo and ΔGo for: (a) 4 Ag(s) + O 2(g) + 4 H+(aq) 4 Ag+(aq) + 2 H 2 O(l) (b) 2 Ag(s) + ½O 2(g) + 2 H+(aq) 2 Ag+(aq) + H 2 O(l)

Calculate Eo and ΔGo for: (a) 4 Ag(s) + O 2(g) + 4 H+(aq) 4 Ag+(aq) + 2 H 2 O(l) (b) 2 Ag(s) + ½O 2(g) + 2 H+(aq) 2 Ag+(aq) + H 2 O(l)

Calculate Eo and ΔGo for: (a) 4 Ag(s) + O 2(g) + 4 H+(aq) 4 Ag+(aq) + 2 H 2 O(l) (b) 2 Ag(s) + ½O 2(g) + 2 H+(aq) 2 Ag+(aq) + H 2 O(l)
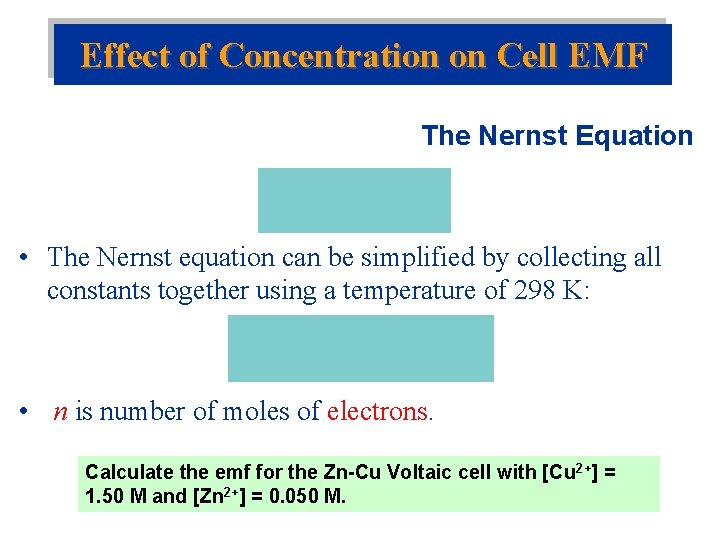
Effect of Concentration on Cell EMF The Nernst Equation • The Nernst equation can be simplified by collecting all constants together using a temperature of 298 K: • n is number of moles of electrons. Calculate the emf for the Zn-Cu Voltaic cell with [Cu 2+] = 1. 50 M and [Zn 2+] = 0. 050 M.
![Calculate the emf for the Zn-Cu Voltaic cell with [Cu 2+] = 1. 50 Calculate the emf for the Zn-Cu Voltaic cell with [Cu 2+] = 1. 50](http://slidetodoc.com/presentation_image/7b759b43754a1e44c1d2fa7422105905/image-34.jpg)
Calculate the emf for the Zn-Cu Voltaic cell with [Cu 2+] = 1. 50 M and [Zn 2+] = 0. 050 M. Zn(s) + Cu 2+(aq) Zn 2+(aq) + Cu(s)
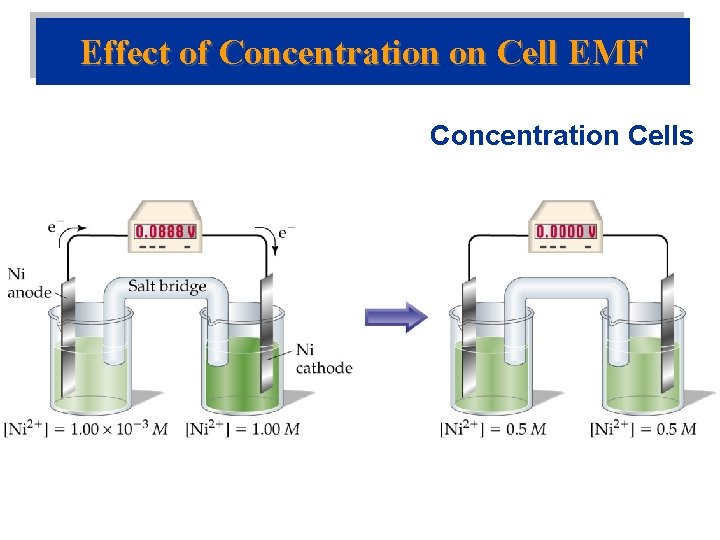
Effect of Concentration on Cell EMF Concentration Cells
Effect of Concentration on Cell EMF and Chemical Equilibrium • A system is at equilibrium when G = 0. • From the Nernst equation, at equilibrium and 298 K (E = 0 V and Q = Keq):
Batteries • A battery is a self-contained electrochemical power source with one or more voltaic cell. • When the cells are connected in series, greater emfs can be achieved.

Batteries Lead-Acid Battery • A 12 V car battery consists of 6 cathode/anode pairs each producing 2 V. • Cathode: Pb. O 2 on a metal grid in sulfuric acid: Pb. O 2(s) + SO 42 -(aq) + 4 H+(aq) + 2 e- Pb. SO 4(s) + 2 H 2 O(l) • Anode: Pb(s) + SO 42 -(aq) Pb. SO 4(s) + 2 e-
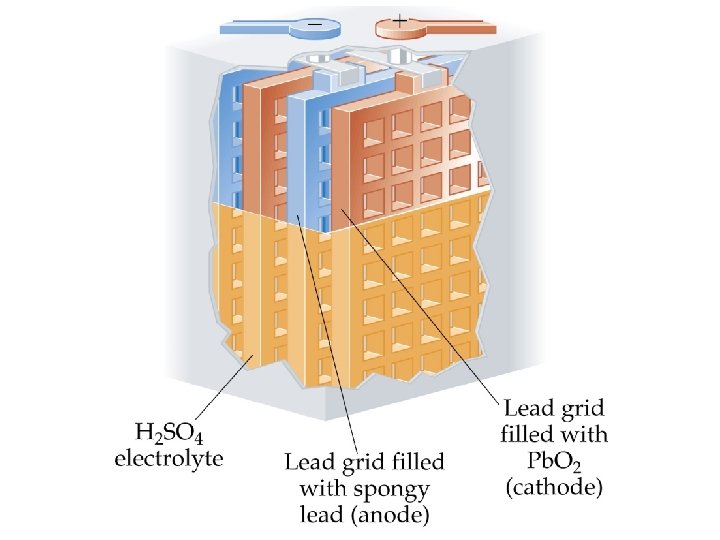
Batteries Lead-Acid Battery • The overall electrochemical reaction is Pb. O 2(s) + Pb(s) + 2 SO 42 -(aq) + 4 H+(aq) 2 Pb. SO 4(s) + 2 H 2 O(l) for which E cell = E red(cathode) - E red(anode) = (+1. 685 V) - (-0. 356 V) = +2. 041 V. • Wood or glass-fiber spacers are used to prevent the electrodes from touching.
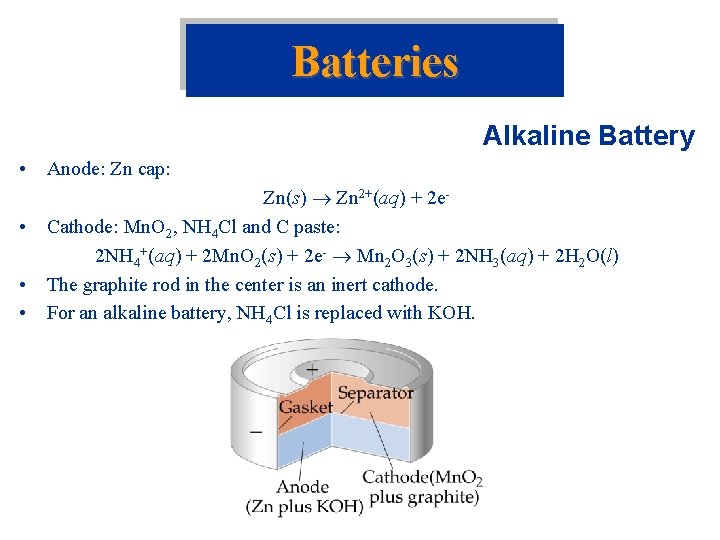
Batteries Alkaline Battery • Anode: Zn cap: • • • Zn(s) Zn 2+(aq) + 2 e. Cathode: Mn. O 2, NH 4 Cl and C paste: 2 NH 4+(aq) + 2 Mn. O 2(s) + 2 e- Mn 2 O 3(s) + 2 NH 3(aq) + 2 H 2 O(l) The graphite rod in the center is an inert cathode. For an alkaline battery, NH 4 Cl is replaced with KOH.
Batteries • • Fuel Cells Direct production of electricity from fuels occurs in a fuel cell. On Apollo moon flights, the H 2 -O 2 fuel cell was the primary source of electricity. Cathode: reduction of oxygen: 2 H 2 O(l) + O 2(g) + 4 e- 4 OH-(aq) Anode: 2 H 2(g) + 4 OH-(aq) 4 H 2 O(l) + 4 e-
Corrosion • • • Corrosion of Iron Since E red(Fe 2+) < E red(O 2) iron can be oxidized by oxygen. Cathode: O 2(g) + 4 H+(aq) + 4 e- 2 H 2 O(l). Anode: Fe(s) Fe 2+(aq) + 2 e-. Dissolved oxygen in water usually causes the oxidation of iron. Fe 2+ initially formed can be further oxidized to Fe 3+ which forms rust, Fe 2 O 3. x. H 2 O(s).
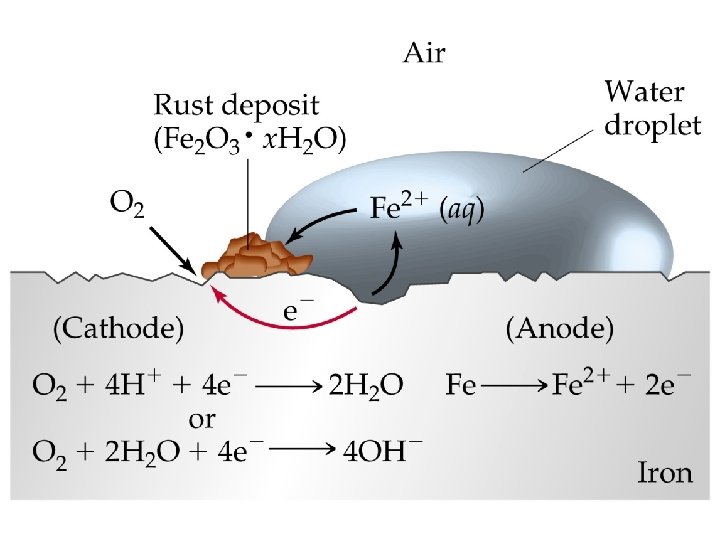

Corrosion of Iron • Oxidation occurs at the site with the greatest concentration of O 2. Preventing Corrosion of Iron • Corrosion can be prevented by coating the iron with paint or another metal. • Galvanized iron is coated with a thin layer of zinc.
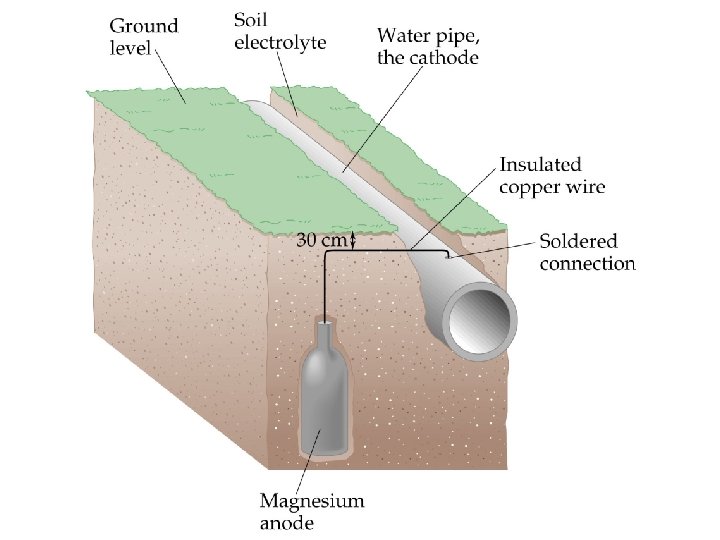
Corrosion Preventing Corrosion of Iron • To protect underground pipelines, a sacrificial anode is added. • The water pipe is turned into the cathode and an active metal is used as the anode. • Often, Mg is used as the sacrificial anode: Mg 2+(aq) +2 e- Mg(s), E red = -2. 37 V Fe 2+(aq) + 2 e- Fe(s), E red = -0. 44 V
Electrolysis of Aqueous Solutions – In electrolytic cells the anode is positive and the cathode is negative. (In galvanic cells the anode is negative and the cathode is positive. )
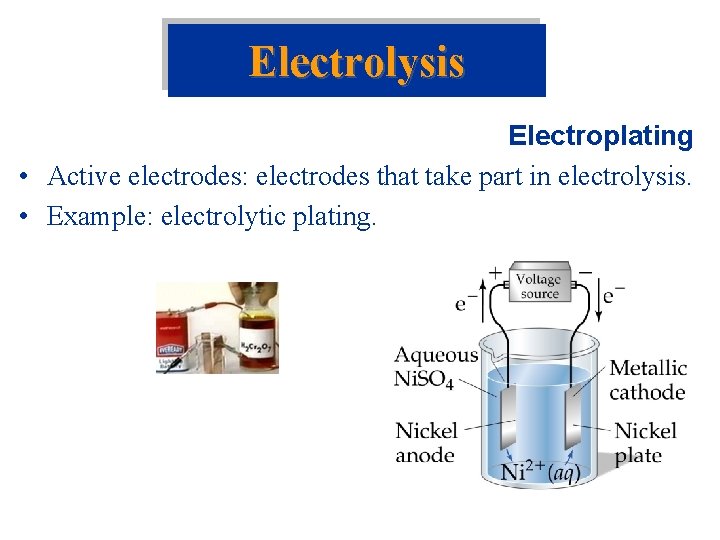
Electrolysis Electroplating • Active electrodes: electrodes that take part in electrolysis. • Example: electrolytic plating.
Electrochemistry Oxidation is the loss of electrons Redox Oxidation States Voltaic Cells Balancing Equations Cell EMF Concentration Cells Nernst Equation Equilibrium Corrosion Batteries Electrolysis
- Slides: 51