Types of Electrolytic Cells Diaphragm Cell Mercury Cell







- Slides: 12

Types of Electrolytic Cells ØDiaphragm Cell ØMercury Cell ØMembrane Cell

DIAPHRAGM CELL ØDiaphragm usually made of asbestos fibers to separate anode and cathode. ØAnode usually of graphite and cathode of cast iron.

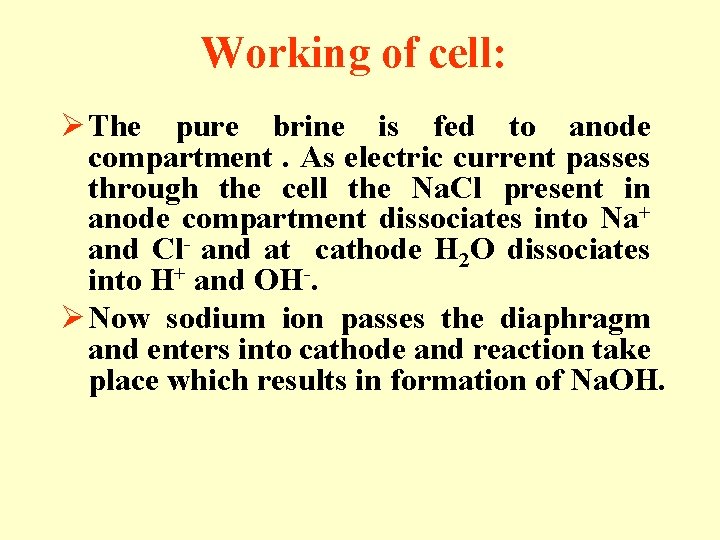
Working of cell: Ø The pure brine is fed to anode compartment. As electric current passes through the cell the Na. Cl present in anode compartment dissociates into Na+ and Cl- and at cathode H 2 O dissociates into H+ and OH-. Ø Now sodium ion passes the diaphragm and enters into cathode and reaction take place which results in formation of Na. OH.

Ø The diaphragm cell can run on fairly dilute impure brine and this gives the product concentration 11% along with Na. Cl. So to increase the concentration from 11% to 50% huge amount of water should be evaporated.

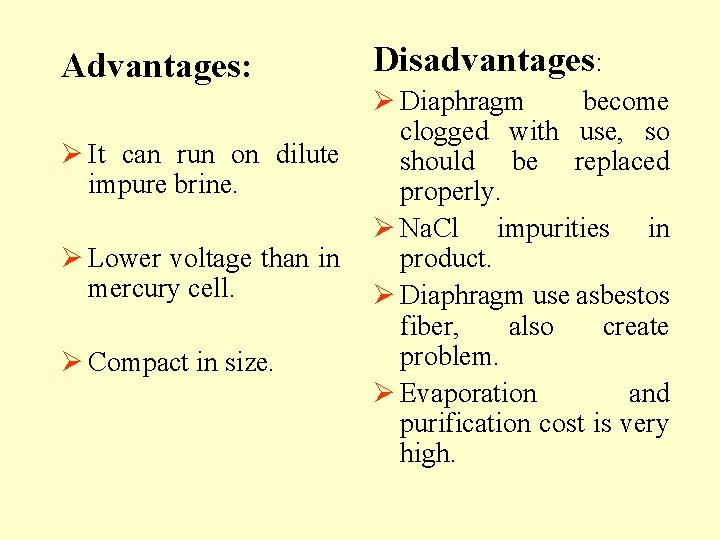
Advantages: Ø It can run on dilute impure brine. Ø Lower voltage than in mercury cell. Ø Compact in size. Disadvantages: Ø Diaphragm become clogged with use, so should be replaced properly. Ø Na. Cl impurities in product. Ø Diaphragm use asbestos fiber, also create problem. Ø Evaporation and purification cost is very high.

MERCURY CELL Ø The anode is either graphite or titanium and cathode is flowing pool of mercury. Ø The electrolysis produce a mercury-sodium alloy which is not decomposed by brine. Ø From anode, chlorine gas is released and amalgam is decomposed in denuding tower.

ØIn denuding tower, water is sprayed from bottom and Na. Hg from top in counter current so we will get maximum yield of Na. OH. ØThe products are extremely pure. The chlorine, along with a little oxygen, generally can be used without further purification.

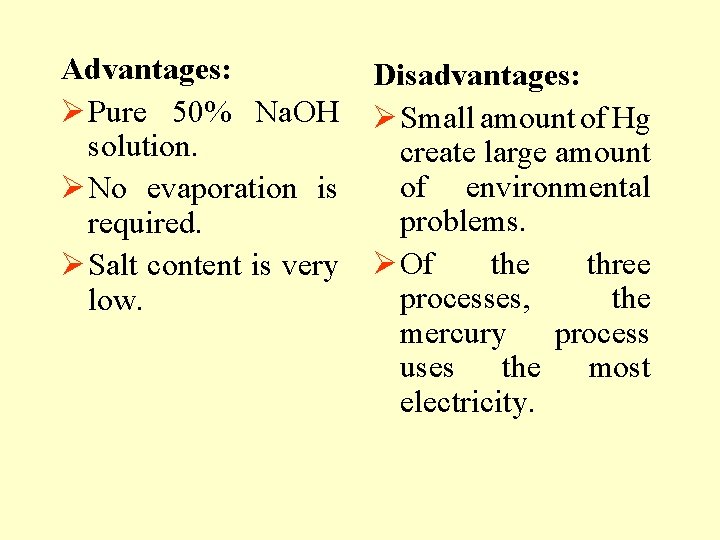
Advantages: Ø Pure 50% Na. OH solution. Ø No evaporation is required. Ø Salt content is very low. Disadvantages: Ø Small amount of Hg create large amount of environmental problems. Ø Of the three processes, the mercury process uses the most electricity.

MEMBRANE CELL Ø Membrane Cells uses a semi permeable membrane to separate anode and cathode. Ø Generally Persulfonic acid polymer membranes are used. Ø The purpose of membrane to allow only sodium ions and exclude OH- and Cl- ions from anode chamber. Ø Membrane cell operates using a more concentrated brine and produce purer and near 30% of Na. OH.

ØSaturated brine solution contains 28% Na. Cl with Mg+2 and Ca+2 ions that clogs the membrane and so salt impurities are removed before charging into cell.

Advantages: Ø Na. OH of 32 -35% concentrate so less water to be evaporated. Ø No release of harmful materials. Disadvantages: Ø High investment cost. Ø Short membrane life. Ø High purity brine require. Ø Large space compare to diaphragm cell.

Comparison of Cells:
 Advantages of diaphragm cell
Advantages of diaphragm cell Voltaic vs electrolytic cells
Voltaic vs electrolytic cells Applications of electrolytic cells
Applications of electrolytic cells Khan academy electrolysis
Khan academy electrolysis Renocell
Renocell Cell notation
Cell notation Applications of electrolytic cell
Applications of electrolytic cell Smt for electrolytic cell
Smt for electrolytic cell Anode positive or negative
Anode positive or negative Electrolytic cell lab
Electrolytic cell lab Electrolytic cell notation
Electrolytic cell notation Cathode
Cathode Olfactory groove keros classification
Olfactory groove keros classification