Chemistry 30 Electrochemical Changes Electrolytic Cells Electrolytic Cells






- Slides: 12

Chemistry 30 Electrochemical Changes Electrolytic Cells

Electrolytic Cells • Opposite of electrochemical cells • Non-spont redox reaction forced to occur by connecting electrodes to a power source • Electrical energy is thereby converted to chemical energy • Called electrolysis

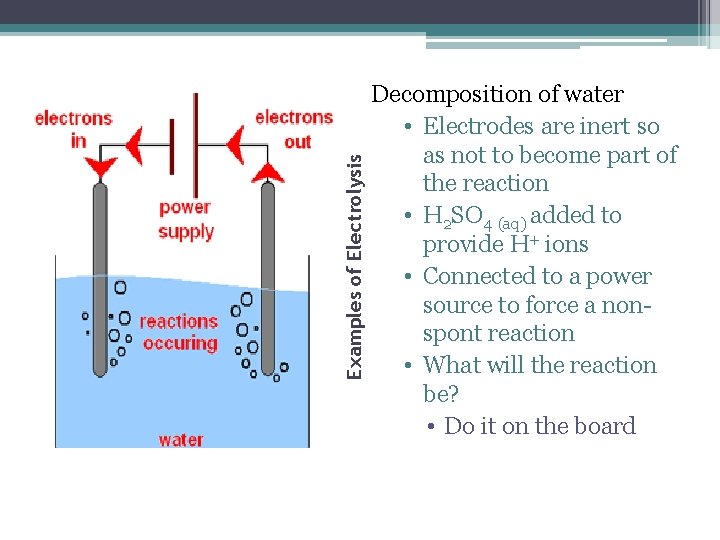
Examples of Electrolysis Decomposition of water • Electrodes are inert so as not to become part of the reaction • H 2 SO 4 (aq) added to provide H+ ions • Connected to a power source to force a nonspont reaction • What will the reaction be? • Do it on the board

Observations • At a low voltage, nothing happens ▫ Until the voltage in the circuit is above 1. 23 V • Bubbles of gas will form at each electrode

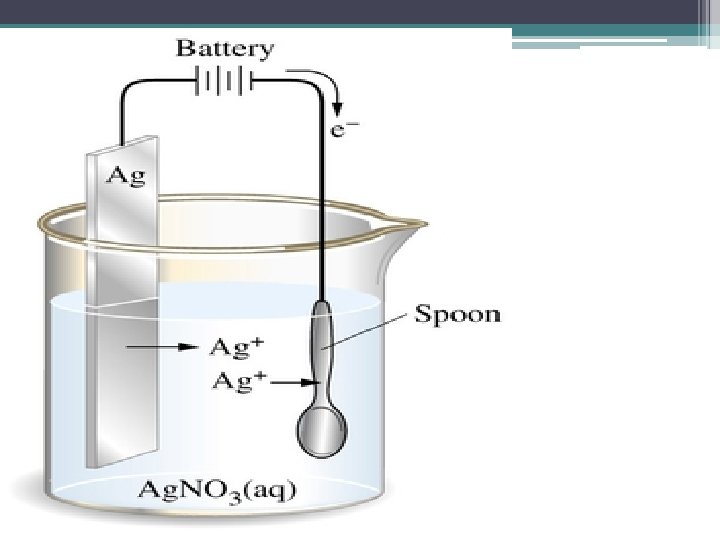
Electroplating • Coating an object with a thin layer of metal to prevent corrosion because the plated metal is a weak RA ▫ So it is oxidized easily • Example ▫ ▫ Silver plating a copper spoon Silver ions are to be converted to Ag(s) in the brine What would this reaction look like? Voltage? What other reasons would there be for doing this?


Commercial Uses • Electrolytic ▫ Substance of value is the one produced from the reaction. �Eg. Plating • Electrochemical ▫ Valued substance = the electricity produced �Eg. Lead storage batteries �Actually used both technologies. How?

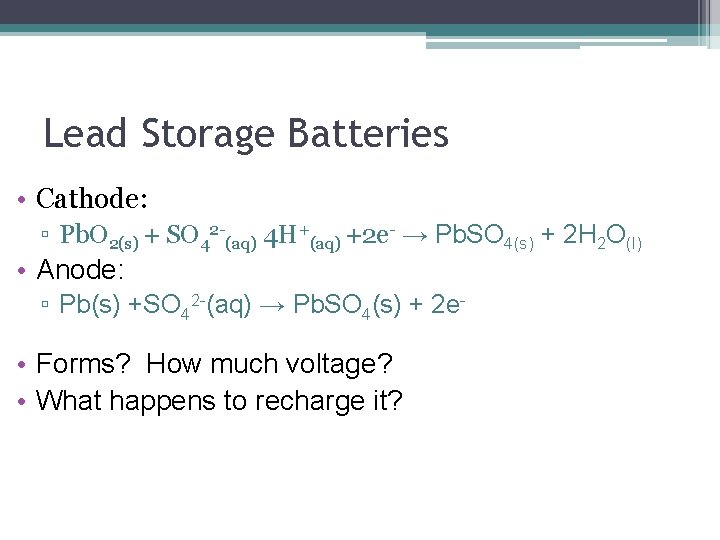
Lead Storage Batteries • Cathode: ▫ Pb. O 2(s) + SO 42 -(aq) 4 H+(aq) +2 e- → Pb. SO 4(s) + 2 H 2 O(l) • Anode: ▫ Pb(s) +SO 42 -(aq) → Pb. SO 4(s) + 2 e- • Forms? How much voltage? • What happens to recharge it?

Electrolytic Cell Notes 1) Redox reaction principles are the same as for electrochemical except that energy must be added for the reaction to occur. ▫ ▫ Half reactions Oxidation at anode, reduction at cathode 2) You still must list your entities and find the SOA and SRA to find which reaction will most likely happen.

Electrolytic Cell Notes 3) You still use the data booklet table, but there are some exceptions. ▫ ▫ ▫ Water should oxidize before Cl-, but if Cl- ions are in aqueous solution, Cl 2(g) is produced before O 2(g) This is called half cell over potential Not covered, we just use the table

Example #2 SNAP Pg 331 • Two graphite electrodes were immersed in a 1. 00 mol/L solution of copper (2) sulfate. Establish the preferential half-reaction for each electrode, the net reaction and the minimum voltage required for electrolysis?

Assignment • Reading ▫ Nelson Pages 639 -651 • Questions ▫ SNAP Pages 331 -332 #’s 1 -6 ▫ Nelson Page 651 #’s 4, 9