Unit 6 Redox Electrochemical Cells Electrochemical Cells An













- Slides: 19

Unit 6: Redox Electrochemical Cells

Electrochemical Cells • An electrochemical cell uses energy released from a spontaneous redox reaction to generate an electric current. • The current is derived from the flow of electrons through metal, called metallic conduction, and the movements of ions in solution, called electrolytic conduction. • A battery consists of a single electrochemical cell or a number of cells connected in series.

How Electrochemical Cells Work • When a piece of zinc metal is placed in an aqueous solution of copper(II) sulfate, a rapid redox reaction takes place. Electrons are transferred from the zinc metal to the copper ions. The reaction is spontaneous since zinc oxidizes more readily than copper. It appears above copper in the activity series: • Zn(s) + Cu 2+(aq) Zn 2+(aq) + Cu(s) Oxidation: Reduction:

• The electrical circuit of an electrochemical cell consists of two parts, an external circuit and an internal circuit.

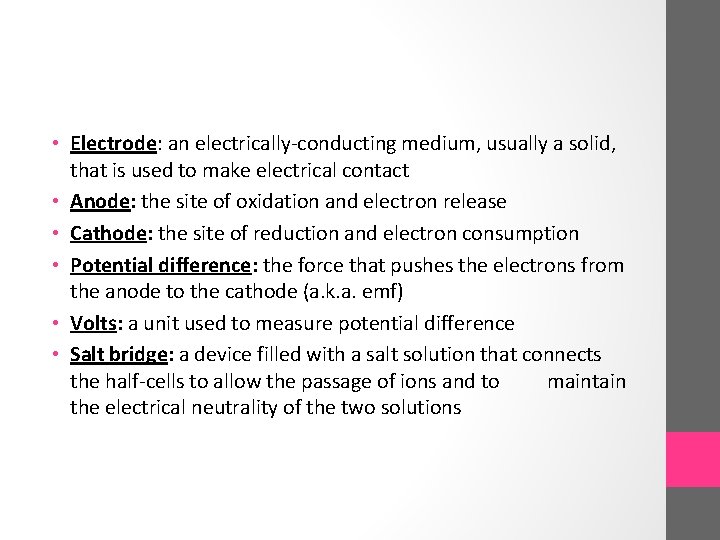
• Electrode: an electrically-conducting medium, usually a solid, that is used to make electrical contact • Anode: the site of oxidation and electron release • Cathode: the site of reduction and electron consumption • Potential difference: the force that pushes the electrons from the anode to the cathode (a. k. a. emf) • Volts: a unit used to measure potential difference • Salt bridge: a device filled with a salt solution that connects the half-cells to allow the passage of ions and to maintain the electrical neutrality of the two solutions

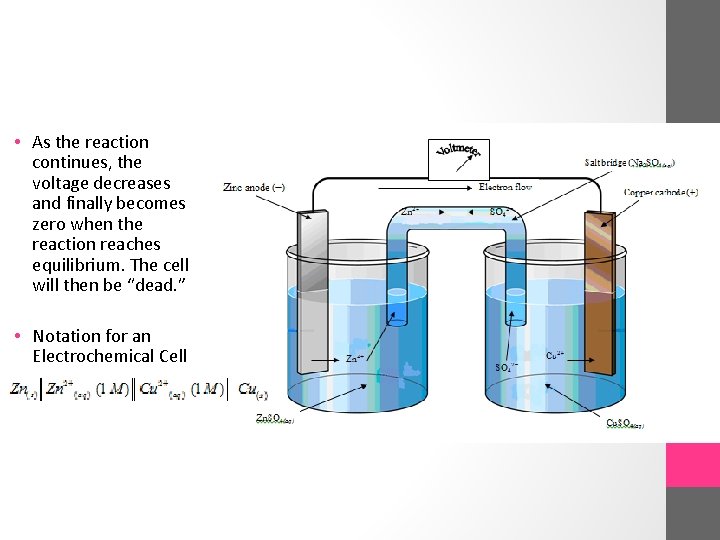
• As the reaction continues, the voltage decreases and finally becomes zero when the reaction reaches equilibrium. The cell will then be “dead. ” • Notation for an Electrochemical Cell

Zn|Zn 2+||Cu 2+|Cu • By convention the anode appears on the left, the cathode on the right. The single vertical lines indicate the contact boundaries between phases in each half-cell. The double vertical lines represent the salt bridge or separator. An electrochemical cell is referred to as a standard cell when the reactant and product ions or molecules are present in concentrations of 1. 0 M.

• https: //www. khanacademy. org/science/chemistry/oxidationreduction/batter-galvanic-voltaic-cell/v/galvanic-cell-voltaiccell

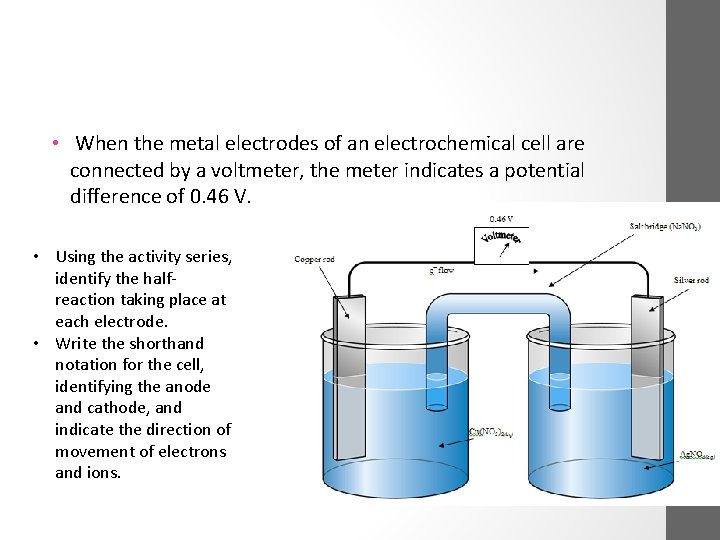
• When the metal electrodes of an electrochemical cell are connected by a voltmeter, the meter indicates a potential difference of 0. 46 V. • Using the activity series, identify the halfreaction taking place at each electrode. • Write the shorthand notation for the cell, identifying the anode and cathode, and indicate the direction of movement of electrons and ions.

Standard Electrode Potentials • An almost unlimited number of half-cells can be constructed and combined into electrochemical cells. However, the reduction potential of each half-cell, the tendency of its ions and molecules to gain electrons, will be different. As a result, the electrodes will have different electrical potentials. • Unfortunately we cannot measure the electrical potential of one half-cell. We can only measure the voltage difference, or emf, between two half-cells by combining them into an electrochemical cell. For example, a cell voltage of 1. 10 V in the standard zinc-copper ion cell tells us that the reduction potential below zinc in the activity series. It also means that the oxidation potential (the tendency to lose electrons) is 1. 10 V greater for zinc than copper.

Standard Half-cell Potentials • The standard hydrogen electrode has been assigned a standard potential (E °) of 0. 00 V. • 2 H+(aq) + 2 e− H 2(g) E ° = 0. 00 V 1 M 100 k. Pa T = 25°C • This electrode is then used as a reference to assign potentials to other half-cells. The measured electromotive force of a standard electrochemical cell containing a hydrogen half-cell is the standard potential (E °) of the second half-cell.

• The standard reduction potential of a half-cell is a measure of the tendency to gain electrons from the hydrogen half-cell. • Ions or molecules with negative reduction potentials gain electrons less easily than hydrogen ions. Those with positive values gain electrons more easily than hydrogen ions. • Lithium ions in aqueous solution have the weakest tendency to gain electrons (E ° = − 3. 05 V); molecular fluorine has the greatest potential to gain electrons (E ° = +2. 87 V). •

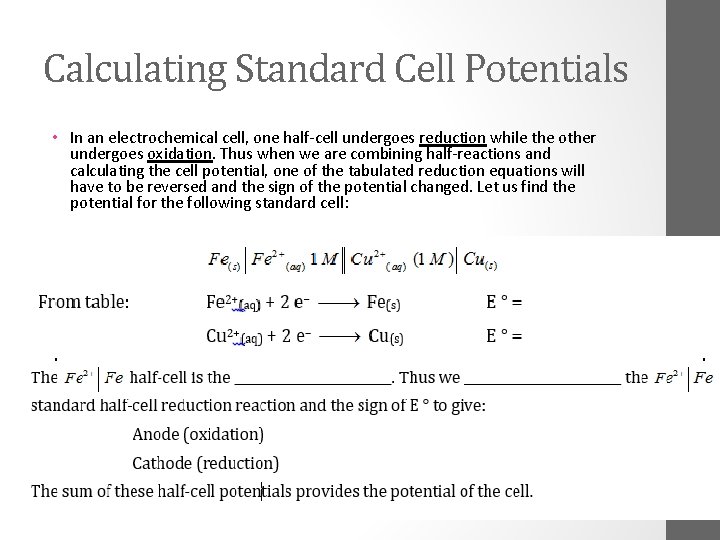
Calculating Standard Cell Potentials • In an electrochemical cell, one half-cell undergoes reduction while the other undergoes oxidation. Thus when we are combining half-reactions and calculating the cell potential, one of the tabulated reduction equations will have to be reversed and the sign of the potential changed. Let us find the potential for the following standard cell: •

• Example • Calculate the potential of the standard cell: Zn|Zn 2+||Cl-|Cl 2, Pt • First we determine the half-reactions from the shorthand notation of the cell. We reverse the Zn 2+ standard half-cell reduction and the sign of E ° given in table thus,

Predicting Spontaneity of Redox Reactions • Reduction potentials can also be used to predict whether a redox reaction is spontaneous or nonspontaneous. A redox reaction is spontaneous when the sum of the potentials of the half-reactions is positive. The reaction is nonspontaneous when the sum is negative.

• Example • Predict whether sulfur dioxide and bromine will react in aqueous solution given the equation: SO 2(g) + Br 2(aq) + 2 H 2 O(l) SO 42−(aq) + 2 Br−(aq) + 4 H+(aq)

Electrolysis • Spontaneous reactions can be moved in the non spontaneous direction (reverse) when a greater voltage than what flows in the forward direction is applied in the reverse direction. When voltage is applied electrons are pushed from what is normally the cathode toward the voltaic cell’s anode. This process is known as electrolysis.

• Ex. The reaction below is spontaneous. It does not normally move in the reverse direction (which is good, otherwise table salt would turn into sodium metal and chlorine gas spontaneously. 2 Na(s) + Cl 2(g) → 2 Na. Cl(s) • If this reaction were to undergo electrolysis (with electric current being passed through molten sodium chloride) the following reaction would result: Electric current + 2 Na. Cl(l) -> 2 Na(l) + Cl 2(g)

• Electrolysis is used in industry to purify metals, such as copper and aluminum, and in electroplating, the process used, for example, to deposit the chrome on the bumper of a 1955 Chevy. A similar process is used to refresh rechargeable batteries.
 Cathode vs anode equation
Cathode vs anode equation Electrochemical deposition
Electrochemical deposition Galvanic corrosion
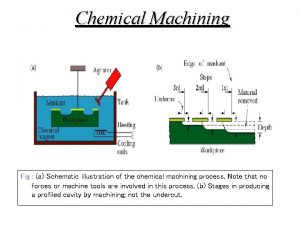
Galvanic corrosion Electrochemical machining animation
Electrochemical machining animation Action potential propagation
Action potential propagation Electrochemical series
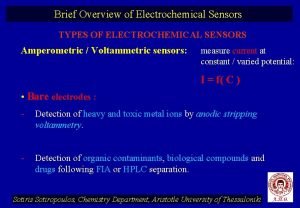
Electrochemical series Types of electrochemical sensors
Types of electrochemical sensors Chloride half equation
Chloride half equation Diffusion limited
Diffusion limited Difference between dry and wet corrosion
Difference between dry and wet corrosion Dse
Dse What are electrochemical series
What are electrochemical series Electrochemical series order
Electrochemical series order Electrochemical impulse
Electrochemical impulse Impedance definition
Impedance definition Module 10 the nervous and endocrine systems
Module 10 the nervous and endocrine systems Types of electrochemical corrosion
Types of electrochemical corrosion Electrochemical machining advantages and disadvantages
Electrochemical machining advantages and disadvantages The stage that creates an “electrochemical gradient”. *
The stage that creates an “electrochemical gradient”. * Breathalyzer redox reaction
Breathalyzer redox reaction