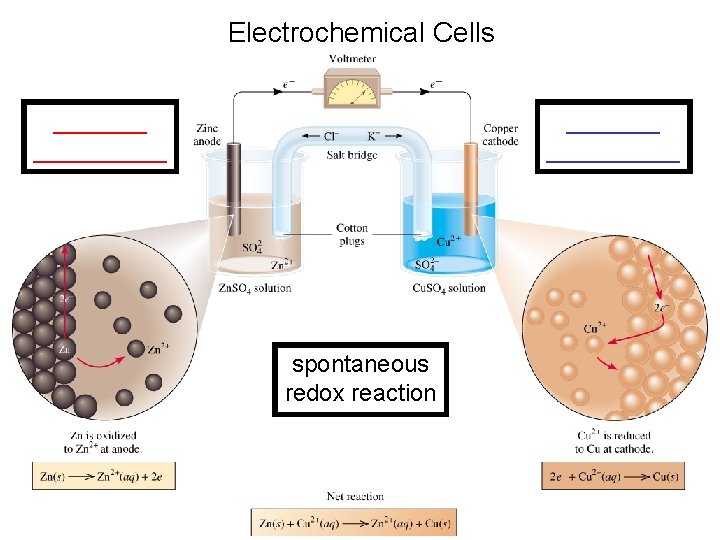
Electrochemical cells Electrochemical Cells spontaneous redox reaction Electrochemical



![The Nernst equation enables us to calculate E as a function of [reactants] and The Nernst equation enables us to calculate E as a function of [reactants] and](https://slidetodoc.com/presentation_image_h2/3a75ca03fffb747c017821a8f9e1545d/image-19.jpg)
![Will the following reaction occur spontaneously at 250 C if [Fe 2+] = 0. Will the following reaction occur spontaneously at 250 C if [Fe 2+] = 0.](https://slidetodoc.com/presentation_image_h2/3a75ca03fffb747c017821a8f9e1545d/image-20.jpg)
- Slides: 30

Electrochemical cells

Electrochemical Cells __________ spontaneous redox reaction

Electrochemical Cells Cell Diagram Zn (s) + Cu 2+ (aq) Cu (s) + Zn 2+ (aq) [Cu 2+] = 1 M & [Zn 2+] = 1 M Zn (s) | Zn 2+ (1 M) || Cu 2+ (1 M) | Cu (s) anode cathode

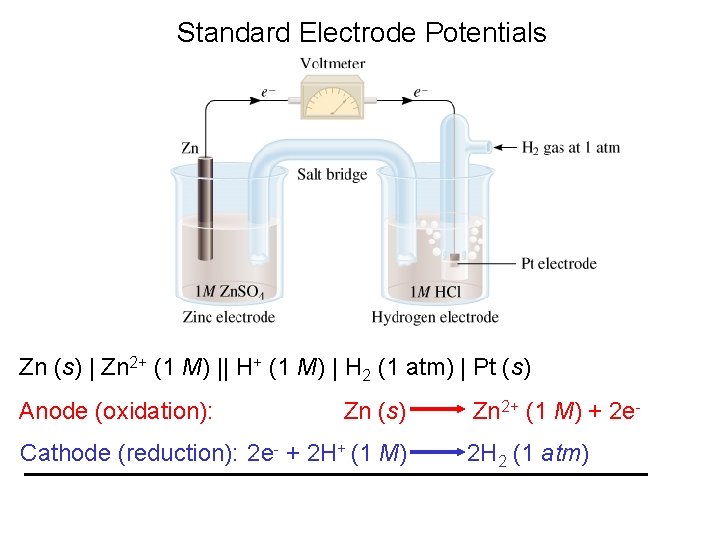
Standard Electrode Potentials Zn (s) | Zn 2+ (1 M) || H+ (1 M) | H 2 (1 atm) | Pt (s) Anode (oxidation): Zn (s) Cathode (reduction): 2 e- + 2 H+ (1 M) Zn 2+ (1 M) + 2 e 2 H 2 (1 atm)

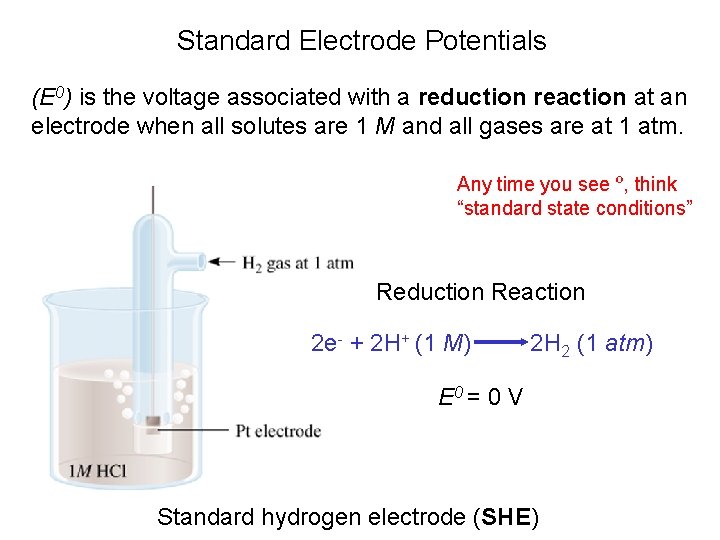
Standard Electrode Potentials (E 0) is the voltage associated with a reduction reaction at an electrode when all solutes are 1 M and all gases are at 1 atm. Any time you see º, think “standard state conditions” Reduction Reaction 2 e- + 2 H+ (1 M) 2 H 2 (1 atm) E 0 = 0 V Standard hydrogen electrode (SHE)

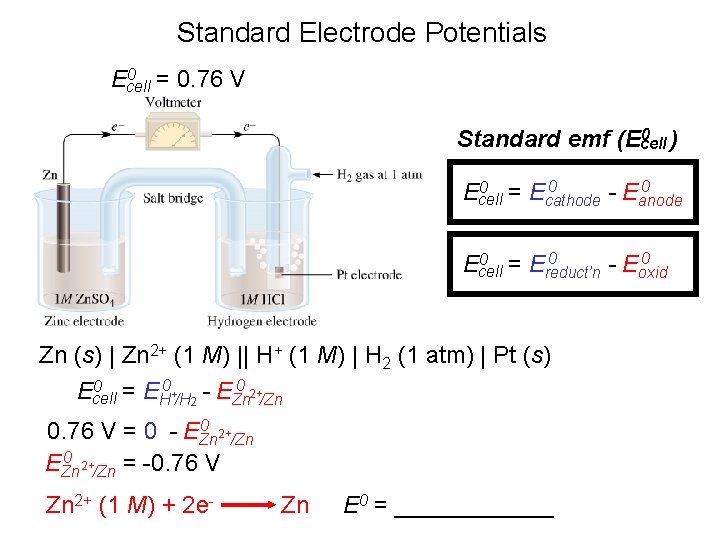
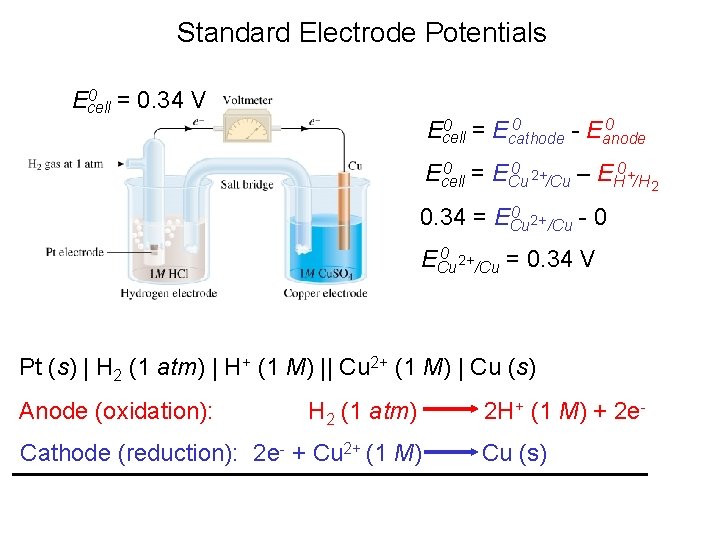
Standard Electrode Potentials 0 = 0. 76 V Ecell 0 ) Standard emf (Ecell 0 0 = E 0 Ecell cathode - Eanode 0 0 = E 0 Ecell reduct’n - Eoxid Zn (s) | Zn 2+ (1 M) || H+ (1 M) | H 2 (1 atm) | Pt (s) 0 = E 0 + - E 0 2+ Ecell H /H 2 Zn /Zn 0 2+ 0. 76 V = 0 - EZn /Zn 0 2+ EZn /Zn = -0. 76 V Zn 2+ (1 M) + 2 e- Zn E 0 = ______

Standard Electrode Potentials 0 = 0. 34 V Ecell 0 0 = E 0 Ecell cathode - Eanode 0 = E 0 2+ 0 Ecell Cu /Cu – EH +/H 2 0 2+ 0. 34 = ECu /Cu - 0 0 2+ ECu /Cu = 0. 34 V Pt (s) | H 2 (1 atm) | H+ (1 M) || Cu 2+ (1 M) | Cu (s) Anode (oxidation): H 2 (1 atm) Cathode (reduction): 2 e- + Cu 2+ (1 M) 2 H+ (1 M) + 2 e. Cu (s)

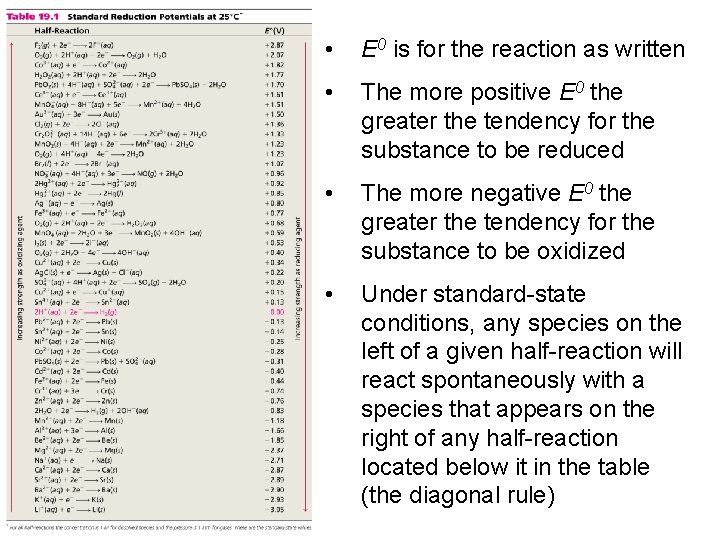
• E 0 is for the reaction as written • The more positive E 0 the greater the tendency for the substance to be reduced • The more negative E 0 the greater the tendency for the substance to be oxidized • Under standard-state conditions, any species on the left of a given half-reaction will react spontaneously with a species that appears on the right of any half-reaction located below it in the table (the diagonal rule)

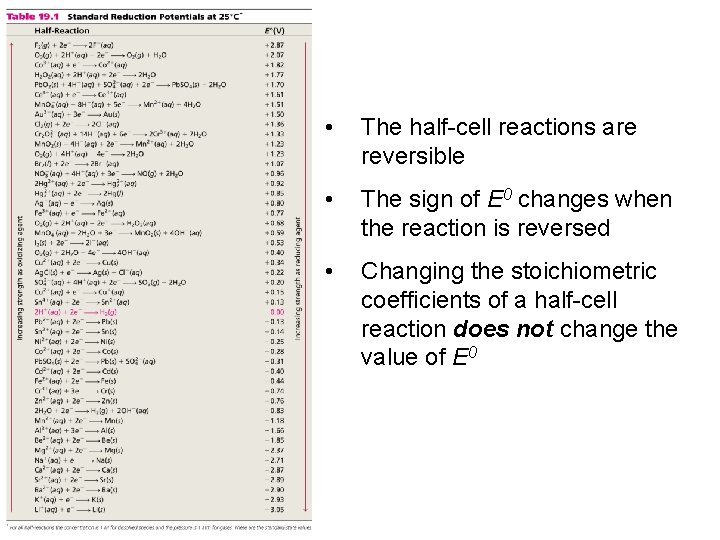
• The half-cell reactions are reversible • The sign of E 0 changes when the reaction is reversed • Changing the stoichiometric coefficients of a half-cell reaction does not change the value of E 0

Can Sn reduce Zn 2+ under standard-state conditions? How do we find the answer? Look up the Eº values in Table. Zn 2+(aq) + 2 e- —> Zn(s) (Is this oxidation or reduction? ) Which reactions in the table will reduce Zn 2+(aq)?

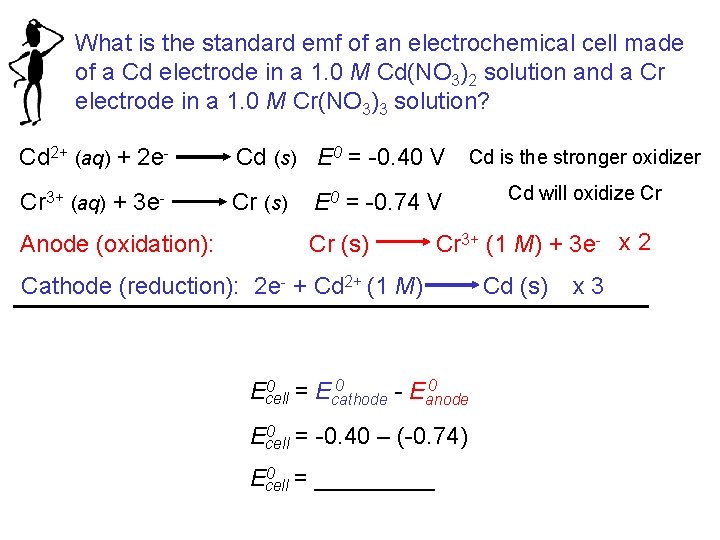
What is the standard emf of an electrochemical cell made of a Cd electrode in a 1. 0 M Cd(NO 3)2 solution and a Cr electrode in a 1. 0 M Cr(NO 3)3 solution? Cd 2+ (aq) + 2 e- Cd (s) E 0 = -0. 40 V Cd is the stronger oxidizer Cr 3+ (aq) + 3 e- Cr (s) Anode (oxidation): E 0 = -0. 74 V Cr (s) Cr 3+ (1 M) + 3 e- x 2 Cathode (reduction): 2 e- + Cd 2+ (1 M) 0 0 = E 0 Ecell E cathode anode 0 = -0. 40 – (-0. 74) Ecell 0 = _____ Ecell Cd will oxidize Cr Cd (s) x 3

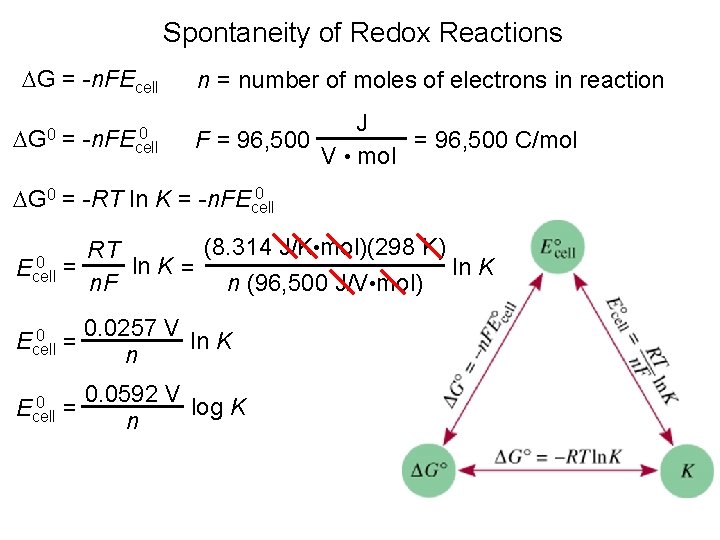
Spontaneity of Redox Reactions G = -n. FEcell G 0 = 0 -n. FEcell n = number of moles of electrons in reaction J F = 96, 500 C/mol V • mol 0 G 0 = -RT ln K = -n. FEcell (8. 314 J/K • mol)(298 K) RT 0 = ln K Ecell n. F n (96, 500 J/V • mol) 0 Ecell = 0 Ecell 0. 0257 V ln K n 0. 0592 V log K = n

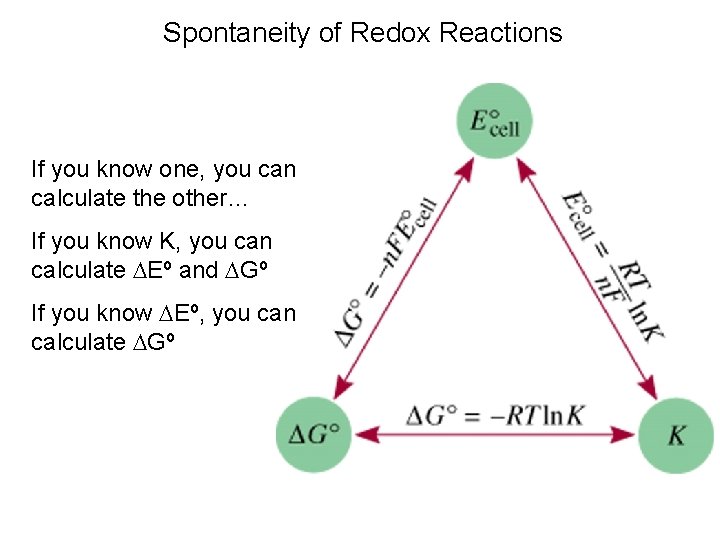
Spontaneity of Redox Reactions If you know one, you can calculate the other… If you know K, you can calculate Eº and Gº If you know Eº, you can calculate Gº

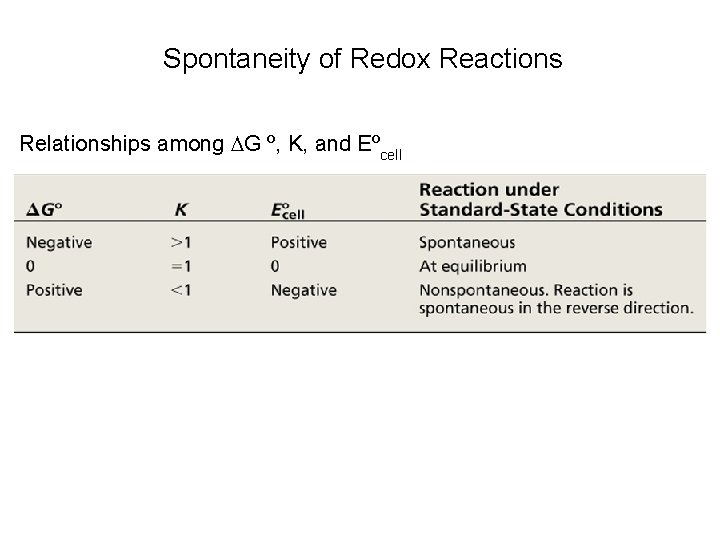
Spontaneity of Redox Reactions Relationships among G º, K, and Eºcell

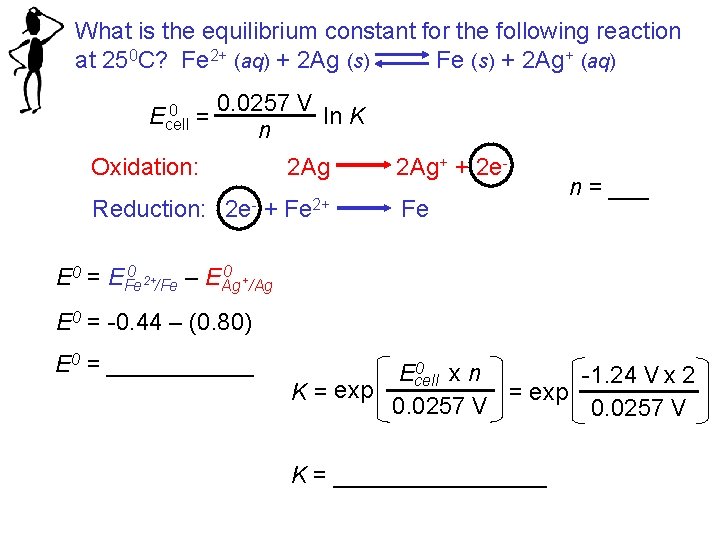
What is the equilibrium constant for the following reaction at 250 C? Fe 2+ (aq) + 2 Ag (s) Fe (s) + 2 Ag+ (aq) 0 Ecell = 0. 0257 V ln K n Oxidation: 2 Ag Reduction: 2 e- + Fe 2+ 2 Ag+ + 2 e. Fe n = ___ 0 0 E 0 = EFe 2+/Fe – EAg +/Ag E 0 = -0. 44 – (0. 80) E 0 = ______ 0 Ecell xn -1. 24 V x 2 = exp K = exp 0. 0257 V K = ________

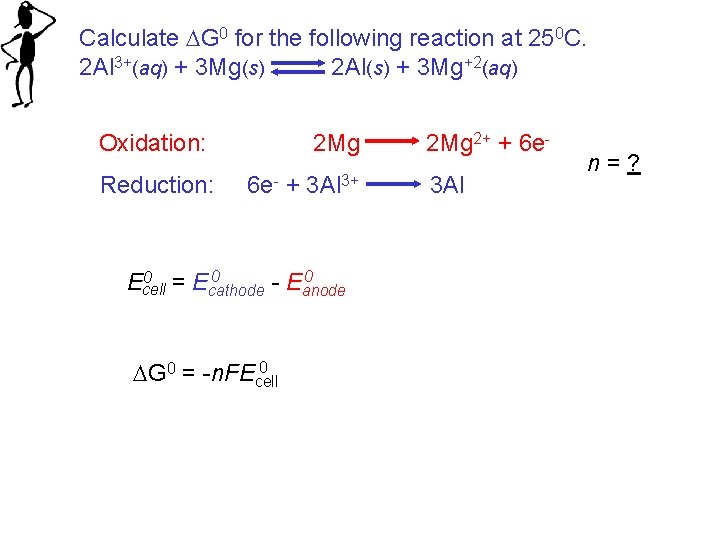
Calculate G 0 for the following reaction at 250 C. 2 Al 3+(aq) + 3 Mg(s) 2 Al(s) + 3 Mg+2(aq) Oxidation: Reduction: 2 Mg 6 e- + 3 Al 3+ 0 0 = E 0 Ecell cathode - Eanode 0 G 0 = -n. FEcell 2 Mg 2+ + 6 e 3 Al n=?

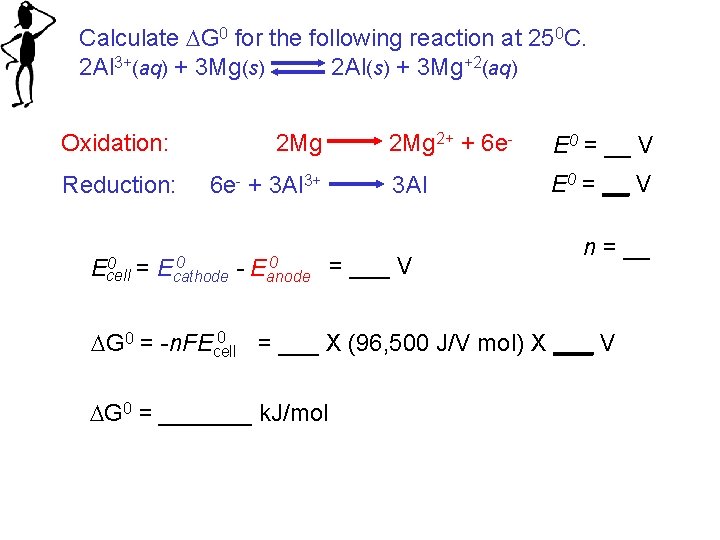
Calculate G 0 for the following reaction at 250 C. 2 Al 3+(aq) + 3 Mg(s) 2 Al(s) + 3 Mg+2(aq) Oxidation: Reduction: 2 Mg 6 e- + 3 Al 3+ 2 Mg 2+ + 6 e- E 0 = __ V 3 Al E 0 = __ V 0 0 = E 0 = ___ V Ecell cathode - Eanode n = __ 0 G 0 = -n. FEcell = ___ X (96, 500 J/V mol) X ___ V G 0 = _______ k. J/mol

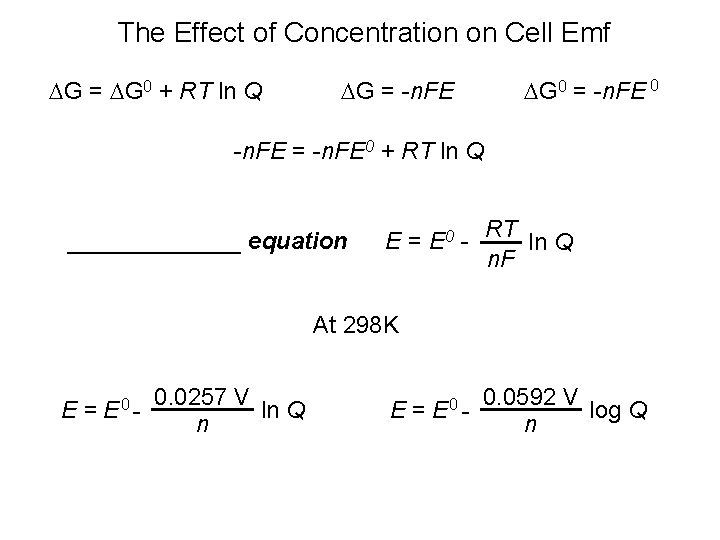
The Effect of Concentration on Cell Emf G = G 0 + RT ln Q G = -n. FE G 0 = -n. FE 0 -n. FE = -n. FE 0 + RT ln Q _______ equation E = E 0 - RT ln Q n. F At 298 K E = E 0 - 0. 0257 V ln Q n E = E 0 - 0. 0592 V log Q n
![The Nernst equation enables us to calculate E as a function of reactants and The Nernst equation enables us to calculate E as a function of [reactants] and](https://slidetodoc.com/presentation_image_h2/3a75ca03fffb747c017821a8f9e1545d/image-19.jpg)
The Nernst equation enables us to calculate E as a function of [reactants] and [products] in a redox reaction.
![Will the following reaction occur spontaneously at 250 C if Fe 2 0 Will the following reaction occur spontaneously at 250 C if [Fe 2+] = 0.](https://slidetodoc.com/presentation_image_h2/3a75ca03fffb747c017821a8f9e1545d/image-20.jpg)
Will the following reaction occur spontaneously at 250 C if [Fe 2+] = 0. 60 M and [Cd 2+] = 0. 010 M? Fe 2+ (aq) + Cd (s) Fe (s) + Cd 2+ (aq) Oxidation: Reduction: Cd 2 e- + Cd 2+ + 2 e- Fe 2+ 2 Fe n = ___ 0 0 E 0 = EFe 2+/Fe – ECd 2+/Cd E 0 = -0. 44 – (-0. 40) E 0 = -0. 04 V 0. 0257 V ln Q n 0. 010 0. 0257 V ln E = -0. 04 V 2 0. 60 E = ______ E = E 0 - E ___ 0 ________

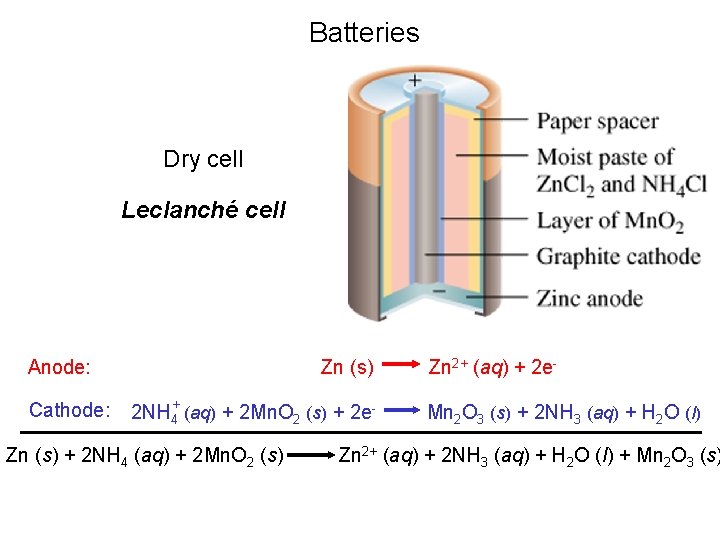
Batteries Dry cell Leclanché cell Anode: Cathode: Zn (s) 2 NH 4+ (aq) + 2 Mn. O 2 (s) + 2 e- Zn (s) + 2 NH 4 (aq) + 2 Mn. O 2 (s) Zn 2+ (aq) + 2 e. Mn 2 O 3 (s) + 2 NH 3 (aq) + H 2 O (l) Zn 2+ (aq) + 2 NH 3 (aq) + H 2 O (l) + Mn 2 O 3 (s)

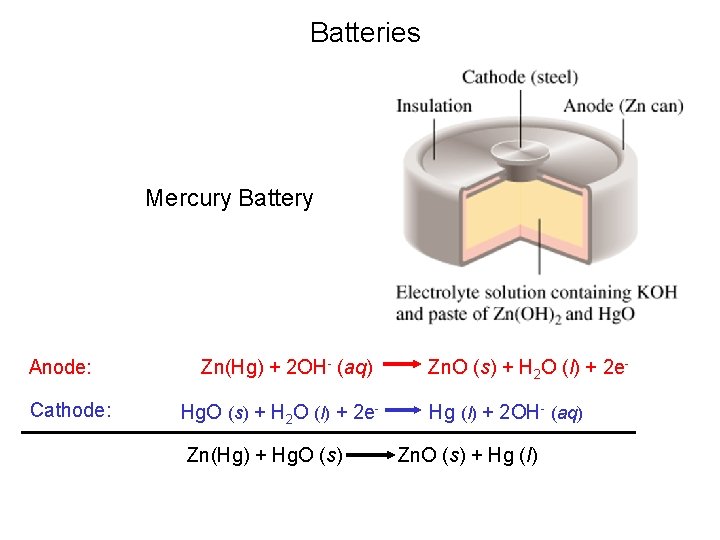
Batteries Mercury Battery Anode: Cathode: Zn(Hg) + 2 OH- (aq) Hg. O (s) + H 2 O (l) + 2 e. Zn(Hg) + Hg. O (s) Zn. O (s) + H 2 O (l) + 2 e. Hg (l) + 2 OH- (aq) Zn. O (s) + Hg (l)

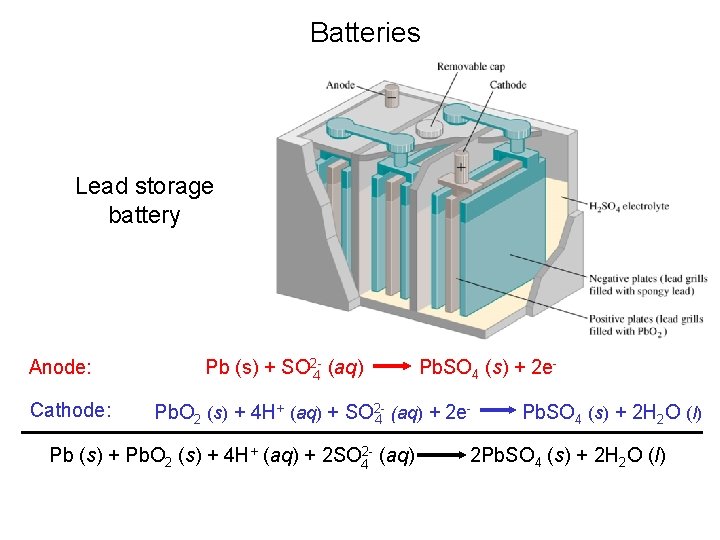
Batteries Lead storage battery Anode: Cathode: Pb (s) + SO 2 -4 (aq) Pb. SO 4 (s) + 2 e- Pb. O 2 (s) + 4 H+ (aq) + SO 24 (aq) + 2 e Pb (s) + Pb. O 2 (s) + 4 H+ (aq) + 2 SO 42 - (aq) Pb. SO 4 (s) + 2 H 2 O (l) 2 Pb. SO 4 (s) + 2 H 2 O (l)

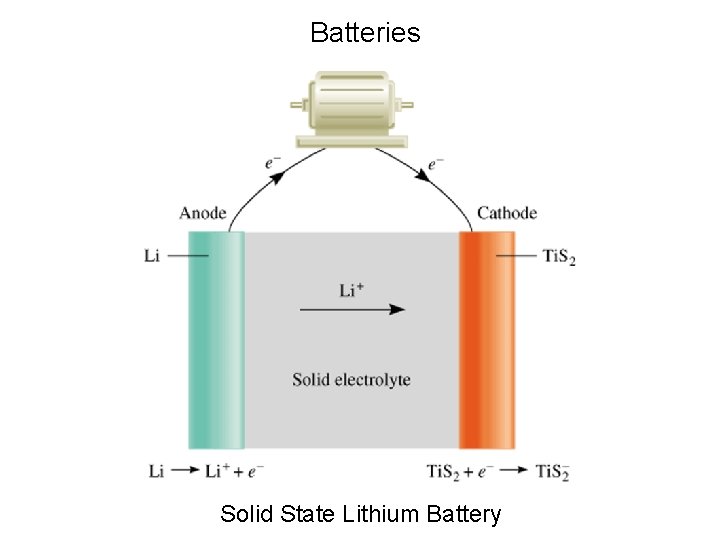
Batteries Solid State Lithium Battery

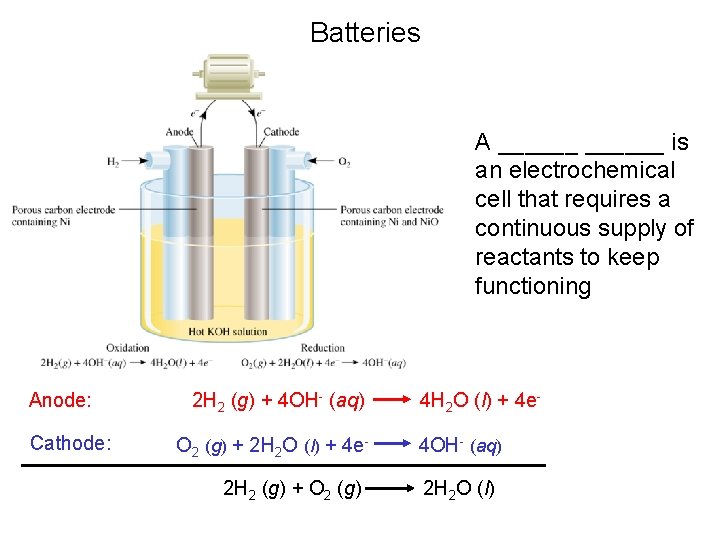
Batteries A ______ is an electrochemical cell that requires a continuous supply of reactants to keep functioning Anode: Cathode: 2 H 2 (g) + 4 OH- (aq) O 2 (g) + 2 H 2 O (l) + 4 e 2 H 2 (g) + O 2 (g) 4 H 2 O (l) + 4 e 4 OH- (aq) 2 H 2 O (l)

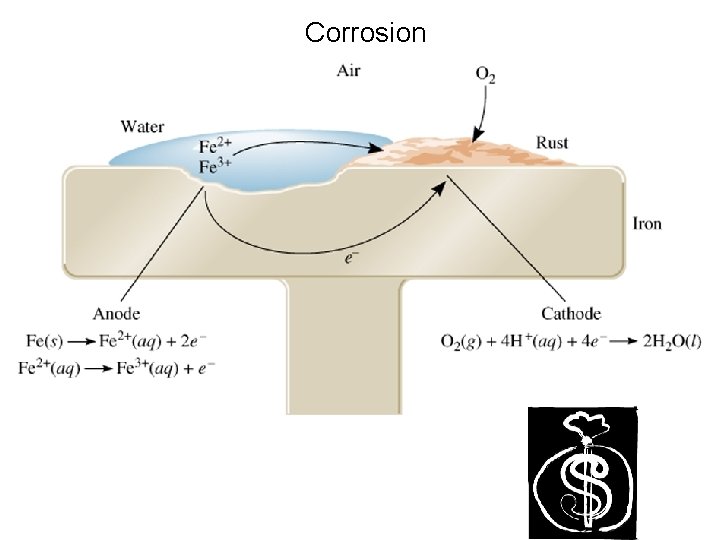
Corrosion

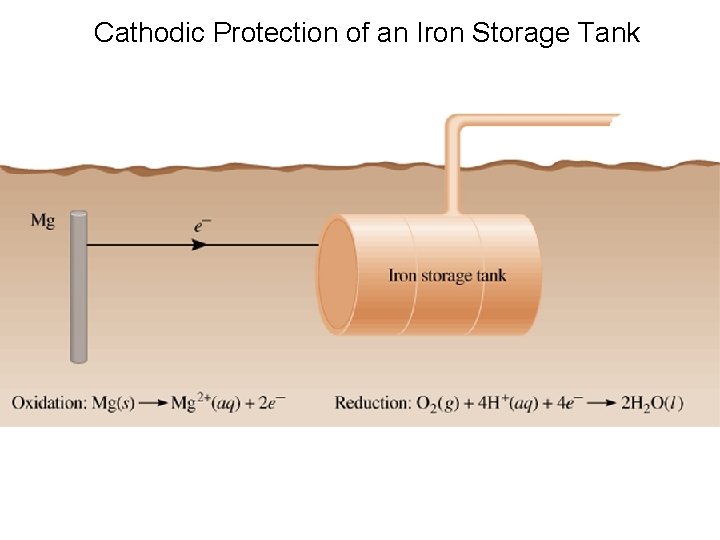
Cathodic Protection of an Iron Storage Tank

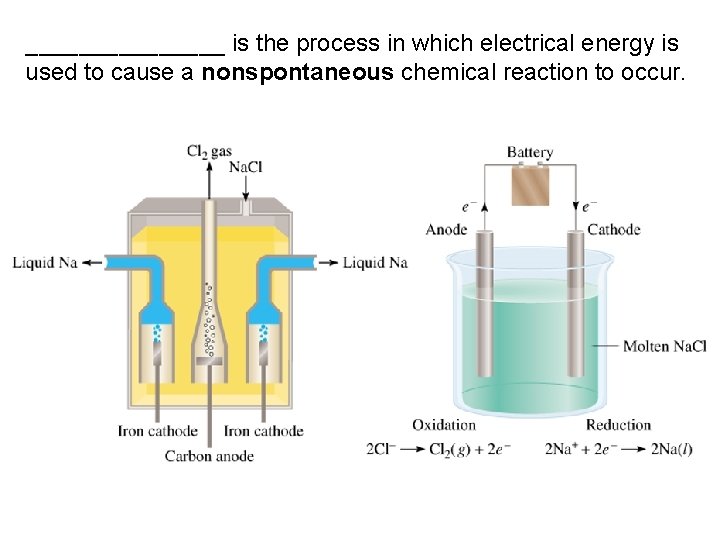
________ is the process in which electrical energy is used to cause a nonspontaneous chemical reaction to occur.

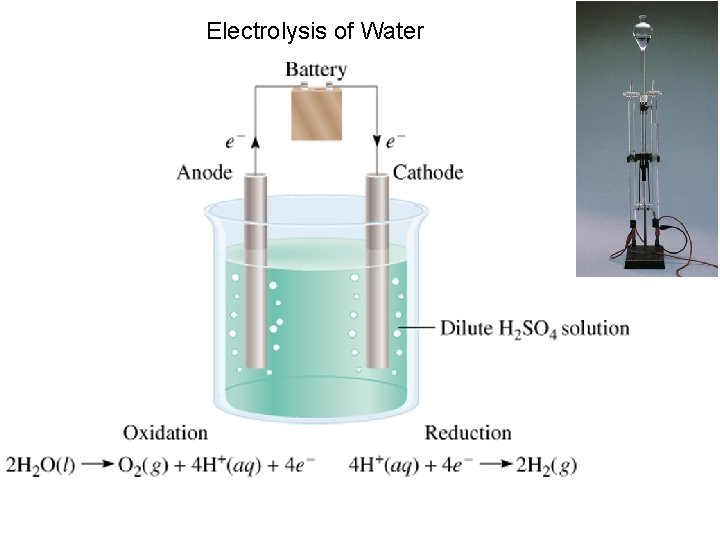
Electrolysis of Water

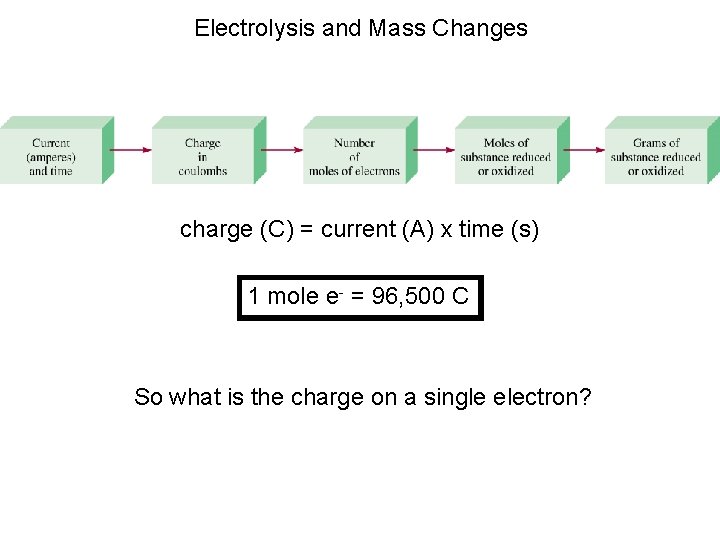
Electrolysis and Mass Changes charge (C) = current (A) x time (s) 1 mole e- = 96, 500 C So what is the charge on a single electron?