Electrochemistry Lesson 8 Electrochemical Cells Electrochemical cells are









- Slides: 15

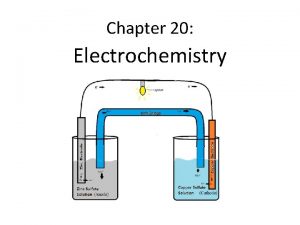
Electrochemistry Lesson 8 Electrochemical Cells

Electrochemical cells are Batteries

Alkaline Batteries KOH

Car Batteries Pb-Acid H 2 SO 4

Mitsubishi i. Mi. EV - Pure Electric Car $ 36, 000 US $ 50, 000 Can Powered by a 330 v Li-Ion Rechargeable battery Plugs into your house and takes 14 hours to charge -100 km for $ 0. 60 Top Speed 130 km/h 63 hp and 133 lb. -ft. of torque

Cell Phone batteries Lithium Ion Rechargeable battery

Lithium Coin Cell

Space Ship Batteries Powered by Radioisotopes

Ni-Metal Hydride

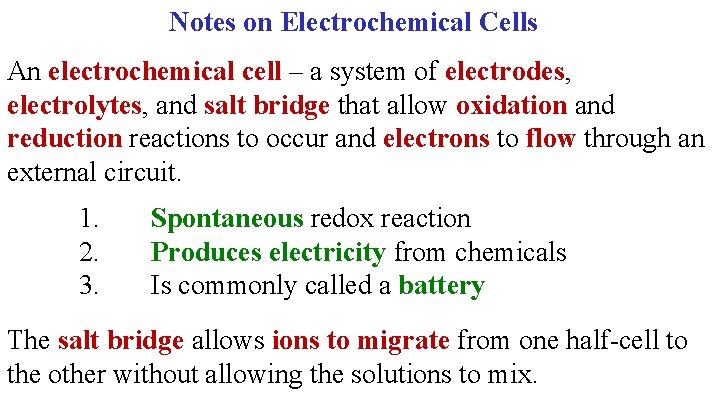
Notes on Electrochemical Cells An electrochemical cell – a system of electrodes, electrolytes, and salt bridge that allow oxidation and reduction reactions to occur and electrons to flow through an external circuit. 1. 2. 3. Spontaneous redox reaction Produces electricity from chemicals Is commonly called a battery The salt bridge allows ions to migrate from one half-cell to the other without allowing the solutions to mix.

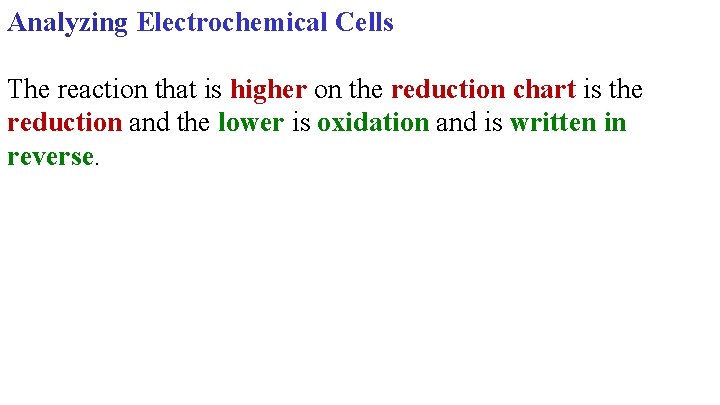
Analyzing Electrochemical Cells The reaction that is higher on the reduction chart is the reduction and the lower is oxidation and is written in reverse.

For any cell Oxidation always occurs at the anode and reduction at the cathode Electrons flow through the wire and go from anode to cathode Anions (- ions) migrate to the anode and cations (+ions) migrate towards the cathode usually through the salt bridge

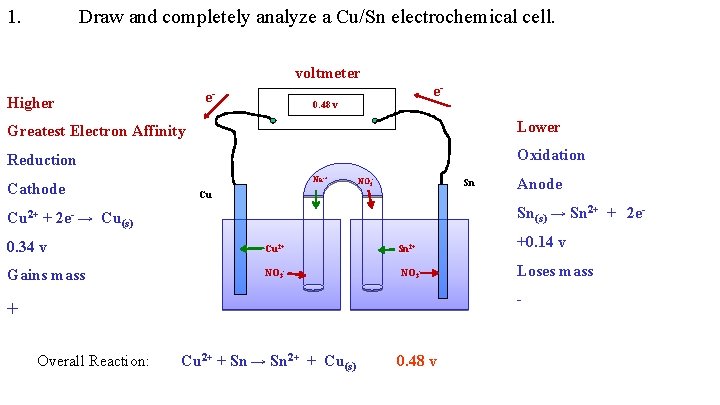
1. Draw and completely analyze a Cu/Sn electrochemical cell. voltmeter e- Higher e- 0. 48 v Greatest Electron Affinity Lower Reduction Oxidation Cathode Na-+ NO 3 - Sn Cu Anode Sn(s) → Sn 2+ + 2 e- Cu 2+ + 2 e- → Cu(s) 0. 34 v Cu 2+ Sn 2+ +0. 14 v Gains mass NO 3 - Loses mass - + Overall Reaction: Cu 2+ + Sn → Sn 2+ + Cu(s) 0. 48 v

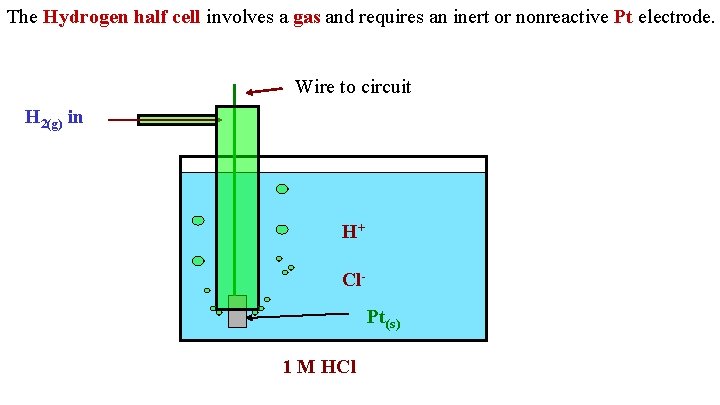
The Hydrogen half cell involves a gas and requires an inert or nonreactive Pt electrode. Wire to circuit H 2(g) in H+ Cl. Pt(s) 1 M HCl

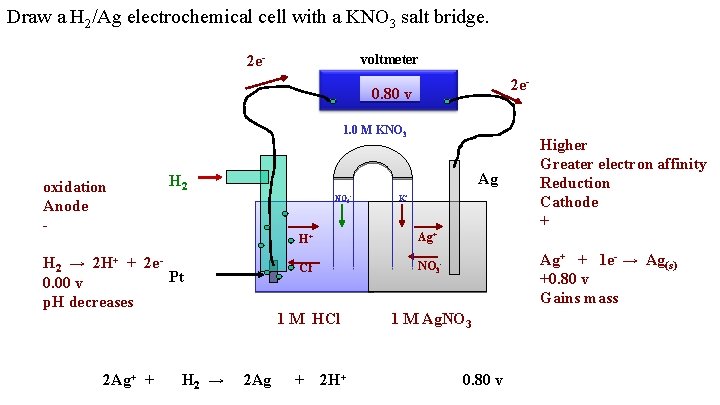
Draw a H 2/Ag electrochemical cell with a KNO 3 salt bridge. voltmeter 2 e 2 e- 0. 80 v 1. 0 M KNO 3 oxidation Anode - H 2 → 2 H+ + 2 e. Pt 0. 00 v p. H decreases Ag NO 3 - K+ H+ Ag+ Cl- NO 3 - 1 M HCl 2 Ag+ + H 2 → 2 Ag + 2 H+ Higher Greater electron affinity Reduction Cathode + Ag+ + 1 e- → Ag(s) +0. 80 v Gains mass 1 M Ag. NO 3 0. 80 v
 Mikael ferm
Mikael ferm Electrochemistry lesson
Electrochemistry lesson Flow of anions and cations in an electrochemical cell
Flow of anions and cations in an electrochemical cell Electrochemical deposition
Electrochemical deposition Electrochemical theory of corrosion
Electrochemical theory of corrosion Electrochemical machining animation
Electrochemical machining animation Refractory period anatomy
Refractory period anatomy Electrochemical series
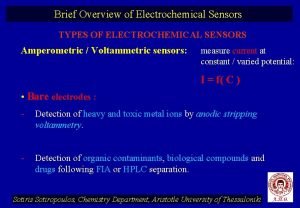
Electrochemical series Types of electrochemical sensors
Types of electrochemical sensors The electrochemical series table
The electrochemical series table Vapor
Vapor Wet or electrochemical corrosion
Wet or electrochemical corrosion Electrochemical etch stop
Electrochemical etch stop What are electrochemical series
What are electrochemical series Electrochemical series order
Electrochemical series order Electrochemical impulse
Electrochemical impulse