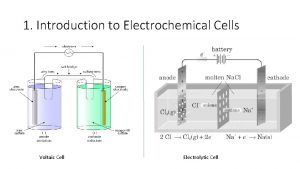
Types of Electrochemical Cells n n Electrolytic Cells












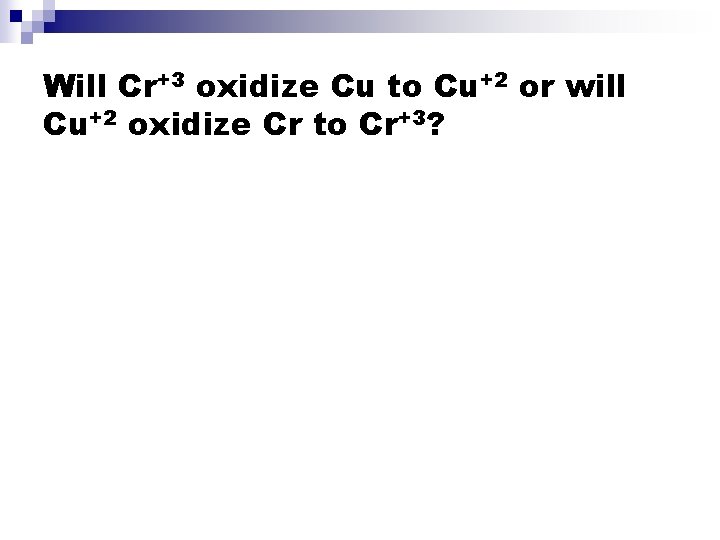
![Problem Calculate E for Fe+3/Fe+2 electrode if the [Fe+2] is 5 times that of Problem Calculate E for Fe+3/Fe+2 electrode if the [Fe+2] is 5 times that of](https://slidetodoc.com/presentation_image_h2/6ae9edd2c41dc124aeaa0cc5c14555b5/image-29.jpg)
- Slides: 33

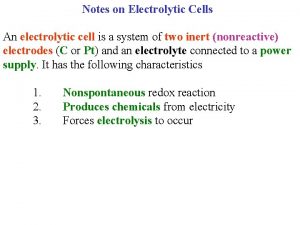
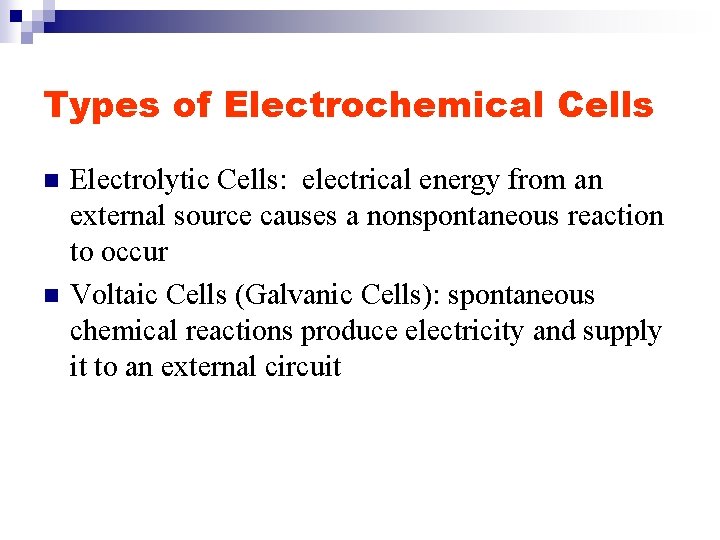
Types of Electrochemical Cells n n Electrolytic Cells: electrical energy from an external source causes a nonspontaneous reaction to occur Voltaic Cells (Galvanic Cells): spontaneous chemical reactions produce electricity and supply it to an external circuit

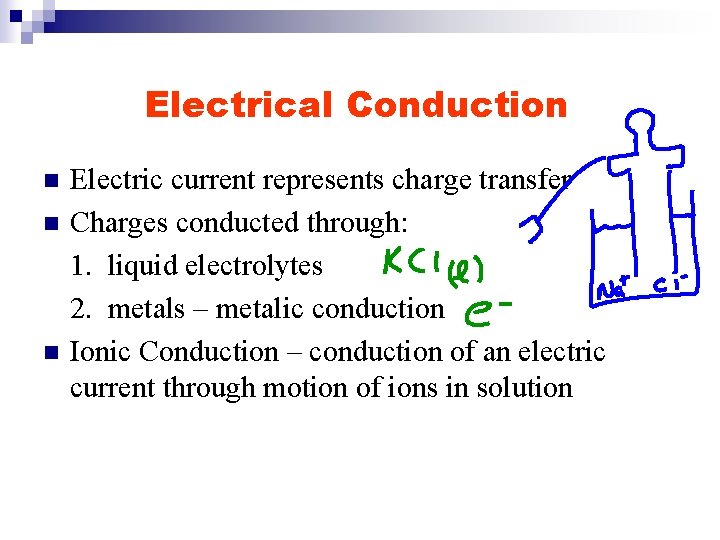
Electrical Conduction n Electric current represents charge transfer Charges conducted through: 1. liquid electrolytes 2. metals – metalic conduction Ionic Conduction – conduction of an electric current through motion of ions in solution

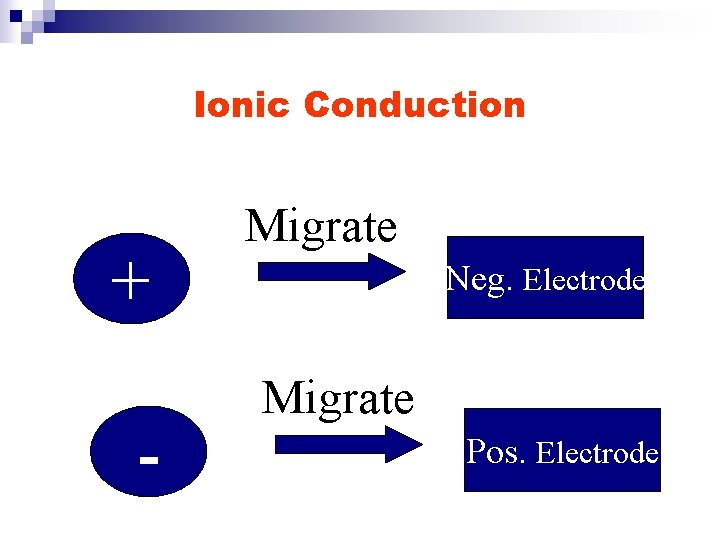
Ionic Conduction + - Migrate Neg. Electrode Migrate Pos. Electrode

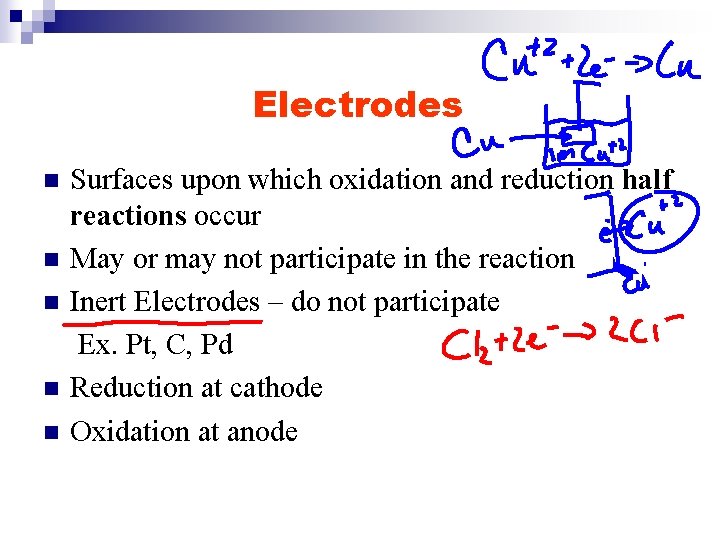
Electrodes n n n Surfaces upon which oxidation and reduction half reactions occur May or may not participate in the reaction Inert Electrodes – do not participate Ex. Pt, C, Pd Reduction at cathode Oxidation at anode

Electrodes RED CAT And AN OX

Ted Talk n http: //ed. ted. com/lessons/electric-vocabulary

Voltaic or Galvanic Cells n n n Spontaneous oxidation – reduction reactions produce electrical energy Two halves of redox reaction are separated Half cell – contains the oxidized and reduced forms of an element or other complex species

Voltaic or Galvanic Cells n n n Salt bridge – completes circuit between the two half cells Salt bridge is any medium through which ions can flow Agar + Salt Gelations 1. Allows electrical contact between two solutions 2. Prevents mixing of electrode solutions 3. Maintains electrical neutrality

Redox Reaction

Redox Reaction

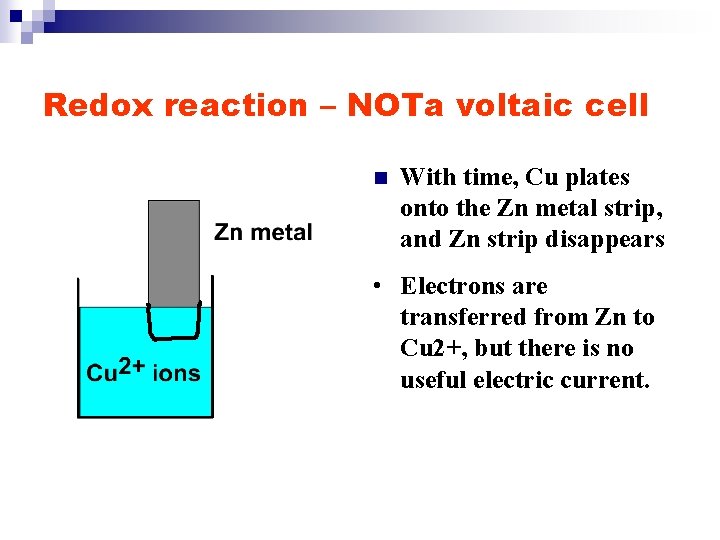
Redox reaction – NOTa voltaic cell n With time, Cu plates onto the Zn metal strip, and Zn strip disappears • Electrons are transferred from Zn to Cu 2+, but there is no useful electric current.

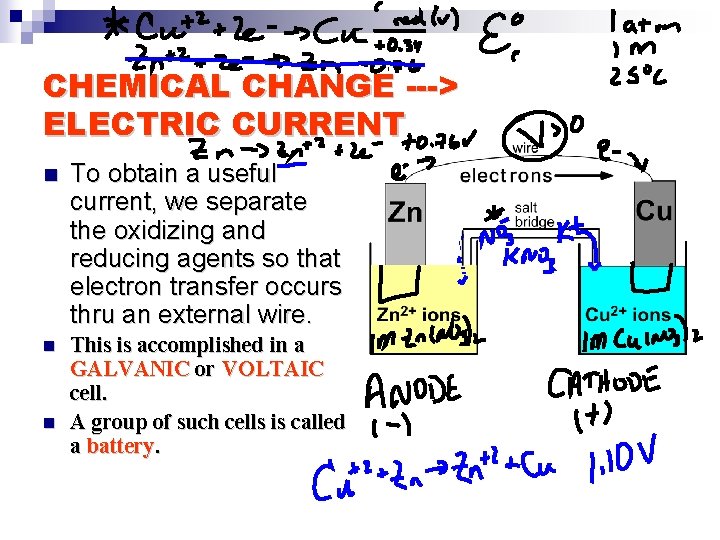
CHEMICAL CHANGE ---> ELECTRIC CURRENT n To obtain a useful current, we separate the oxidizing and reducing agents so that electron transfer occurs thru an external wire. n This is accomplished in a GALVANIC or VOLTAIC cell. A group of such cells is called a battery. n

Voltaic Cell links n n http: //www. chembio. uoguelph. ca/educmat/chm 19 105/galvanic 1. htm http: //www. youtube. com/watch? v=0 o. Sq. PDD 2 r. M A

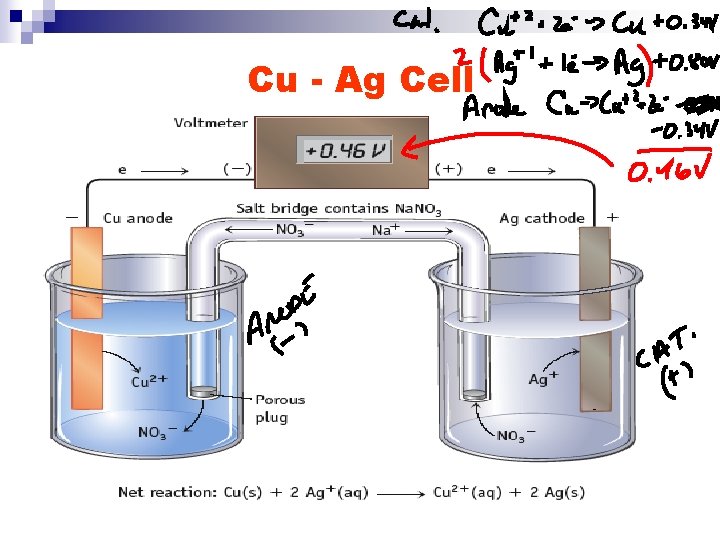
Cu - Ag Cell

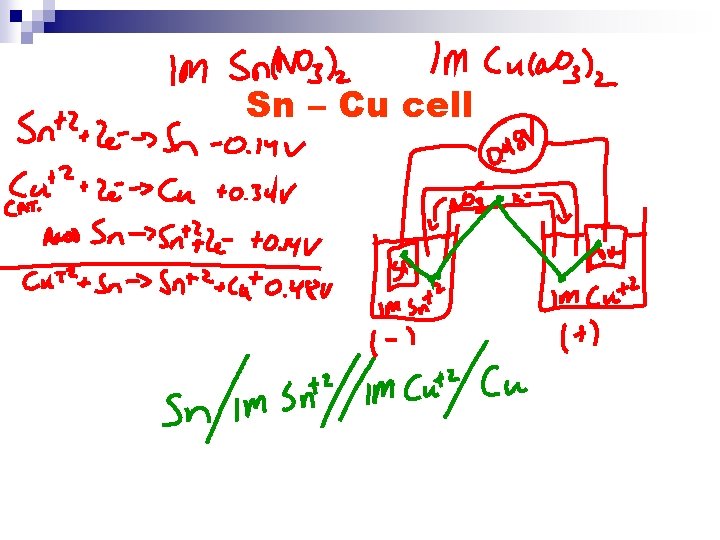
Sn – Cu cell

Summary of Zn, Cu, Ag n Zn – Cu Cu electrode – cathode Cu+2 is more easily reduced than Zn+2 Zn is a stronger reducing agent than Cu n Ag – Cu Cu electrode – anode Ag+ is more easily reduced than Cu+2 Cu is a stronger reducing agent than Ag n Cathode – Anode are dictated by species present

Summary of Zn, Cu, Ag n Strength as oxidizing agents Zn+2 < Cu+2 < Ag+ n Strength as reducing agents Zn > Cu > Ag

Standard Electrode Potentials n n Magnitude of a cell’s potential measures the spontaneity of its redox reaction Higher cell potentials indicate a greater driving force Want to separate total cell potentials into individual potentials of the two half reactions Determine tendencies for redox reactions

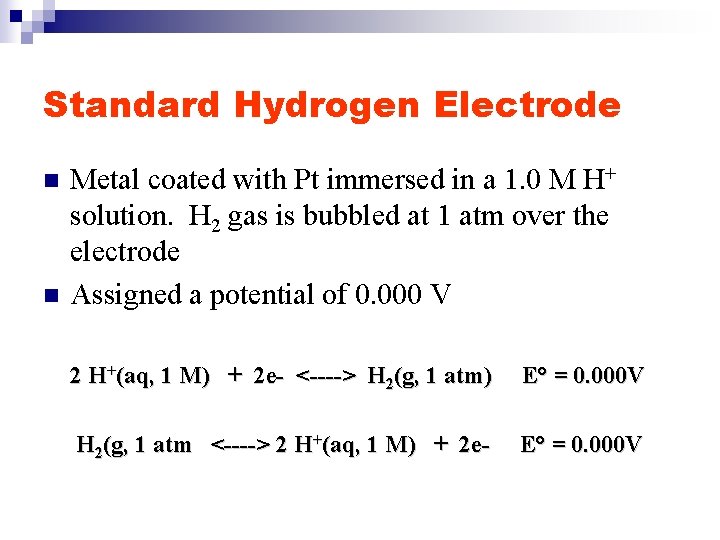
Standard Hydrogen Electrode n n n “Every oxidation needs a reduction” e- must go somewhere Therefore it is impossible to determine experimentally the potential of a single electrode Establish an arbitrary standard electrode Standard Hydrogen Electrode, SHE

Standard Hydrogen Electrode n n Metal coated with Pt immersed in a 1. 0 M H+ solution. H 2 gas is bubbled at 1 atm over the electrode Assigned a potential of 0. 000 V 2 H+(aq, 1 M) + 2 e- <----> H 2(g, 1 atm) E° = 0. 000 V H 2(g, 1 atm <----> 2 H+(aq, 1 M) + 2 e- E° = 0. 000 V

Cu – SHE Cell

Zn – SHE Cell

Zn – Cu Cell

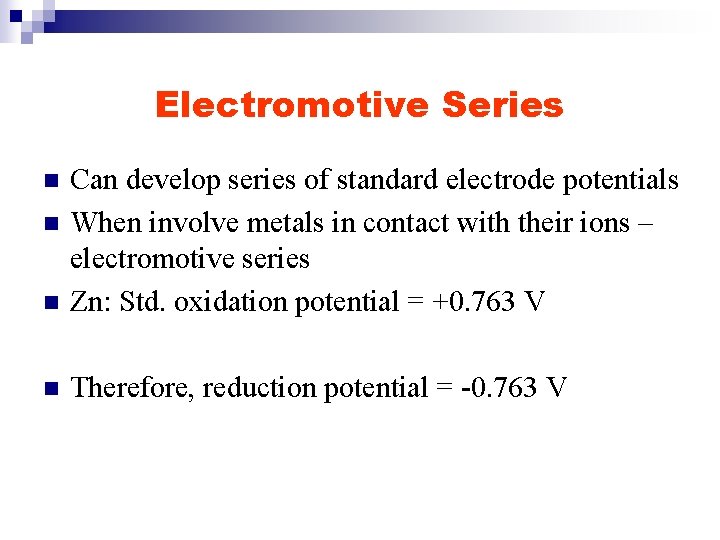
Electromotive Series n Can develop series of standard electrode potentials When involve metals in contact with their ions – electromotive series Zn: Std. oxidation potential = +0. 763 V n Therefore, reduction potential = -0. 763 V n n

Electromotive Series n n n International convention is to use reduction half reactions Indicates tendencies of electrodes to behave as cathodes toward SHE If E° < 0. 0 V, then electrode acts as anode versus SHE

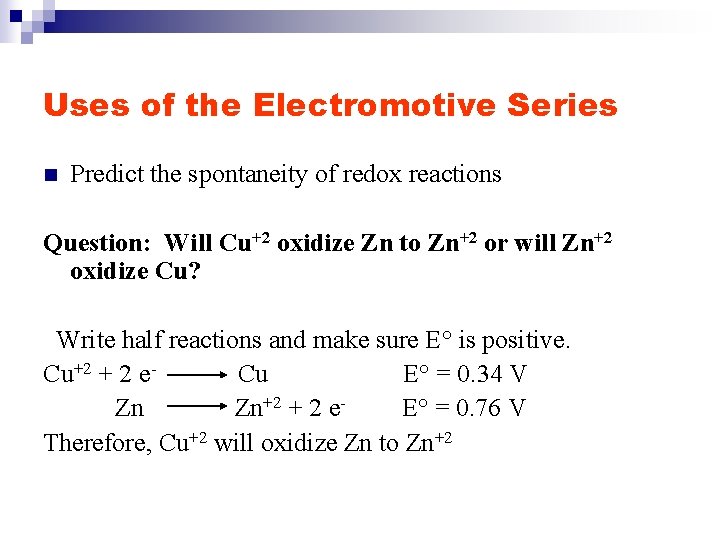
Uses of the Electromotive Series n Predict the spontaneity of redox reactions Question: Will Cu+2 oxidize Zn to Zn+2 or will Zn+2 oxidize Cu? Write half reactions and make sure E° is positive. Cu+2 + 2 e. Cu E° = 0. 34 V Zn Zn+2 + 2 e. E° = 0. 76 V Therefore, Cu+2 will oxidize Zn to Zn+2
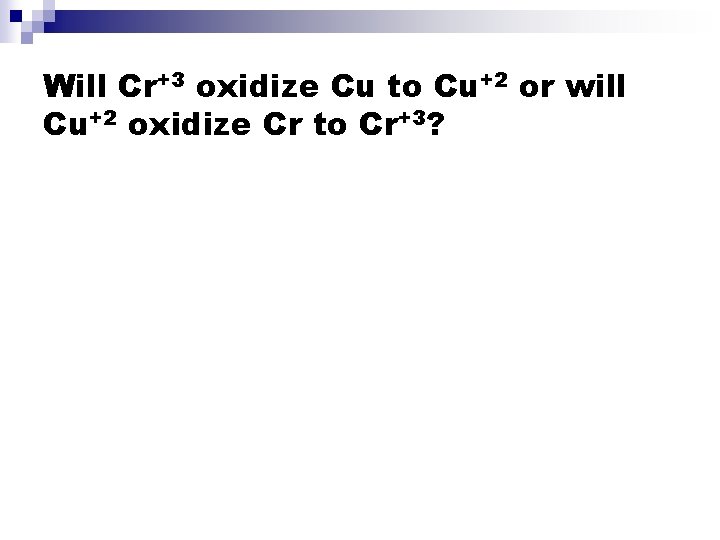
Will Cr+3 oxidize Cu to Cu+2 or will Cu+2 oxidize Cr to Cr+3?

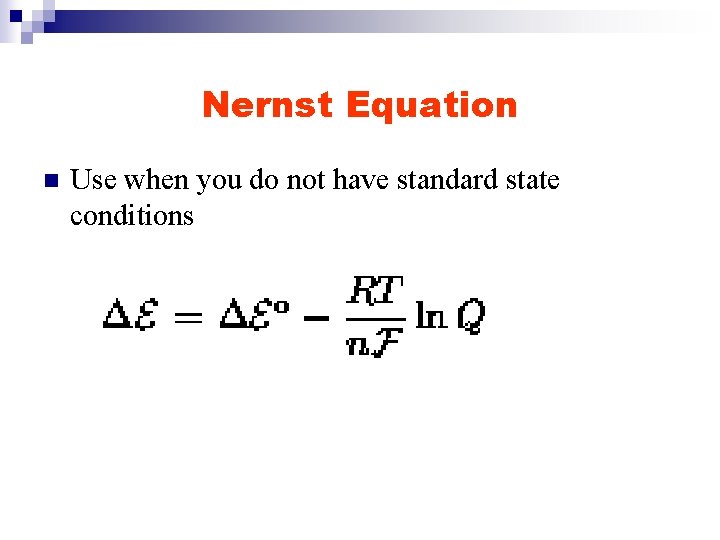
Nernst Equation n Use when you do not have standard state conditions
![Problem Calculate E for Fe3Fe2 electrode if the Fe2 is 5 times that of Problem Calculate E for Fe+3/Fe+2 electrode if the [Fe+2] is 5 times that of](https://slidetodoc.com/presentation_image_h2/6ae9edd2c41dc124aeaa0cc5c14555b5/image-29.jpg)
Problem Calculate E for Fe+3/Fe+2 electrode if the [Fe+2] is 5 times that of [Fe+3].

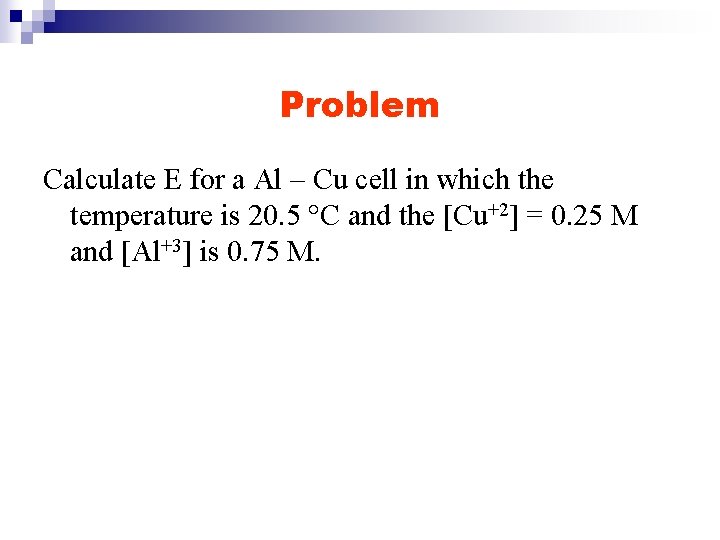
Problem Calculate E for a Al – Cu cell in which the temperature is 20. 5 °C and the [Cu+2] = 0. 25 M and [Al+3] is 0. 75 M.

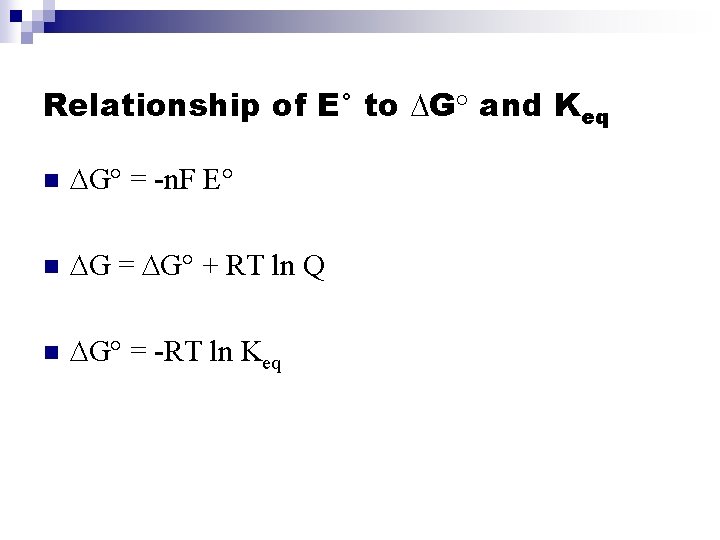
Relationship of E° to DG° and Keq n DG° = -n. F E° n DG = DG° + RT ln Q n DG° = -RT ln Keq

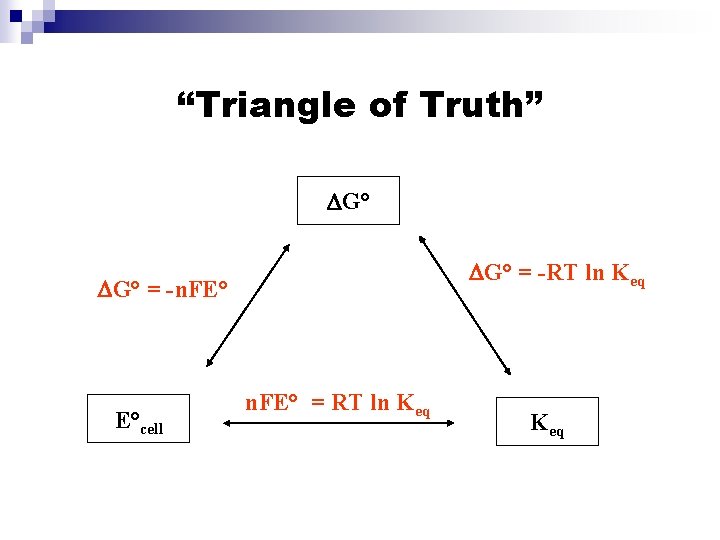
“Triangle of Truth” DG° = -RT ln Keq DG° = -n. FE° E°cell n. FE° = RT ln Keq

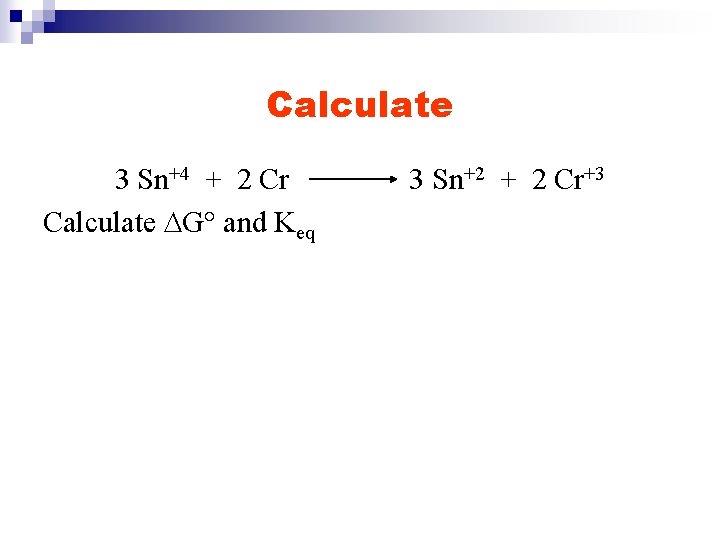
Calculate 3 Sn+4 + 2 Cr Calculate DG° and Keq 3 Sn+2 + 2 Cr+3
 Application of electrolytic cell
Application of electrolytic cell Voltaic vs electrolytic cell
Voltaic vs electrolytic cell Khan academy balancing equations
Khan academy balancing equations Types of electrochemical sensors
Types of electrochemical sensors Types of electrochemical corrosion
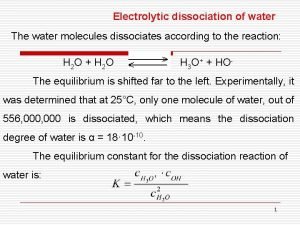
Types of electrochemical corrosion Dissociated meaning
Dissociated meaning Renocell
Renocell Electrolytic cell animation
Electrolytic cell animation Applications of electrolytic cell
Applications of electrolytic cell Example of electrolytic decomposition reaction
Example of electrolytic decomposition reaction Electrolytic etching
Electrolytic etching Electrolytic capacitor polarity
Electrolytic capacitor polarity Smt for electrolytic cell
Smt for electrolytic cell Anode positive or negative
Anode positive or negative Electrolytic cell lab
Electrolytic cell lab Oxidation reduction
Oxidation reduction Anode is positive in electrolytic cell
Anode is positive in electrolytic cell Electrolytic etching metallography
Electrolytic etching metallography Cathode vs anode equation
Cathode vs anode equation Electrochemical deposition
Electrochemical deposition Electrochemical theory of corrosion
Electrochemical theory of corrosion Electrochemical machining animation
Electrochemical machining animation Action potential propagation
Action potential propagation Electrochemical series
Electrochemical series Chloride half equation
Chloride half equation Vapor
Vapor Mechanism of wet corrosion
Mechanism of wet corrosion Etch stop techniques
Etch stop techniques What are electrochemical series
What are electrochemical series Electrochemical series order
Electrochemical series order Electrochemical impulse
Electrochemical impulse Electrochemical impedance spectroscopy
Electrochemical impedance spectroscopy The body's speedy electrochemical communication network
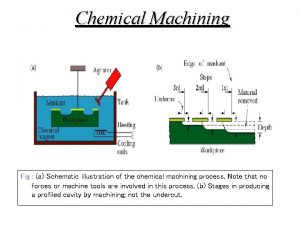
The body's speedy electrochemical communication network Chemical machining applications
Chemical machining applications