Topic 9 OxidationReduction 9 2 Electrochemical Cells Electrolytic






- Slides: 26

Topic 9 – Oxidation-Reduction 9. 2 – Electrochemical Cells Electrolytic Cells 1

Electrolytic vs. Voltaic Cells How do voltaic and electrolytic cells differ? 2

Electrolytic vs. Voltaic Cells The process in which electrical energy is used to bring about a chemical change is called electrolysis. • You are already familiar with some results of electrolysis, such as gold-plated jewelry, chrome-plated automobile parts, and silver-plated dishes. 3

Electrolytic vs. Voltaic Cells The apparatus in which electrolysis is carried out is an electrolytic cell. • An electrolytic cell uses electrical energy (direct current) to make a non-spontaneous redox reaction proceed to completion. • An electrolytic cell is an electrochemical cell used to cause a chemical change through the application of electrical energy. 4

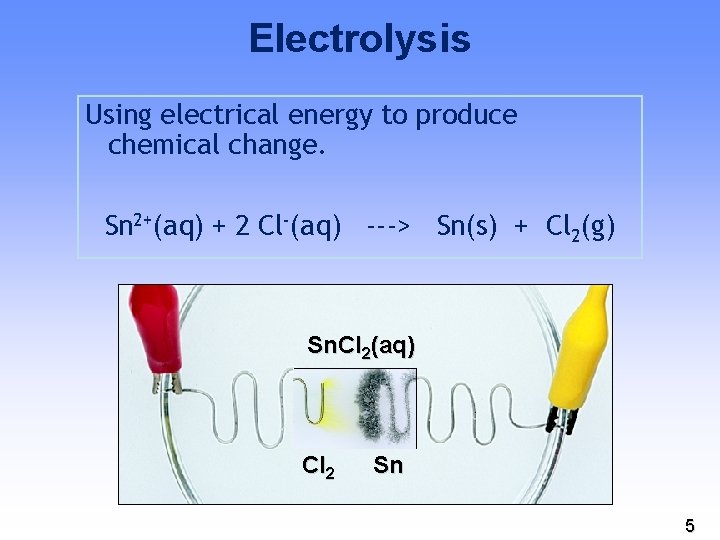
Electrolysis Using electrical energy to produce chemical change. Sn 2+(aq) + 2 Cl-(aq) ---> Sn(s) + Cl 2(g) Sn. Cl 2(aq) Cl 2 Sn 5

In electrolysis, an electric current is used to do which of the following? A. Cause a chemical change B. Produce a battery C. Generate heat D. Run a motor Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved. 6

In electrolysis, an electric current is used to do which of the following? A. Cause a chemical change B. Produce a battery C. Generate heat D. Run a motor 7

Electrolytic Cells Driving Nonspontaneous Processes What are some applications that use electrolytic cells? 8

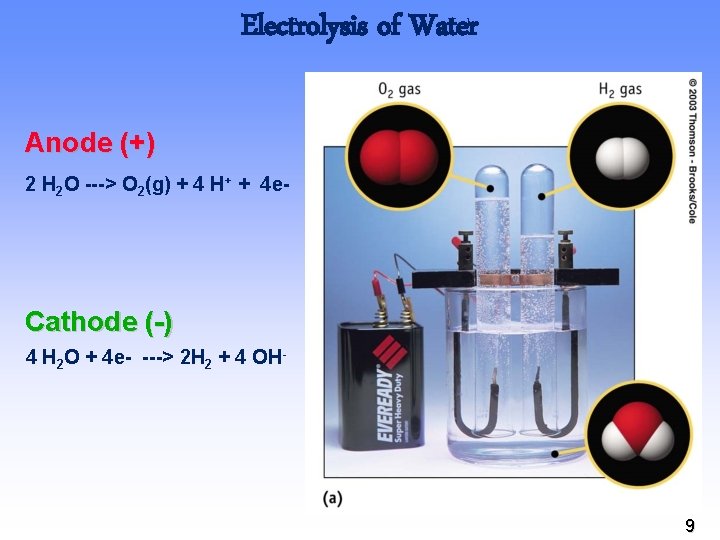
Electrolysis of Water Anode (+) 2 H 2 O ---> O 2(g) + 4 H+ + 4 e- Cathode (-) 4 H 2 O + 4 e- ---> 2 H 2 + 4 OH- 9

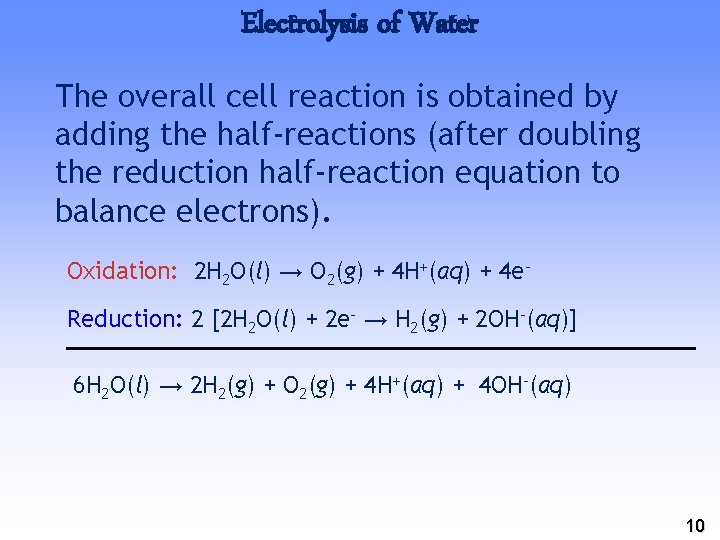
Electrolysis of Water The overall cell reaction is obtained by adding the half-reactions (after doubling the reduction half-reaction equation to balance electrons). Oxidation: 2 H 2 O(l) → O 2(g) + 4 H+(aq) + 4 e– Reduction: 2 [2 H 2 O(l) + 2 e– → H 2(g) + 2 OH–(aq)] 6 H 2 O(l) → 2 H 2(g) + O 2(g) + 4 H+(aq) + 4 OH–(aq) 10

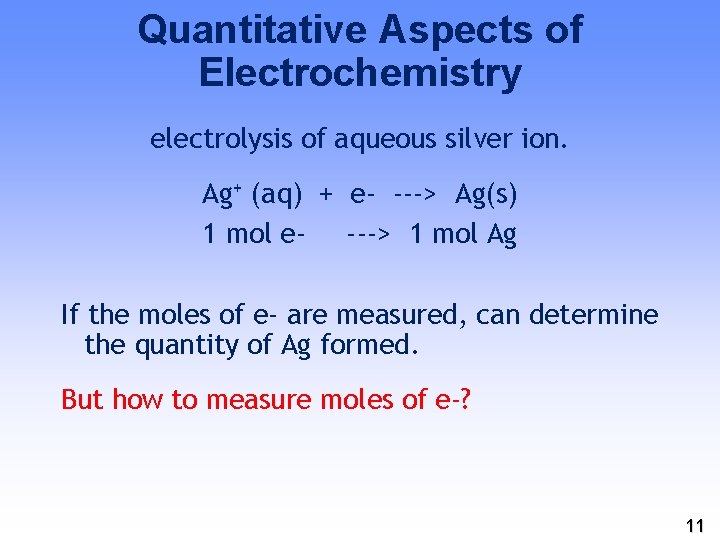
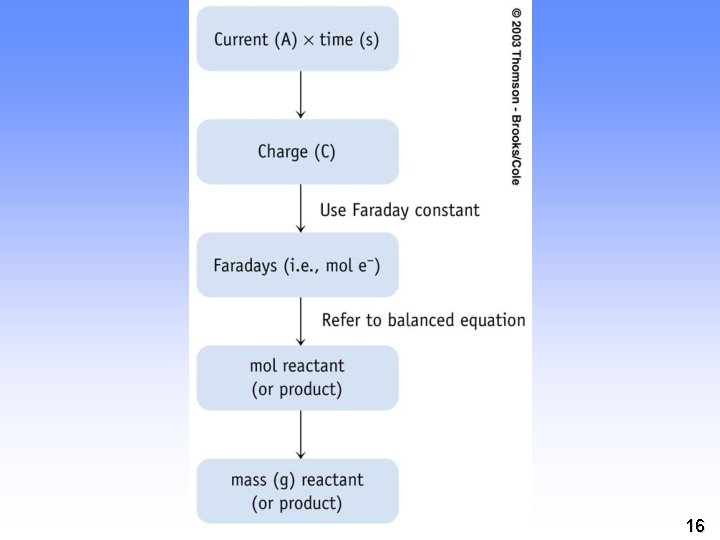
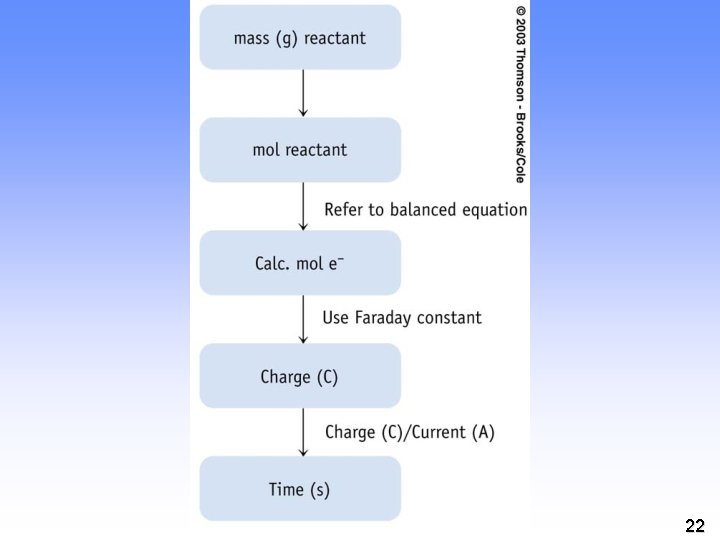
Quantitative Aspects of Electrochemistry electrolysis of aqueous silver ion. Ag+ (aq) + e- ---> Ag(s) 1 mol e- ---> 1 mol Ag If the moles of e- are measured, can determine the quantity of Ag formed. But how to measure moles of e-? 11

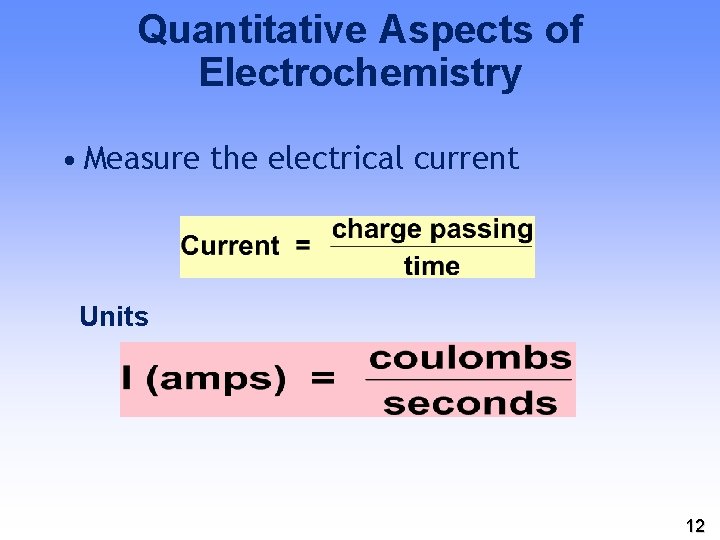
Quantitative Aspects of Electrochemistry • Measure the electrical current Units 12

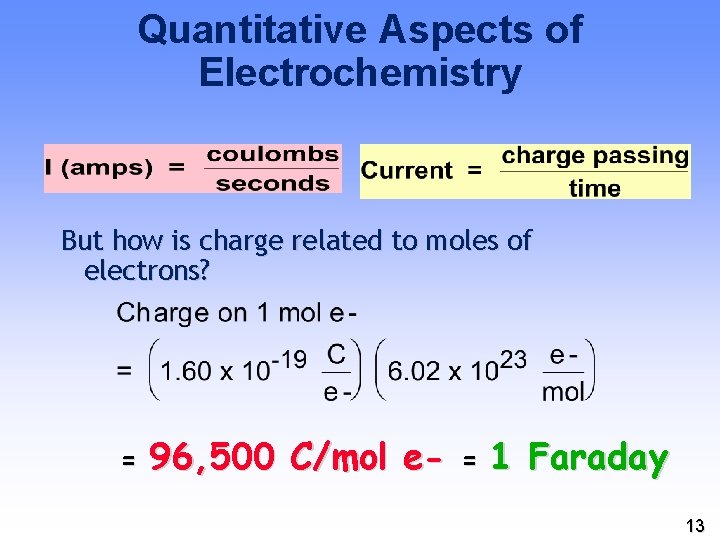
Quantitative Aspects of Electrochemistry But how is charge related to moles of electrons? = 96, 500 C/mol e- = 1 Faraday 13

Michael Faraday 1791 -1867 Originated the terms anode, cathode, anion, cation, electrode. Discoverer of • electrolysis • magnetic props. of matter • electromagnetic induction • benzene and other organic chemicals 14

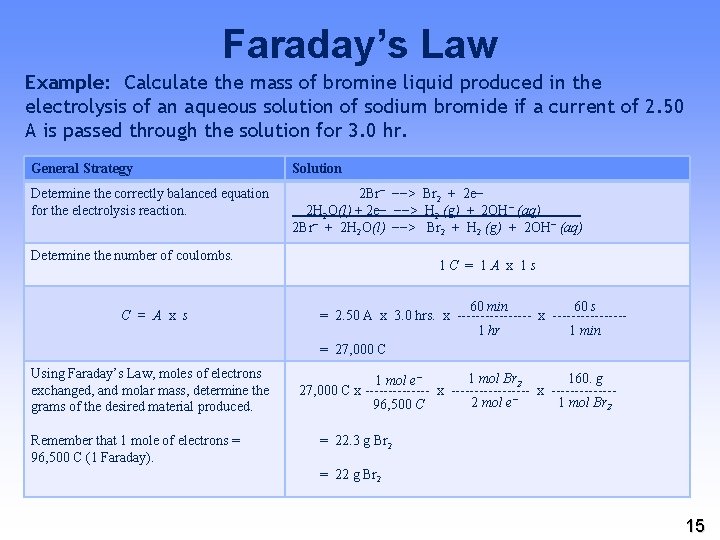
Faraday’s Law Example: Calculate the mass of bromine liquid produced in the electrolysis of an aqueous solution of sodium bromide if a current of 2. 50 A is passed through the solution for 3. 0 hr. General Strategy Solution Determine the correctly balanced equation for the electrolysis reaction. 2 Br− −−> Br 2 + 2 e− 2 H 2 O(l) + 2 e− −−> H 2 (g) + 2 OH− (aq) 2 Br− + 2 H 2 O(l) −−> Br 2 + H 2 (g) + 2 OH− (aq) Determine the number of coulombs. C = A x s Using Faraday’s Law, moles of electrons exchanged, and molar mass, determine the grams of the desired material produced. Remember that 1 mole of electrons = 96, 500 C (1 Faraday). 1 C = 1 A x 1 s 60 min 60 s = 2. 50 A x 3. 0 hrs. x --------1 hr 1 min = 27, 000 C 1 mol Br 2 160. g 1 mol e− 27, 000 C x ----------------- x -------2 mol e− 1 mol Br 2 96, 500 C = 22. 3 g Br 2 = 22 g Br 2 15

16

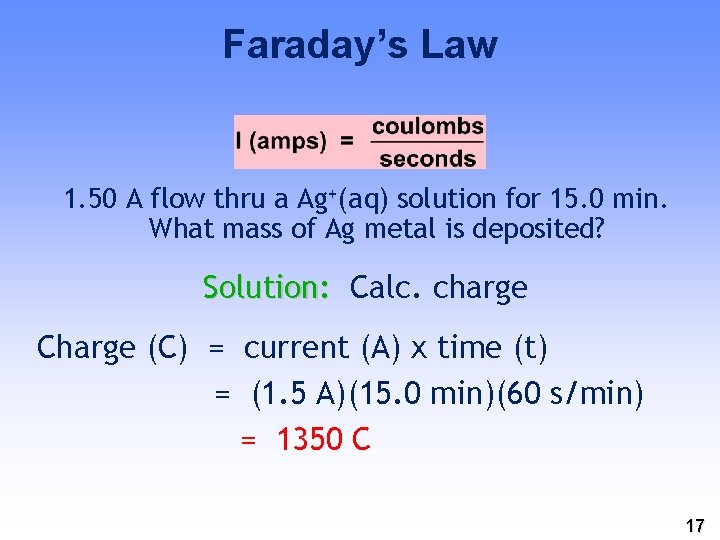
Faraday’s Law 1. 50 A flow thru a Ag+(aq) solution for 15. 0 min. What mass of Ag metal is deposited? Solution: Calc. charge Charge (C) = current (A) x time (t) = (1. 5 A)(15. 0 min)(60 s/min) = 1350 C 17

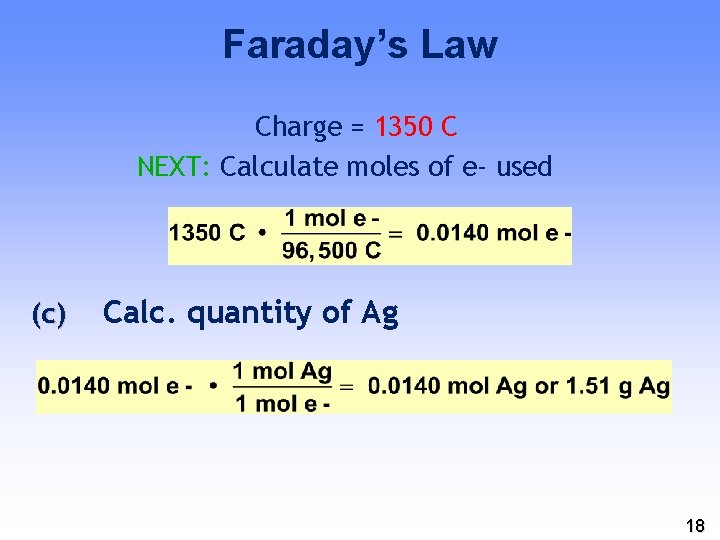
Faraday’s Law Charge = 1350 C NEXT: Calculate moles of e- used (c) Calc. quantity of Ag 18

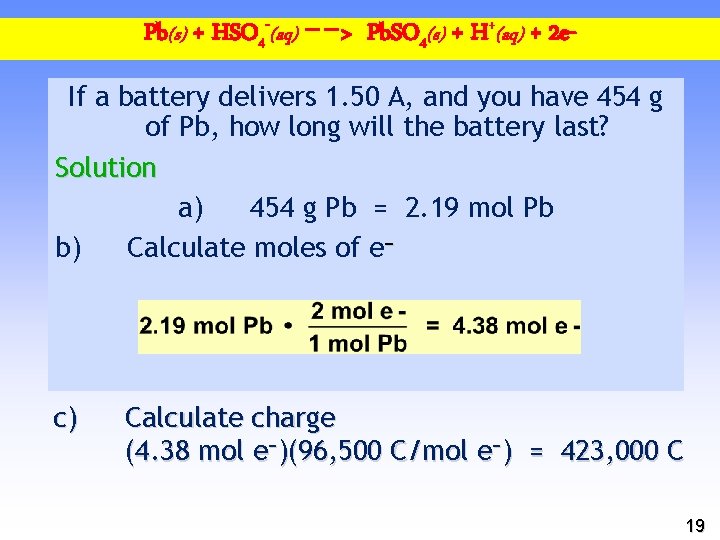
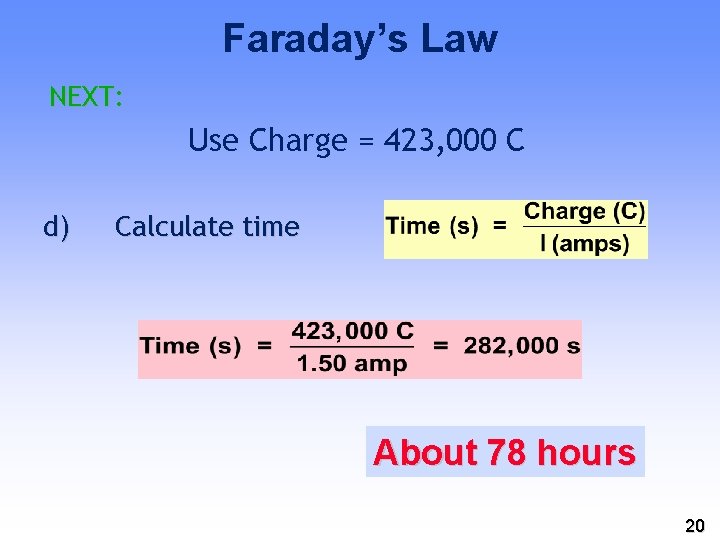
Pb(s) + HSO 4 -(aq) −−> Pb. SO 4(s) + H+(aq) + 2 e. If a battery delivers 1. 50 A, and you have 454 g of Pb, how long will the battery last? Solution a) 454 g Pb = 2. 19 mol Pb b) Calculate moles of e− c) Calculate charge (4. 38 mol e−)(96, 500 C/mol e−) = 423, 000 C 19

Faraday’s Law NEXT: Use Charge = 423, 000 C d) Calculate time About 78 hours 20

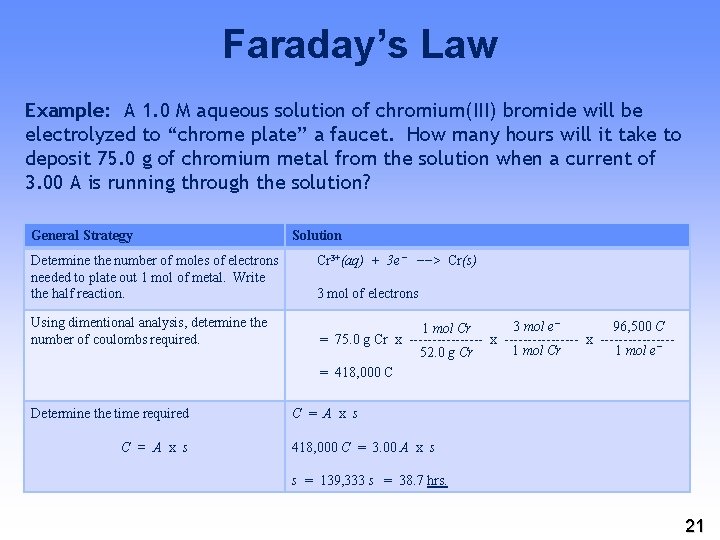
Faraday’s Law Example: A 1. 0 M aqueous solution of chromium(III) bromide will be electrolyzed to “chrome plate” a faucet. How many hours will it take to deposit 75. 0 g of chromium metal from the solution when a current of 3. 00 A is running through the solution? General Strategy Solution Determine the number of moles of electrons needed to plate out 1 mol of metal. Write the half reaction. Cr 3+(aq) + 3 e− −−> Cr(s) Using dimentional analysis, determine the number of coulombs required. 3 mol e− 96, 500 C 1 mol Cr = 75. 0 g Cr x ---------------- x --------1 mol Cr 1 mol e− 52. 0 g Cr = 418, 000 C Determine the time required C = A x s 3 mol of electrons C = A x s 418, 000 C = 3. 00 A x s s = 139, 333 s = 38. 7 hrs. 21

22

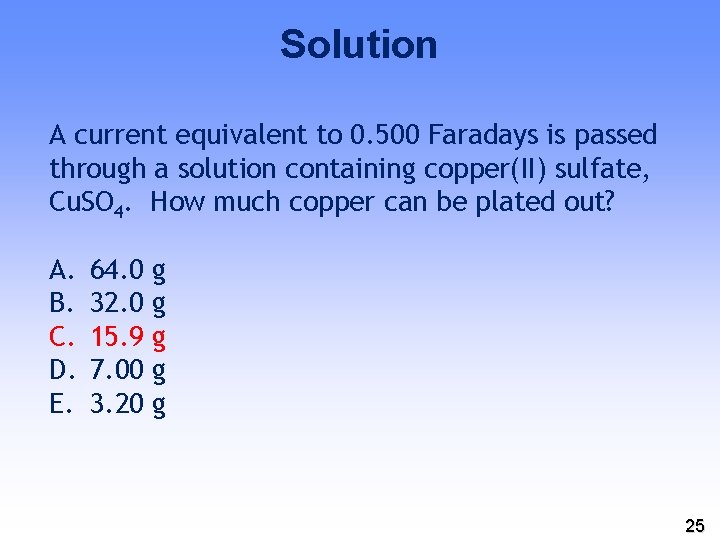
YOUR TURN A current equivalent to 0. 500 Faradays is passed through a solution containing copper(II) sulfate, Cu. SO 4. How much copper can be plated out? A. B. C. D. E. 64. 0 32. 0 15. 9 7. 00 3. 20 g g g 23

Solution A current equivalent to 0. 500 Faradays is passed through a solution containing copper(II) sulfate, Cu. SO 4. How much copper can be plated out? Cu 2+ Cu + 2 e− 0. 500 Faradays = 0. 500 mol e− x ½ mol Cu = 0. 250 mol Cu x 63. 55 g/mol = 15. 89 g 24

Solution A current equivalent to 0. 500 Faradays is passed through a solution containing copper(II) sulfate, Cu. SO 4. How much copper can be plated out? A. B. C. D. E. 64. 0 32. 0 15. 9 7. 00 3. 20 g g g 25

Homework Review Sections 9. 1 and 9. 2. Review questions at the end of Topic 9. Complete Kerboodle quiz 9. 2. Study for TEST. 26