Electrochemistry Introduction Voltaic Cells Electrochemical Cell Electrochemical device






- Slides: 17

Electrochemistry Introduction Voltaic Cells

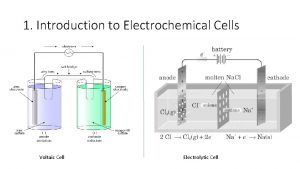
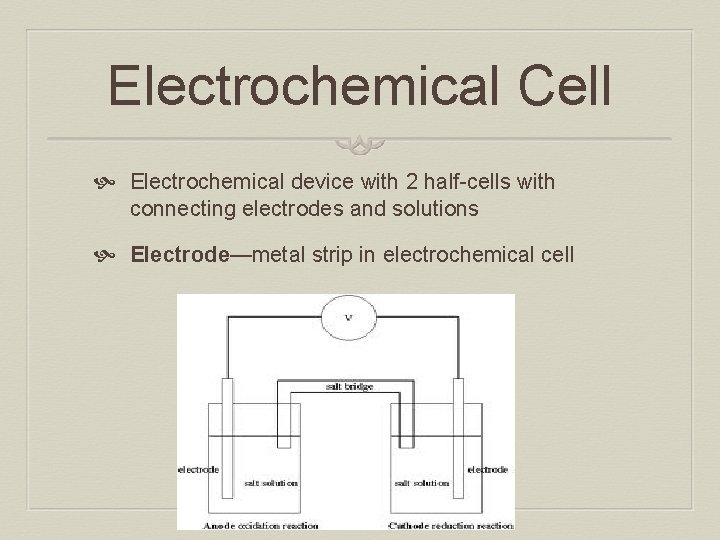
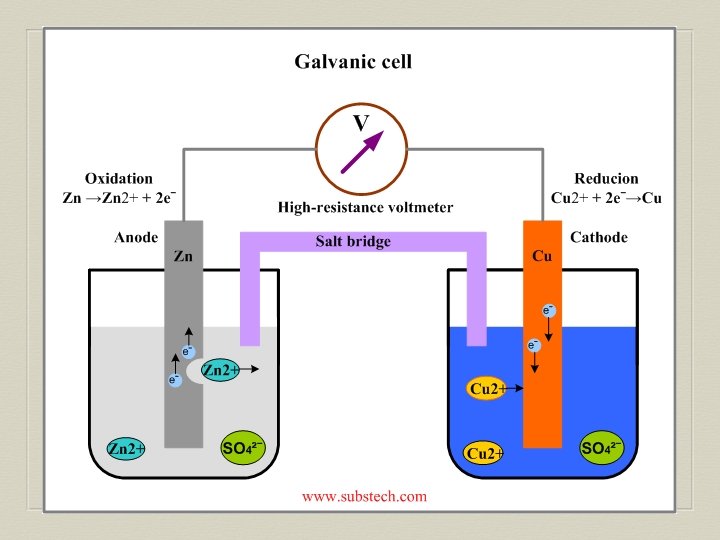
Electrochemical Cell Electrochemical device with 2 half-cells with connecting electrodes and solutions Electrode—metal strip in electrochemical cell

2 types of electrochemical cells 1) Voltaic Cells 2) Electrolytic Cells Still dealing with oxidation-reduction reactions Physical separation of oxidation and reduction processes

1) Voltaic Cells “simple battery” Electric current generated from a redox reaction in an electrochemical cell Pathway of electron transfer Redox reactions in this cell are always SPONTANEOUS (ΔG < 0 ) Physically separates oxidation process from reduction process


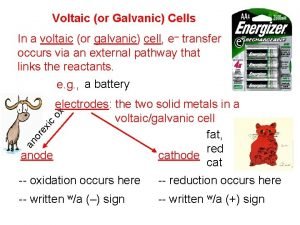
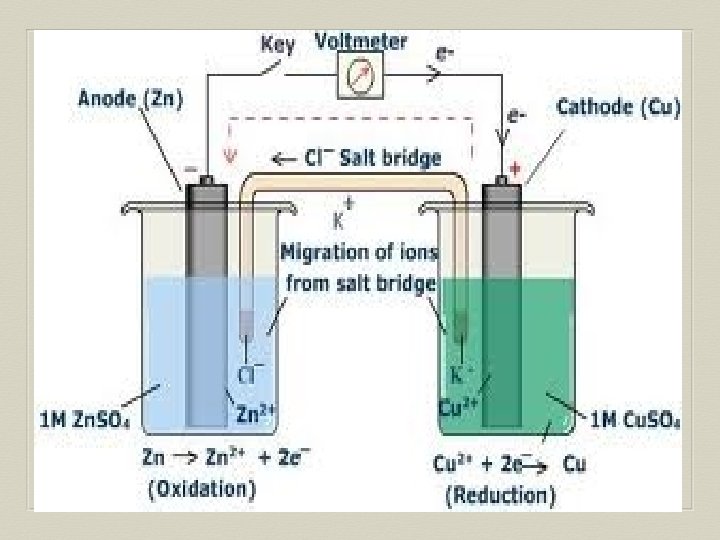
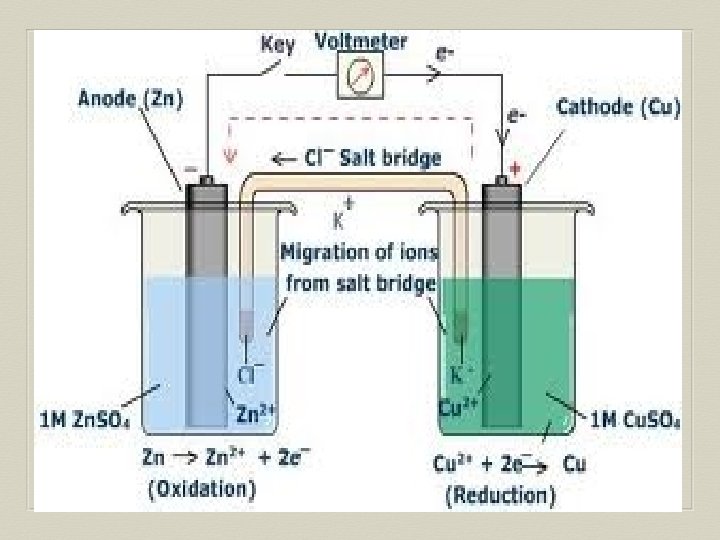
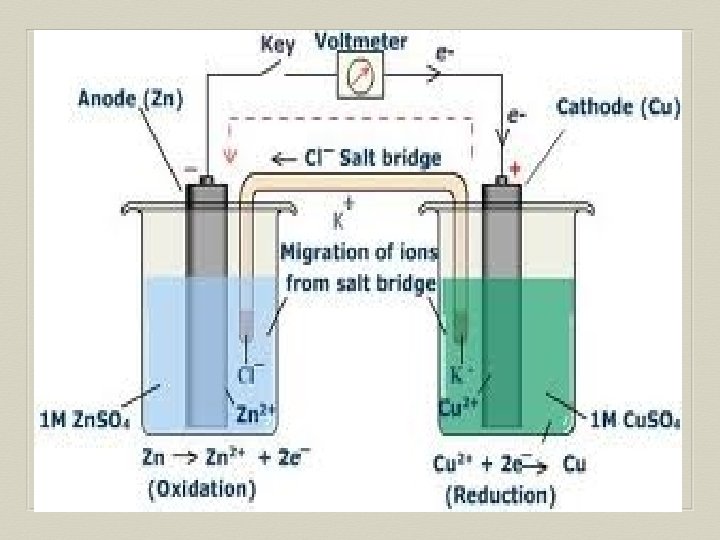
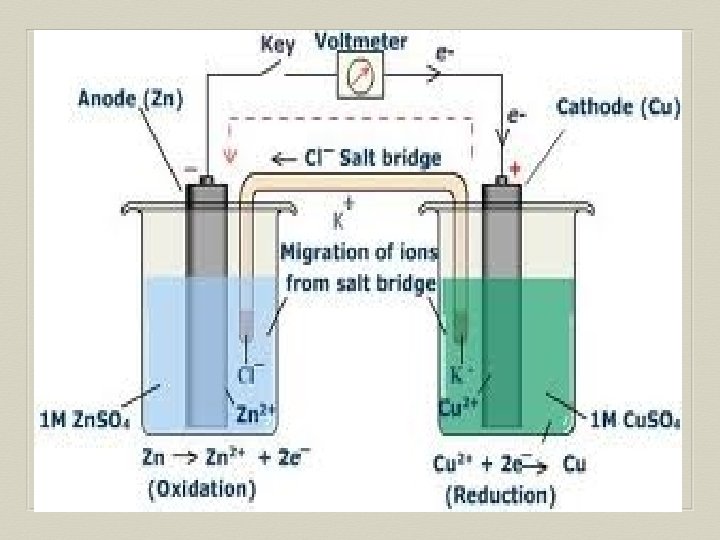
Voltaic Cell—Oxidation Process Anode Electrode where oxidation occurs Negative charge, source of electrons Metal electrode dissolves and metallic ions form in solution Electrons released into solution and buildup NEGATIVE charge at electrode---electrons migrate out through connecting wire GIVING UP ELECTRONS !!


Voltaic Cell—Reduction Process Cathode Electrode where reduction occurs Positive charge, electron receiver, ion source Metallic ions (positive ions) move to electrode surface and accept electrons coming from the anode through the connecting wire. Metallic ions converted to solid metal on the electrode


Salt Bridge U-shaped tube containing a soluble salt in a saturated solution (ex. KNO 3) Salt solution MUST be soluble, not form precipitate Maintains electrical neutrality within cell solutions Electrons do NOT go through bridge, only through wire Salt dissociates into ions, ions move to balance charges Negative ions move to ANODE, minimize POSITIVE charge Positive ions move to CATHODE, minimize NEGATIVE charge No acting role in redox reaction


General Points for Voltaic Cells Electrons move from ANODE (-) to CATHODE (+) Electrons have HIGH potential energy at anode, not cathode Naturally favor a state of low potential energy Electron movement through the connecting wire generates an electric current that can be utilized.


Cell Potential (Ecell) Also called “cell voltage” Difference between the electric potential between the electrodes in an electrochemical cell Voltaic cell’s potential to do work on the environment through the generation of an electric current Magnitude indicates amount of current generated through redox reaction occurring in the electrochemical cell Measured by voltmeter V = J/C

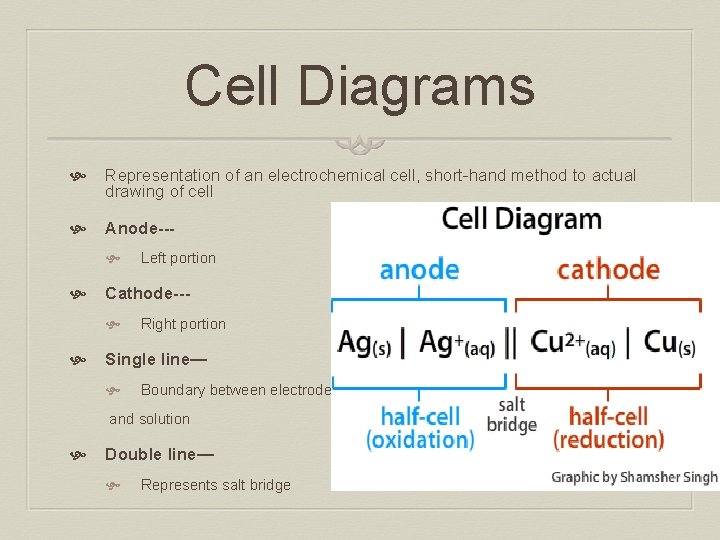
Cell Diagrams Representation of an electrochemical cell, short-hand method to actual drawing of cell Anode-- Cathode-- Left portion Right portion Single line— Boundary between electrode and solution Double line— Represents salt bridge

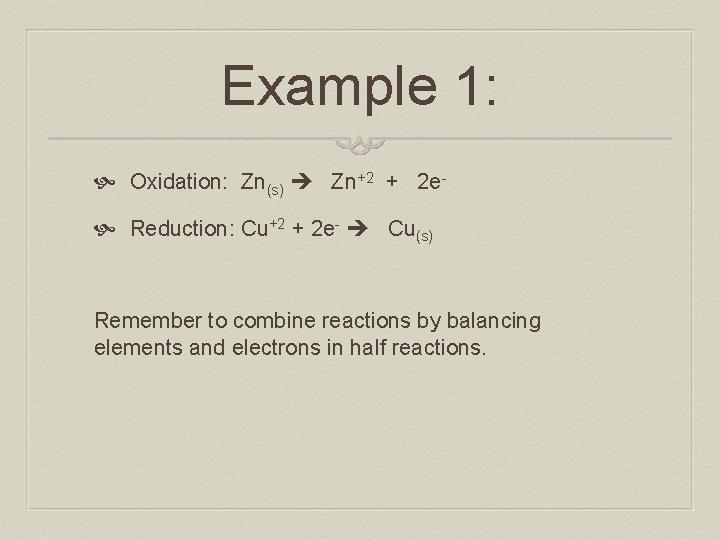
Example 1: Oxidation: Zn(s) Zn+2 + 2 e Reduction: Cu+2 + 2 e- Cu(s) Remember to combine reactions by balancing elements and electrons in half reactions.

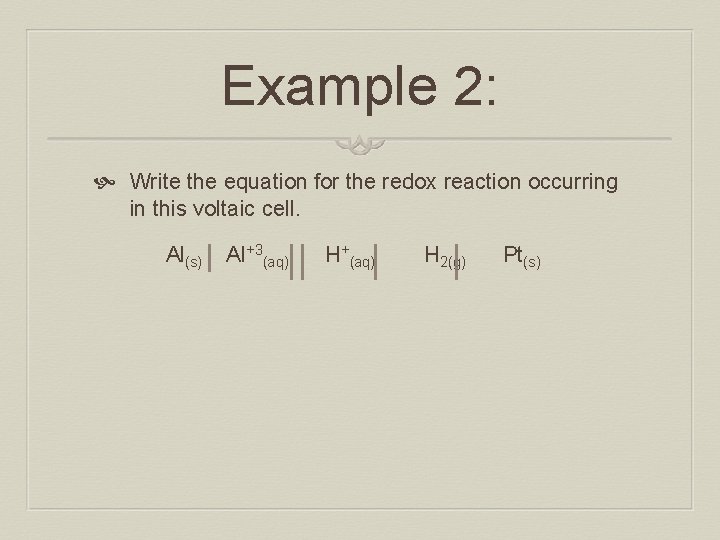
Example 2: Write the equation for the redox reaction occurring in this voltaic cell. Al(s) Al+3(aq) H+(aq) H 2(g) Pt(s)
 Voltaic cells example #2 worksheet answers
Voltaic cells example #2 worksheet answers Chapter 21 electrochemistry
Chapter 21 electrochemistry Voltaic and galvanic cells
Voltaic and galvanic cells Lithium ion battery reaction equation
Lithium ion battery reaction equation Voltaic cell components
Voltaic cell components Voltaic cell electron flow
Voltaic cell electron flow Primary battery and secondary battery
Primary battery and secondary battery Voltaic cell virtual lab
Voltaic cell virtual lab Flow of anions and cations in an electrochemical cell
Flow of anions and cations in an electrochemical cell Balance redox
Balance redox Hardware output
Hardware output Redox half reactions
Redox half reactions Voltaic fundamental
Voltaic fundamental Voltaic fundamentals
Voltaic fundamentals Voltaic fundamentals
Voltaic fundamentals Voltaic aim
Voltaic aim Voltaic aim
Voltaic aim Intro to electrochemistry
Intro to electrochemistry