UNIT 4 CORROSION ITS PREVENTION Mr V M





























- Slides: 63


UNIT: 4 CORROSION & ITS PREVENTION Mr. V. M. Bansode Mechanical Dept. DYPIEMR

ISSUES TO ADDRESS. . . v Why does corrosion occur? v How do temperature and environment affect corrosion rate? v How do we suppress corrosion?

Objective ü What Is Corrosion ü Review Of Basic Metallurgy ü Various Forms Of Corrosion ü Corrosion Monitoring And Inspection ü Fundamentals Of Corrosion Prevention ü Corrosion Prevention Methods 1. Corrosion Prevention Using Inhibitors 2. Corrosion Prevention Using Coatings 3. Corrosion Prevention By Removal Of Corrosive Gases 4. Corrosion Prevention By Use Of Proper Construction Materials / Metals. ( With few Case Studies ) 5. Corrosion Prevention–miscellaneous Methods

Introduction to Corrosion

Ø Any iron article if exposed to moist air, gets rusted or if brass component is exposed to atmosphere it gets covered with green oxide film. Ø The chemical or electrochemical reactions between metals & environment such as air, water, sea water, acid, alkali, gas leads to corrosion. Ø These reactions occurs at different temp. , at different rate & produce different forms of products by consuming metal, which leads to decay or deterioration of metals. Ø Corrosion = deterioration or destruction of metal

corrosion � It is the destruction of metal through unwanted or unintentional chemical or electrochemical reaction which occur between surface of metal & environment. � It is a slow process but it is persistent. � No any metal can withstand corrosive attack in all environment. � All metal will corrode under certain condition & it will become useless or will get destroyed. � Due to corrosion material losses it strength & fails ultimately. � Corrosion involves loss of electron by metal atoms (oxidation) & gain of electrons by other atom (reduction)

Effects of corrosion �Loss of process efficiency. �Life of components gets reduced. �Replacement of corroded equipment is must. �Machine failure occurs leads to probability of plant shut down. �Possibility of accidents & hazards. So it is necessary to understand the mechanism of corrosion so that it can be prevented or minimized.

Types of corrosion �According to the corrosive environment it is classified into various types such as: 1. Direct chemical /dry/ atmospheric corrosion: chemical reaction between metal & atmospheric gases to which it is exposed. 2. Electrochemical /wet/ immersed corrosion: electrochemical reaction between metal & electrolyte (aqueous salt solution, acid or alkali)

Formation & growth of film �Adsorption: secondary attraction forces between the residual valency of the metal atoms at the surface and oxygen molecules. �Chemisorption: The oxygen molecules at the surface gradually enter into chemical combination with the surface metal atoms by electron transfer or sharing mechanism. �Growth of film a)Growth of non-porous film b)Growth of porous film

Growth laws 1. Parabolic law 2. Logarithmic law 3. Cubic law 4. Linear law

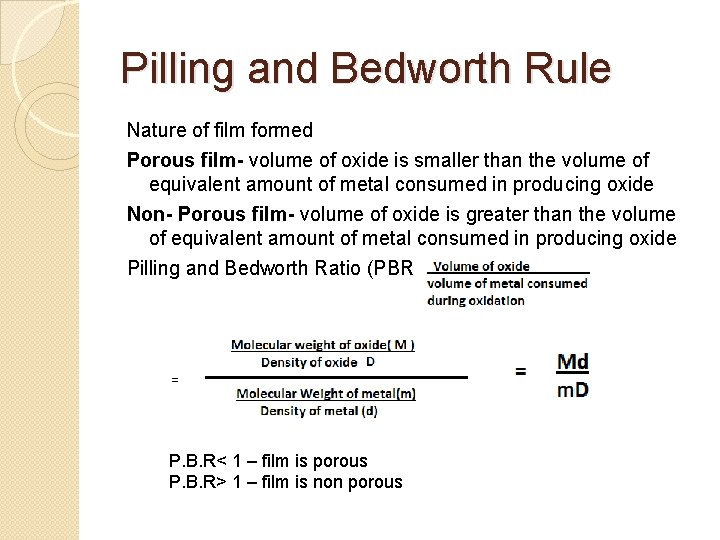
Pilling and Bedworth Rule Nature of film formed Porous film- volume of oxide is smaller than the volume of equivalent amount of metal consumed in producing oxide Non- Porous film- volume of oxide is greater than the volume of equivalent amount of metal consumed in producing oxide Pilling and Bedworth Ratio (PBR) = P. B. R< 1 – film is porous P. B. R> 1 – film is non porous

Direct chemical /dry/ atmospheric corrosion � This type of corrosion occurs when metal is exposed to gaseous atmosphere like hydrogen, oxygen, nitrogen, halogen, etc. � Extent of corrosion depends upon: 1. Chemical affinity: if it is high then corrosion will be high. 2. Protective value of film: if film formed due to corrosion is protective then rate of corrosion decreases. 3. Nature of formed film: if film is non porous then it is protective. 4. Adhesion between film & metal surface: if adhesion is high then protective value will be more. 5. Conductivity of film: for non-porous film corrosion depends on electronic & ionic conductivities of film. Lower the conductivity, less will be the corrosion.

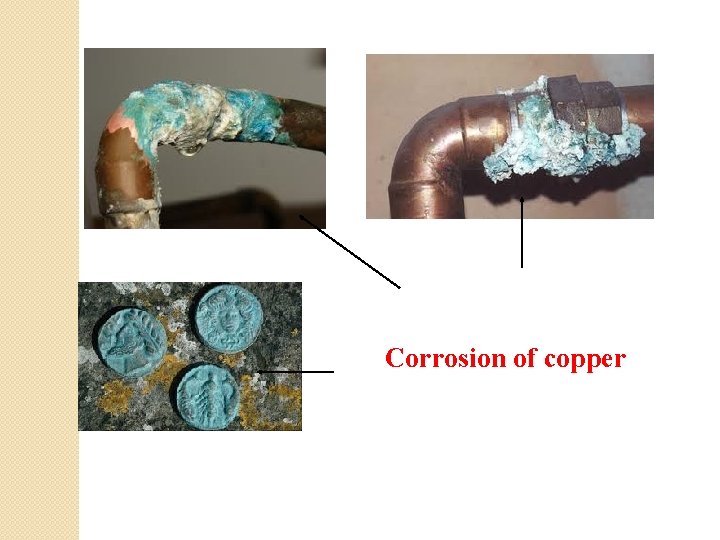
Common examples of direct corrosion

Tarnishing of silverware Rusting of iron & steel

Corrosion of copper

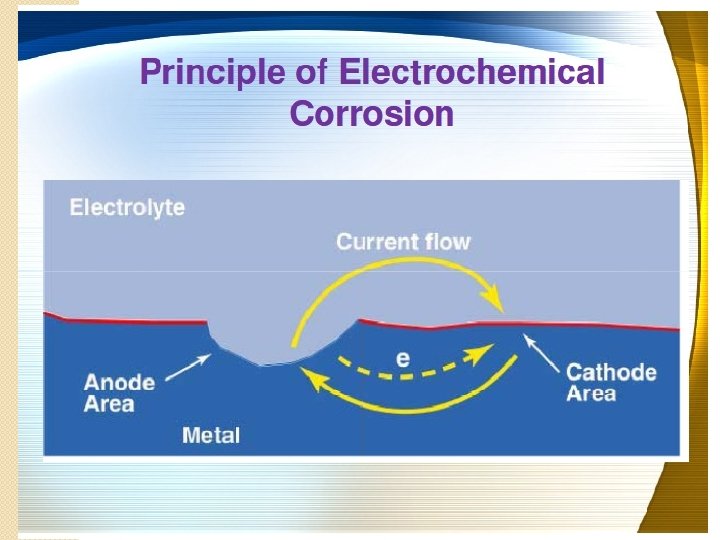
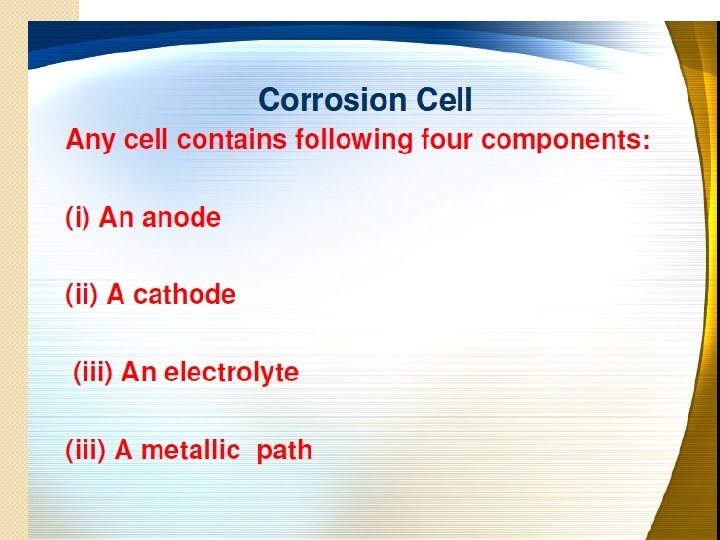
Electrochemical corrosion � It is also known as wet / indirect / � This type of corrosion occurs immersed corrosion. mainly under wet or moist conditions through electrochemical reaction. � Conditions for wet corrosion are: 1. When conducting liquid is in contact with metal. 2. When 2 dissimilar metals are immersed or single metal is dipped partially in aqueous solution of acid, base or salt & involves transfer of electrons. 3. In both the cases there should be flow of electricity from certain areas of metal surface to other areas through the electrolyte. 4. Potential difference must be there in between two dissimilar metals or between two different areas on metal surface. 5. Due to the chemical & structural difference in metal & its environment metal surface develop anodic & cathodic area. 6. Corrosion occurs due to existence of anodic & cathodic area between which current flows through electrolyte.

� At anode= liberation of free electrons occurs (oxidation), thus metal decay or get destroy at anode. � At cathode= gain of electrons (reduction) no any decay or destruction of metal at cathode area. � According to Nernst's theory, all metals donate electrons called as electrode potential. � Different metals have different electrode potentials when placed in same solution at same temperature. � Electrode potential= potential difference developed between metal ion in solution & electrons on metal surface at equilibrium. � Electrode potential depends on: 1. Chemical nature of metal. 2. Nature of electrolyte. 3. Temperature of solution to some extent.



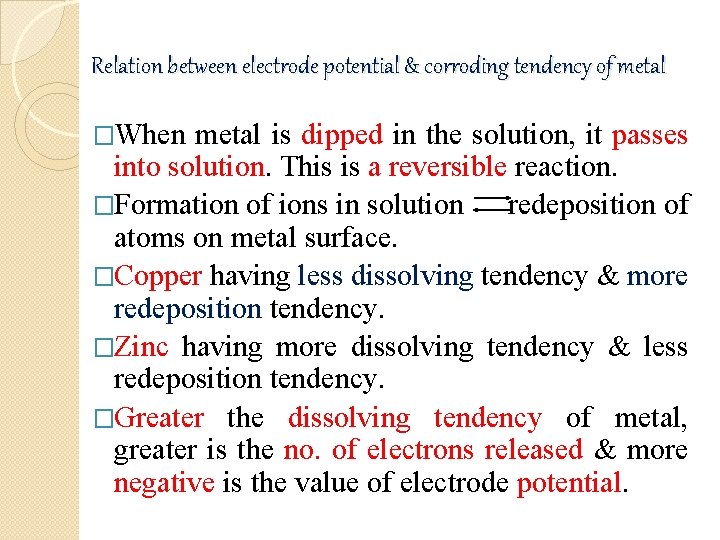
Relation between electrode potential & corroding tendency of metal �When metal is dipped in the solution, it passes into solution. This is a reversible reaction. �Formation of ions in solution redeposition of atoms on metal surface. �Copper having less dissolving tendency & more redeposition tendency. �Zinc having more dissolving tendency & less redeposition tendency. �Greater the dissolving tendency of metal, greater is the no. of electrons released & more negative is the value of electrode potential.

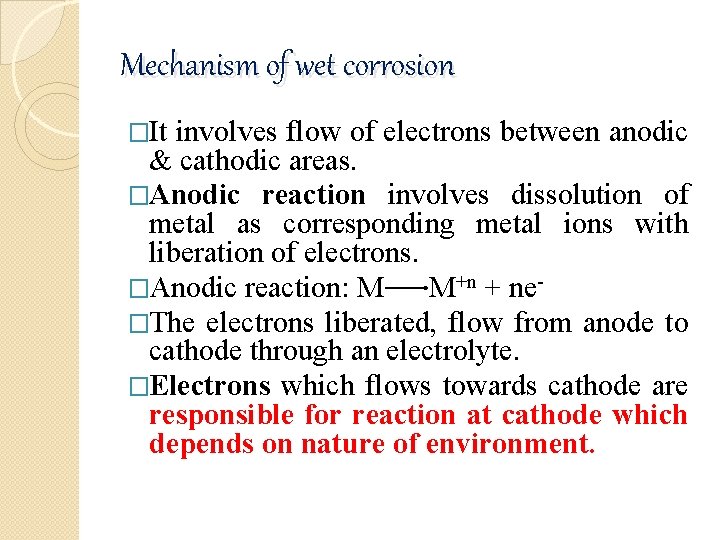
Mechanism of wet corrosion �It involves flow of electrons between anodic & cathodic areas. �Anodic reaction involves dissolution of metal as corresponding metal ions with liberation of electrons. �Anodic reaction: M M+n + ne�The electrons liberated, flow from anode to cathode through an electrolyte. �Electrons which flows towards cathode are responsible for reaction at cathode which depends on nature of environment.

Mechanism of wet corrosion � Cathodic reaction (absence � Hydrogen ions combine of oxygen) with electrons & form hydrogen gas. � 2 H+ + 2 e-1 H 2(g) � In acidic medium (presence of oxygen) � 2 H+ + 4 e-1 + O 2 2 H 2 O � In neutral or alkaline medium (presence of oxygen): � 2 H 2 O + O 2 + 4 e-1 4 OH� Electrochemical reaction involves any one of the following mechanism 1. Hydrogen evolution 2. Oxygen absorption

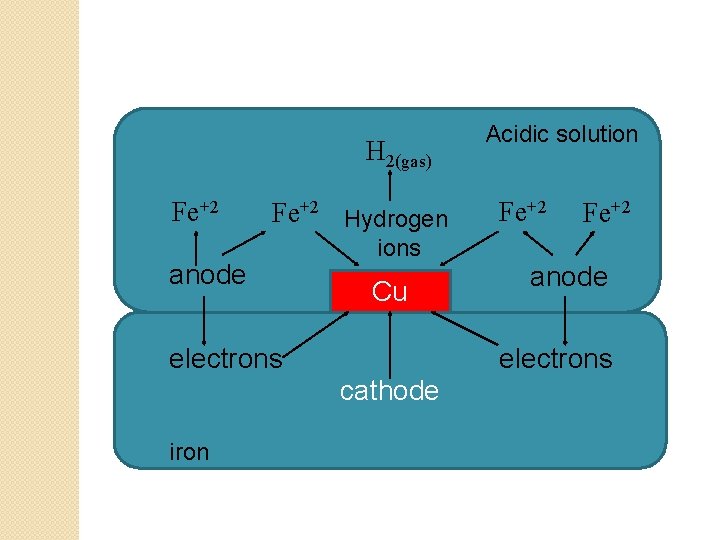
Hydrogen evolution mechanism �This type of electrochemical corrosion occurs usually in acidic environment like industrial waste, solution of non oxidizing acids. �Example: steel tank containing acid industrial waste & small copper scrap are in contact. �Pieces of copper & steel tank are in contact with each other in presence of acid electrolyte give rise to an electrochemical cell. �In this cell, steel= anode & copper= cathode. �Steel tank portion which is in contact with copper gets corrode

� At anode (at steel tank): � Iron passes into solution as Fe+2 ions � Fe Fe+2 + 2 e-1 � Free electrons are accumulated at cathode. � At cathode (at copper pieces): � Hydrogen ions from acidic electrolyte take up the free electrons & hydrogen gas formed is liberated in the form of bubbles at cathode. � 2 H+ + 2 e-1 H 2(g) (reduction ) � Net cell reaction: Fe + 2 H+ Fe+2 + H 2(g) � Corrosion by this mechanism involves displacement of hydrogen ions from acidic electrolyte by metal ions. � All metals above hydrogen in electrochemical series will have tendency to get dissolved in acidic electrolyte with simultaneous liberation of hydrogen. � Anode must have greater area than that of the cathode for this type of corrosion.

H 2(gas) Fe+2 Hydrogen anode ions Cu electrons Fe+2 anode electrons cathode iron Acidic solution

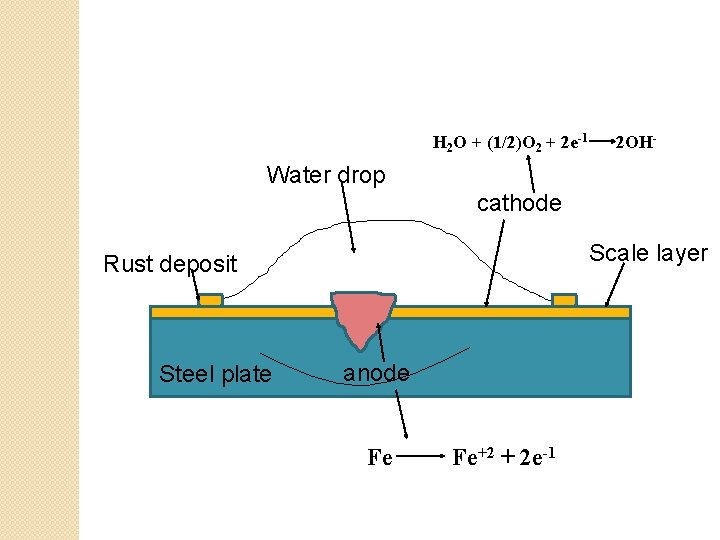
Oxygen absorption mechanism �This type of electrochemical corrosion occurs when electrolyte is neutral aqueous solution or alkaline solution containing dissolved oxygen. �Example: steel plate lying on the ground & exposed to atmosphere. �In due course of time, oxide layer will form on surface of steel plate & drops of water may be collected over a small crack on oxide film present on surface of steel. �In this case water act as an electrolyte, iron plate underlying through the crack act as anode & oxide scale on steel surface act as cathode.

� Reactions involved in this type of corrosion at anode & cathode are as follows: � At anode: Fe Fe+2 + 2 e-1 � At cathode: electrons flow from anode to cathode that is from crack to oxide scale on surface of steel plate. These electrons are interrupted by dissolved oxygen present in electrolyte, forming hydroxyl ion as: H 2 O + (1/2)O 2 + 2 e-1 2 OH� Fe+2 & OH- ions at cathode diffuses & combine to form ferrous hydroxide. � Outward diffusion of Fe+2 ions are more rapid due to its small size as compared to OH� Thus although the corrosion takes place at anode, rust deposition occurs at cathode. � Fe+2 + 2 OHFe(OH)2 � If enough oxygen is present in electrolyte, ferrous hydroxide is easily oxidized to ferric hydroxide. � If oxygen is limited, then corrosion product may be black anhydrous Fe 3 O 4. � In oxygen absorption mechanism cathode area is more than that of the anode

H 2 O + (1/2)O 2 + 2 e-1 2 OH- Water drop cathode Scale layer Rust deposit Steel plate anode Fe Fe+2 + 2 e-1

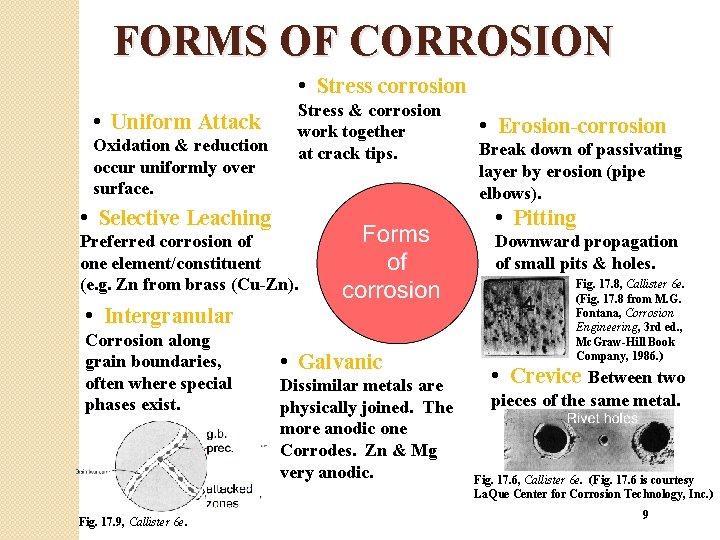
FORMS OF CORROSION • Stress corrosion • Uniform Attack Oxidation & reduction occur uniformly over surface. Stress & corrosion work together at crack tips. • Erosion-corrosion Break down of passivating layer by erosion (pipe elbows). • Selective Leaching • Pitting Preferred corrosion of one element/constituent (e. g. Zn from brass (Cu-Zn). Downward propagation of small pits & holes. • Intergranular Corrosion along grain boundaries, often where special phases exist. Fig. 17. 9, Callister 6 e. • Galvanic Dissimilar metals are physically joined. The more anodic one Corrodes. Zn & Mg very anodic. Fig. 17. 8, Callister 6 e. (Fig. 17. 8 from M. G. Fontana, Corrosion Engineering, 3 rd ed. , Mc. Graw-Hill Book Company, 1986. ) • Crevice Between two pieces of the same metal. Fig. 17. 6, Callister 6 e. (Fig. 17. 6 is courtesy La. Que Center for Corrosion Technology, Inc. ) 9

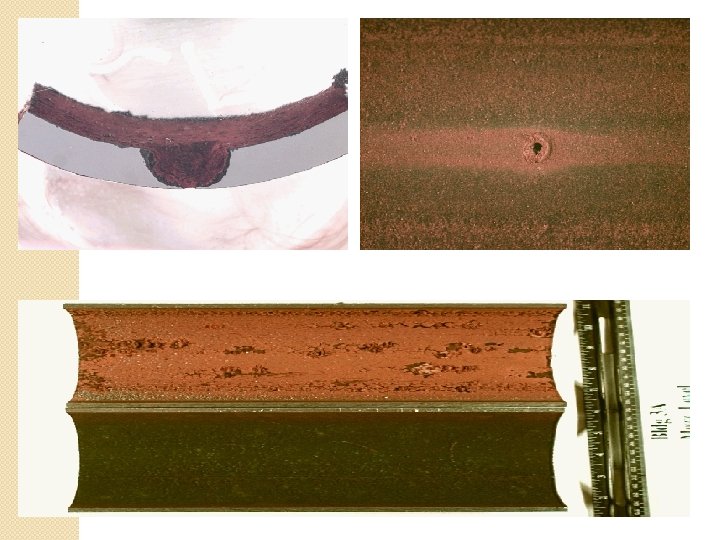
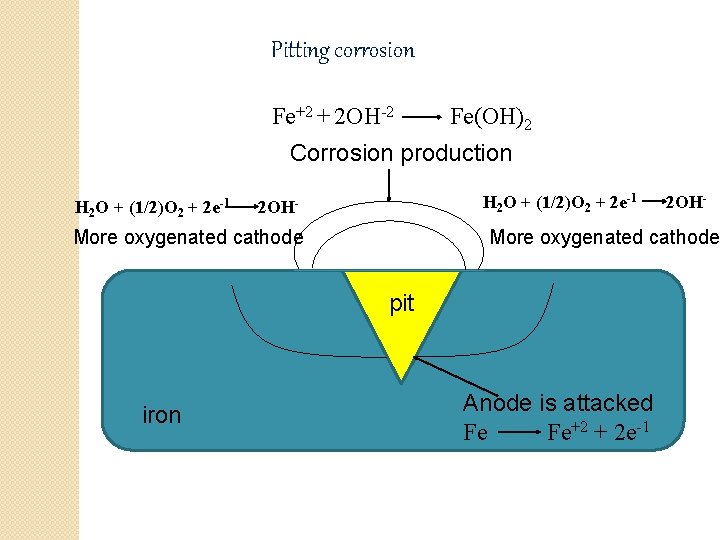
Pitting corrosion �It is localized, accelerated attack. �It produces pinholes, pits, cavities in the metal. �Pitting corrosion is very destructive because of the pit may penetrate deep into metal. �This type of corrosion is caused due to breakdown or cracking of protective film on metal at specific points. �Breakdown of protective film occurs due to various mechanical factors such as scratches, local straining of metal due to non uniform stresses, sliding under load, chemical attack, presence of impurity on metal. �It results into the formation of small anodic & large cathodic area. �Pits appear in conical or hemispherical shapes. �Corrosion product may form cap over pit cavities.

Pitting corrosion Fe+2 + 2 OH-2 Fe(OH)2 Corrosion production H 2 O + (1/2)O 2 + 2 e-1 2 OH- More oxygenated cathode pit iron 2 OH- Anode is attacked Fe Fe+2 + 2 e-1

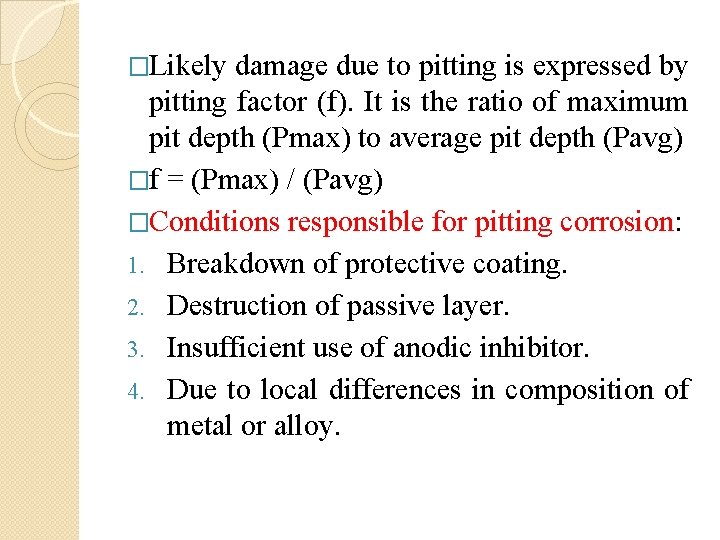
�Likely damage due to pitting is expressed by pitting factor (f). It is the ratio of maximum pit depth (Pmax) to average pit depth (Pavg) �f = (Pmax) / (Pavg) �Conditions responsible for pitting corrosion: 1. Breakdown of protective coating. 2. Destruction of passive layer. 3. Insufficient use of anodic inhibitor. 4. Due to local differences in composition of metal or alloy.

Stress corrosion This is produced due to combined effect of mechanical stress & corrosive environment on metal. � Stressed area become anodic with respect to other areas & get corroded by forming stress cells. � If stresses are tensile it leads to cracking of material in due coarse of time when exposed to environment. Hence it is called as stress corrosion cracking. � Tensile stress present in material may be residual (internal) or applied (external) �

�Internal stresses developed because of cold working & due to non-uniform & rapid cooling, welding poor design, precipitation of second phase. �Due to application of external loads on the component, external stresses are produced. �When stress exceed minimum level of stress i. e. critical level of stress cracking occurs.

Critical level of stress depends on factors: � Composition of the alloy � Grain size, microstructure � Surface of the component � Type of environment � Service temperature. � For different materials, & geometry of component type of environment is different: 1. Carbon steel crack in nitrate solutions. 2. Cu & its alloy crack in ammonia & mercury environment 3. Austenitic stainless steel crack in solution containing high chloride iron.

Season cracking �Stress corrosion cracking of copper alloys mainly brass is called as season cracking. �When humidity is very high, chances of cracking is also high, hence called as season cracking. �Single phase alpha brass susceptible to this type of failure. �Cracks may be intergranular or transgranular depends on environment & magnitude of present stress.

Cavitation corrosion �It occurs by simultaneous action of corrosion & cavitation. �If liquid passes from high pressure to low pressure, the air or vapor bubbles collapse against solid surface. �This causes corrosion of material by pitting. �It is observed in ship propellers, rotating pumps, valves. �Cavitation corrosion can be minimized by: 1. Use of suitable hydraulic design. 2. Use of high strength corrosion resistant alloy. 3. Use of protective coatings

Erosion corrosion (impingement corrosion) � This type of corrosion is caused by combined effect of the abrading action of turbulent flow of gases, vapours liquid & mechanical rubbing action of solid over a metal surface. � It is caused due to breakdown of protective film at the spot of impingement & its subsequent inability to repair itself under existing abrading condition. � The abrading action removes protective film from localized spots on the metal surface, there by resulting in the formation of differential aeration cell. � The result of this corrosion is localized which may lead to formation of pit at anodic point of the cell. � This type of corrosion can occur in any metal or alloy. � This is occurred in the region where flow of electrolyte is disturbed. � Example: bend in pipes, pump parts, condenser tubes � Abrasive particles & air bubbles in liquid increase the chances & intensity of erosion corrosion.

Intergranular corrosion � This type of corrosion occurs in granular metals & in homogeneous alloys. � This corrosion proceeds along the grain boundaries & is microscopic in nature. � Intergranular corrosion occurs along grain boundaries & region where material is sensitive to the corrosion attacks. � Corrosive liquid attack only grain boundaries & leaves grain interior untouched or attack only slightly. � This type of corrosion occurs due to the grain boundaries contain that material which shows electrode potential more anodic than that of grain center in corroding medium. � This leads to precipitation of certain compounds at grain boundaries.

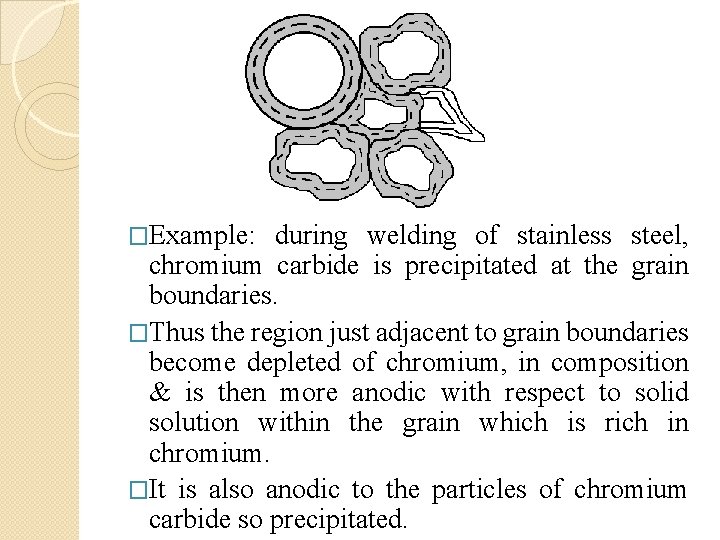
�Example: during welding of stainless steel, chromium carbide is precipitated at the grain boundaries. �Thus the region just adjacent to grain boundaries become depleted of chromium, in composition & is then more anodic with respect to solid solution within the grain which is rich in chromium. �It is also anodic to the particles of chromium carbide so precipitated.

�For given metal or alloy, possibility of stress corrosion depends upon oxygen content & p. H value of electrolyte. �This results in brittle type of failure which may be intergranular or transgranular in character. �Stress corrosion cracking can be eliminated by reducing internal stresses with the help of heat treatment like stress relief annealing or full annealing. �Shot peening is also used or alloying can be done.

Control/prevention : � Proper selection of material , design and fabrication � Modification of corrosive environment � Purification and alloying � Cathodic protection � Application of protective coatings � Application of inhibitors

Proper selection of material: � Pure metal have less corrosion resistance than impure metal � Metal having less electrode potential. � Coarser grain structure have higher corrosion resistance. � Cold worked materials and materials with high internal stresses � Single phase alloys have better corrosion resistance than two phase. � The corrosion resistance of isolated metals is more than the metals in coupling. If two or more metals coupled together i. e brought in contact , one of them become anodic and get corroded by galvanic corrosion. � By addition of suitable alloying elements in metals corrosion resistance can be increased.

� Avoid sharp corners – paint tends to be thinner at sharp corners and often starts to fail. � Provide for easy drainage (tanks) – avoid remaining liquids collect at bottom. E. g steel is resistant against concentrated sulfuric acid. But if remaining liquid is exposed to air, acid tend to absorb moisture, resulting in dilution and rapid attack occurs. � Avoid localized heating during heat transfer operations – localized heating and high corrosion rates. Hot spots also tend to produce stresses – SCC failures. � Design to exclude air – except for active-passive metals and alloys because they require O 2 for protective films. � Most general rule : AVOID HETEROGENEITY!!!

Alteration of Environment Dehumidification and purification of environment: Ø Dehumidification process of reducing moisture from air to such extent that corrosion become negligible. Ø By adding desiccant (sillica gel and activated alumina) or lowering tempering dehumidification can be achieve. Ø These methods only applicable to restricted spaces i. e. in closed areas Ø e. g computer room, electron microscope room, fine surfaces of steel preparation room. Addition of alkaline neutralizers to acidic electrodes: Ø The corrosion by acidic solutions is reduced by neutralising the acidic character of electrolyte by addition of substances called as alkaline neutralizers. Ø Commonly used alkaline neutralizers are ammonia, sodium hydroxide, lime, sodium salts of petroleum phenoles and triethanolamine. Ø The amount added should be sufficient to maintains PH

Alteration of Environment – Deaeration and Deactivation ◦ Corrosion can be reduced by eliminating oxygen from the electrolyte. The oxygen can be eliminated by ether mechanical(Deaeration) or chemical method (Deactivation) ◦ e. g boiler feed water was deaerated by passing it through a large mass of scrap steel. .

Cathodic and anodic protection: Cathodic protection: � Principle : In electrochemical corrosion, anode is the one which undergoes corrosion and cathode remain protected from corrosion. In cathodic protection, the metal to be proteted is forced to behave like cathode. � Cathodic protection is of two types: a) Sacrificial anodic / galvanic protection b) Immersed current cathodic protection

Sacrificial anodic / galvanic protection � Here structure to be protected is connected to a small block or piece of more active/anodic metal. � The more active metal work as anode and the corrosion attack is concentrated at active metal and corrosion rate is slow. � The main structure to be protected act as cathode and remain protected. � The more active metal so employed, is called sacrificial anode. Metals commonly used for anode Mg, Zn, Al and their alloys.

Advantages: �No external power is required �Easy to install �Anode can be radialy added �Minimum maintenance and cathodic interference problems. Disadvantages: Ø Limited driving potential Ø Lower current output Ø Poorly coated structures may require many anodes

Applications: �Buried pipe line and underground cables �Marine structure, ship hulls, and water tanks.

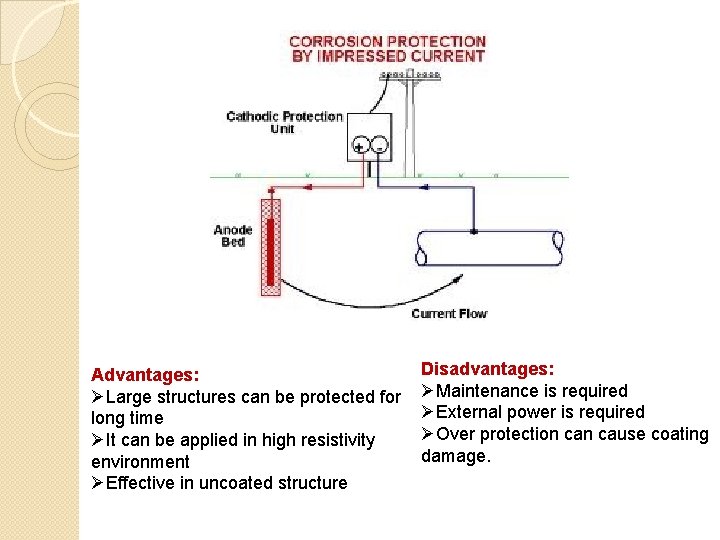
Immersed current cathodic protection � Corrosion occurs in metal depending on environment to which it is exposed � In this method an immersed external current is applied in opposite direction to the metal to nullify the corrosion current. � By doing so, the metal subjected to corrosion is converted to anode to cathode thus protected. � Usually DC current is used. � It is connected with an insoluble anode. e. g. graphite, high silica iron, stainless steel, platinum

Advantages: ØLarge structures can be protected for long time ØIt can be applied in high resistivity environment ØEffective in uncoated structure Disadvantages: ØMaintenance is required ØExternal power is required ØOver protection cause coating damage.

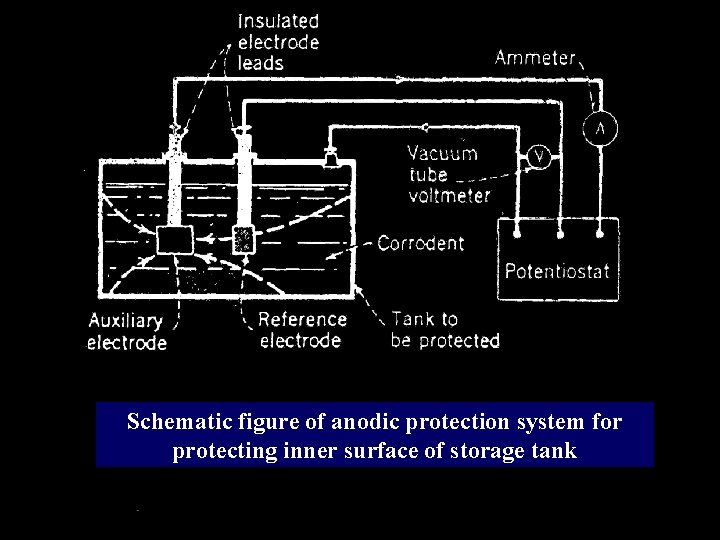
Anodic protection: � Passivity : is the phenomenon in which a metal or an alloy exhibits higher corrosion resistance than expected from its position in the electrochemical series by forming highly protective thin film on the surface. � The metal or alloy which gets radially passivated are protected by the growth of protective oxide surface film by application of anodic current on metal in suitable oxidizing medium. In this method the part of metal to be protected from corrosion is made more anodic by applying external immersed DC current in the same direction as that of corrosion current, Thus making more anodic. � Principle:

Anodic protection: ØAs a result, a thin oxide film is formed which protects the surface from further corrosion ØMetallic part which is to be protected from corrosion is connected to potentiostat. Potentiostat matains a constant potential and has a reference electrode(calomel) attached to it ØAn auxiliary electrode which does not suffer much corrosion in the oxidizing environment (platinum) act as cathode. ØThere is formation of thin corrosion resistant oxide layer which protect the metal from corrosion.

Schematic figure of anodic protection system for protecting inner surface of storage tank

Advantages � Greater throwing power than cathodic protection , hence quite complex structure can be protected. � Method can be applied to severely corroding medium like acids � Low current density � Less cost Disadvantages � Applicable only to those metals which exhibits passivity � Corrosion rate is reduce but it is not reduced to zero Applications � Stainless steel containers used for storage corrosive chem � Chemical reactors , tanks and pipe carrying corrosive liqu

Metallic coating • There are two factors involved in the protection of the underlying metal by a metallic coating. One is the mechanical isolation of the metal from the corroding environment and the other is the galvanic reaction of the coating metal and the base metal. • If the coating metal is higher in the galvanic series than the base metal, discontinuities in the coating are not a problem ( because base metal is cathodic and hence protected )

For example Ø Zinc and Cadmium afford galvanic protection to the steel. ØNickel and Chromium platting give an attractive appearances and excellent corrosion qualities to tin plate hence it is used in food containers. Various methods used to apply metallic coating are as follows : Ø Hot dipping Ø Electroplating Ø Metal cladding Ø High temperature diffusion Ø Metal spraying etc.

Non-Metallic Coating • The most common non metallic coating are paints, enamels, porcelain and chemical coatings. • Now a days coating of plastic, pitch are also used. • Paint is the most widely used protection against corrosion. Sometimes a rust inhibiting action is provided by read lead. • Bituminous coating are very useful for protecting underground tanks and pipes. • Chemical coatings are produced either by chemical dipping in suitable solutions or by chemical reaction.

Application of inhabitor • A corrosion inhibitor is a substance which when added in small quantities to the aqueous corrosive environment, effectively decrease the corrosion of metal. It can be anodic or cathodic. Anodic inhibitors • Anodic inhibitors decrease the anodic reaction or metal dissolution. • Oxidizing substance like chromates, nitrates, phosphates, borax etc are used as anodic inhibitors for the protection of iron and steel.

• Here oxygen works as the anodic inhibitors and forms a protective film by forming ferric hydroxide by the oxidation of ferrous hydroxide, thereby reducing the corrosion rate. • However, this type of corrosion control may be dangerous, since several local attack can occur, if certain area are left unprotected by depletion of inhibitors. Cathodic inhibitors • They prevent evolution of hydrogen (in case of acidic solution ) and oxygen absorption (in case of neutral solution). Such inhibitors try to shied cathodic areas. • In acidic solution, organic inhibitors like amines, mercaptans are added which get absorbed on the cathodic

Surface area of metal, preventing diffusion of H+ ion to cathode and by increasing over voltage of hydrogen. • Antimony or arsenic oxides are also used as inhibitors because they form adherent film of metallic arsenic/antimony at cathode, there by increasing hydrogen overvoltage, restricting the liberation of hydrogen. • In neutral solution, inhibitors like Mg, Zn, Ni salts are used. They react with hydroxyl ion (at cathode) forming corresponding insoluble hydroxide which are deposited on the cathode forming impermeable barriers.