9 2 Electrochemical cells Two types of electrochemical
















- Slides: 28

9. 2 Electrochemical cells

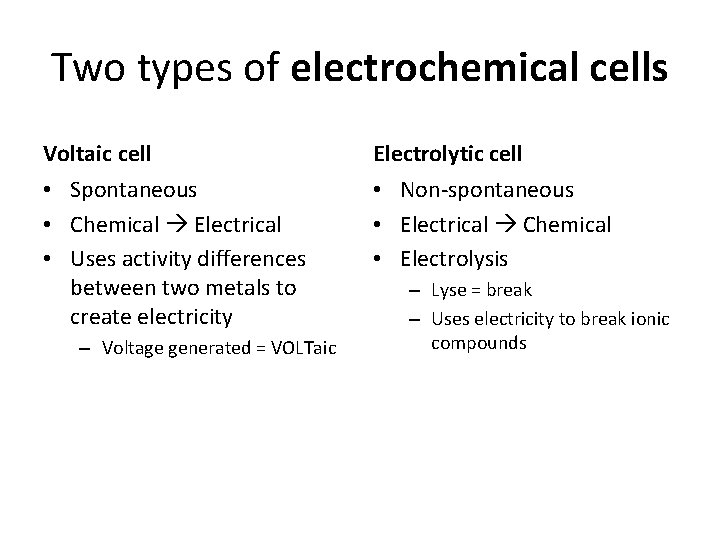
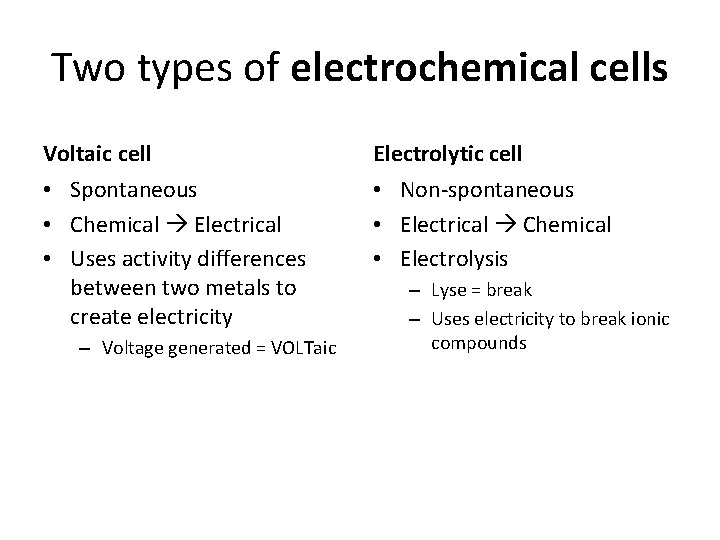
Two types of electrochemical cells Voltaic cell Electrolytic cell • Spontaneous • Chemical Electrical • Uses activity differences between two metals to create electricity • Non-spontaneous • Electrical Chemical • Electrolysis – Voltage generated = VOLTaic – Lyse = break – Uses electricity to break ionic compounds

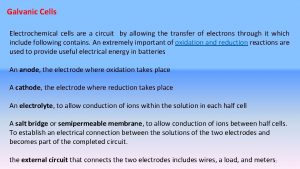
Voltaic (Galvanic) cells • Voltaic cells convert energy from spontaneous, exothermic chemical processes to electrical energy. • Oxidation occurs at the anode (negative electrode) and reduction occurs at the cathode (positive electrode) in a voltaic cell.


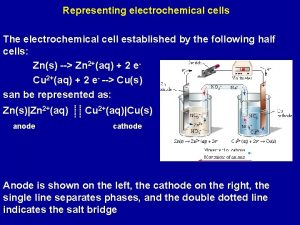
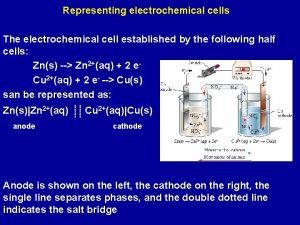
What is a voltaic cell? A device for obtaining electrical energy from a spontaneous chemical reaction 2 e- Zinc Half-Cell: Zn(s) ⇌ Zn 2+ (aq) + 2 e- Copper Half-Cell: Cu(s) ⇌ Cu 2+ (aq) + Zn is higher in the reactivity series, loses e-s more readily & is a better reducing agent If the half cells are connected, then e-s will flow through the external wire from the zinc half-cell to the copper half-cell

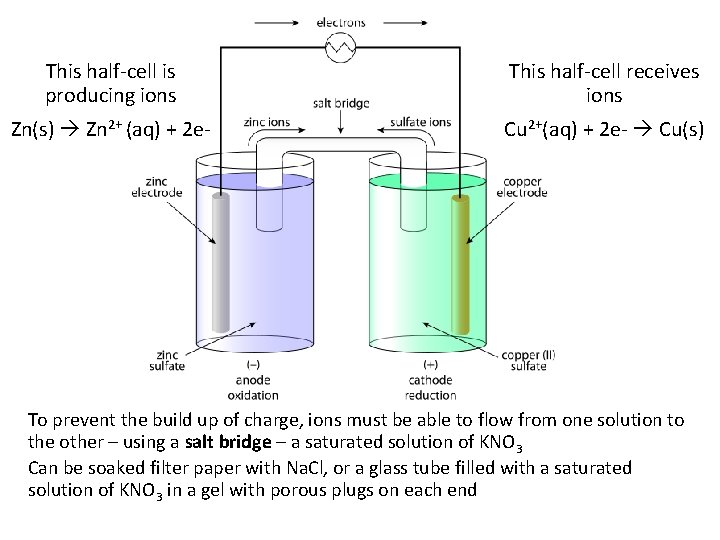
This half-cell is producing ions This half-cell receives ions Zn(s) Zn 2+ (aq) + 2 e- Cu 2+(aq) + 2 e- Cu(s) To prevent the build up of charge, ions must be able to flow from one solution to the other – using a salt bridge – a saturated solution of KNO 3 Can be soaked filter paper with Na. Cl, or a glass tube filled with a saturated solution of KNO 3 in a gel with porous plugs on each end

Voltaic cell explained • https: //www. youtube. com/watch? v=A 0 VUsoe T 9 a. M

Voltaic Cells – Virtual Lab • http: //group. chem. iastate. edu/Greenbowe/se ctions/projectfolder/flashfiles/electro. Chem/v oltaic. Cell. EMF. html

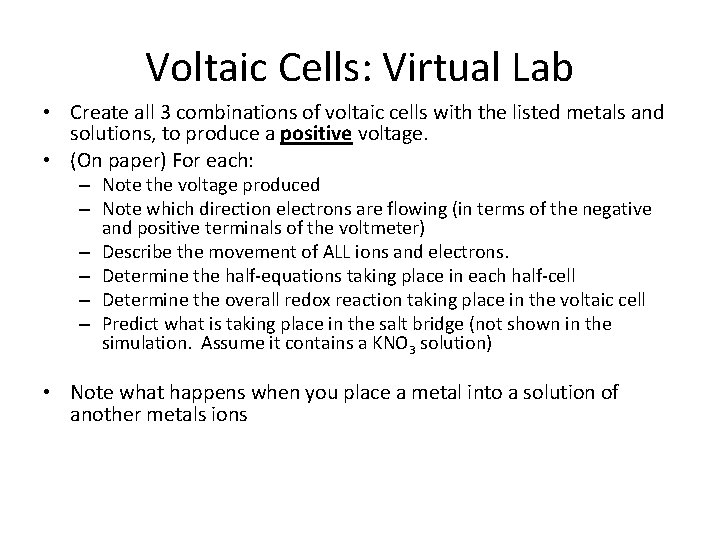
Voltaic Cells: Virtual Lab • Create all 3 combinations of voltaic cells with the listed metals and solutions, to produce a positive voltage. • (On paper) For each: – Note the voltage produced – Note which direction electrons are flowing (in terms of the negative and positive terminals of the voltmeter) – Describe the movement of ALL ions and electrons. – Determine the half-equations taking place in each half-cell – Determine the overall redox reaction taking place in the voltaic cell – Predict what is taking place in the salt bridge (not shown in the simulation. Assume it contains a KNO 3 solution) • Note what happens when you place a metal into a solution of another metals ions

Voltaic Cells: Virtual Lab • In your group, draw a diagram of your selected voltaic cell. • Indicate ion and electron movement throughout all parts of the cells. • Identify the positive and negative electrodes, as well as the anode and cathode.

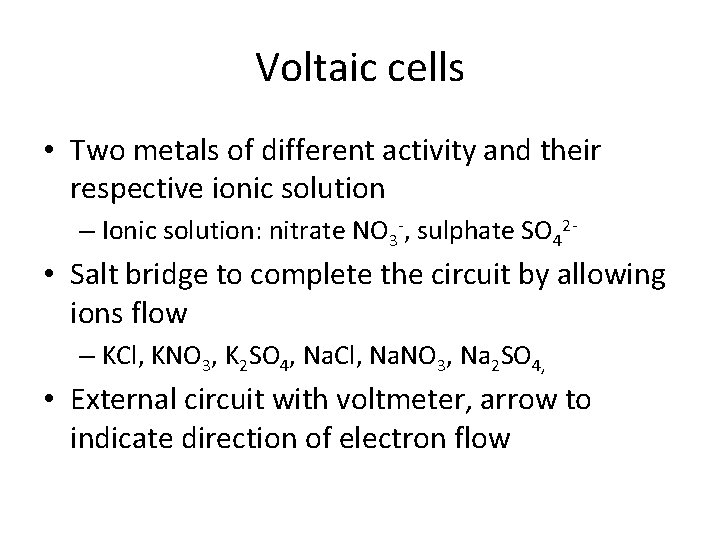
Voltaic cells • Two metals of different activity and their respective ionic solution – Ionic solution: nitrate NO 3 -, sulphate SO 42 - • Salt bridge to complete the circuit by allowing ions flow – KCl, KNO 3, K 2 SO 4, Na. Cl, Na. NO 3, Na 2 SO 4, • External circuit with voltmeter, arrow to indicate direction of electron flow

Voltaic cell • More active metal will preferentially lose electrons. Solid metal electrode will form ions going into the solution. Electrode will lose mass. • Less active metal’s ions in solution will be forced to accept electrons to form solid metal, which will deposit on the metal electrode. Electrode will gain mass.

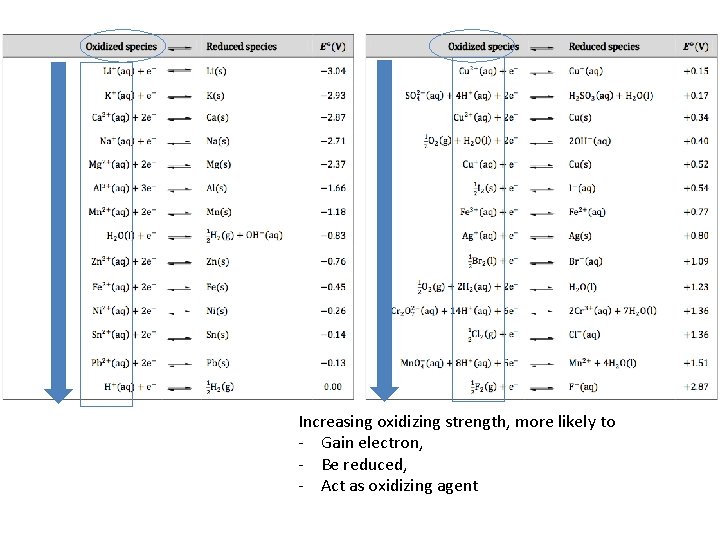
Increasing reducing strength, more active, more likely to - Lose electron, - Be oxidized, - Act as reducing agent

Increasing oxidizing strength, more likely to - Gain electron, - Be reduced, - Act as oxidizing agent


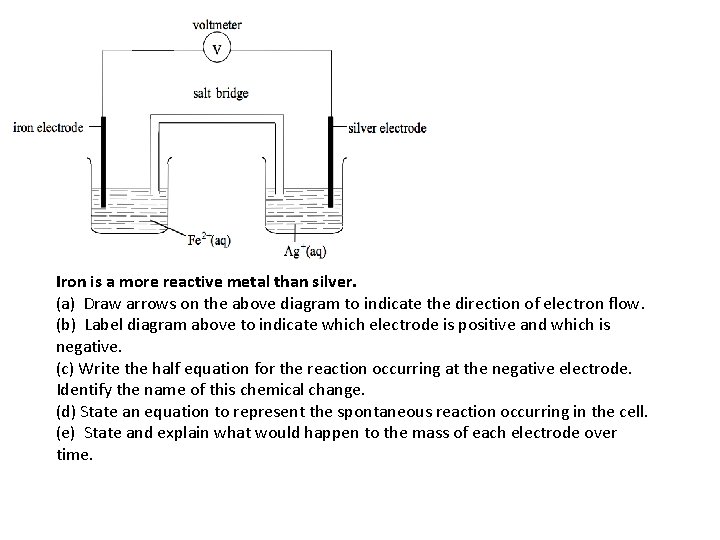
Iron is a more reactive metal than silver. (a) Draw arrows on the above diagram to indicate the direction of electron flow. (b) Label diagram above to indicate which electrode is positive and which is negative. (c) Write the half equation for the reaction occurring at the negative electrode. Identify the name of this chemical change. (d) State an equation to represent the spontaneous reaction occurring in the cell. (e) State and explain what would happen to the mass of each electrode over time.

Electrolytic cell • Electrolytic cells convert electrical energy to chemical energy, by bringing about nonspontaneous processes. • Oxidation occurs at the anode (positive electrode) and reduction occurs at the cathode (negative electrode) in an electrolytic cell.

Two types of electrochemical cells Voltaic cell Electrolytic cell • Spontaneous • Chemical Electrical • Uses activity differences between two metals to create electricity • Non-spontaneous • Electrical Chemical • Electrolysis – Voltage generated = VOLTaic – Lyse = break – Uses electricity to break ionic compounds

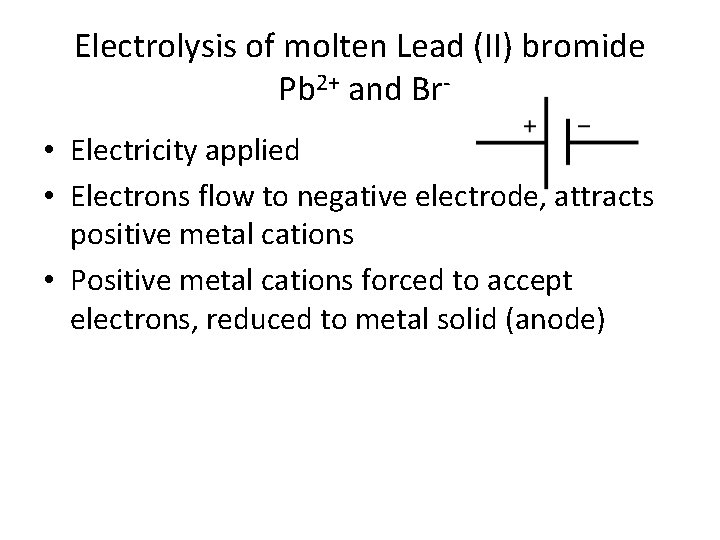
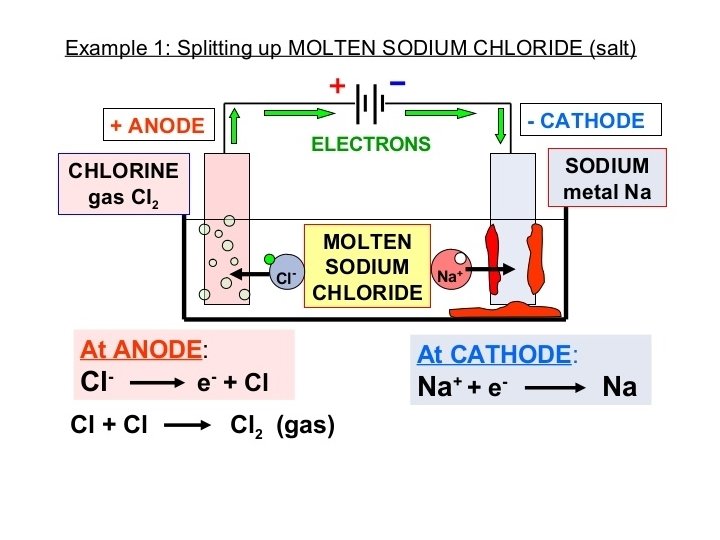
Electrolytic cell (Electrolysis) • Electrical energy is applied to a molten ionic compound immersed in a container with two unreactive electrodes (usually graphite) – Ionic compound melted into liquid state so that the ions can move. • Electrical energy = flow of electrons • Example: Molten lead (II) bromide Pb. Br 2 • Cation: Pb 2+; Anion Br-


Electrolysis of molten Lead (II) bromide Pb 2+ and Br • Electricity applied • Electrons flow to negative electrode, attracts positive metal cations • Positive metal cations forced to accept electrons, reduced to metal solid (anode)

Electrolysis of molten Lead (II) bromide Pb 2+ and Br • Positive electrode attracts negative non-metal anions • Negative non-metal anions forced to give up electrons to complete the circuit • Negative non-metal anions loses electrons, oxidized to element state • The examples you will encounter usually involve – 2 Cl-(aq) Cl 2 (g) + 2 e– 2 Br-(aq) Br 2 (l) + 2 e– 2 I-(aq) I 2 (s) + 2 e-

Electrolysis labelled diagram: replace “electrolyte” with the molten ionic compound in question

A few notes on electrolytic cells • Know the symbol for drawing battery • Suitable material for electrodes are non-reactive – Graphite – Platinum


• LEO GER – Lose Electron Oxidation, Gain Electron Reduction – Ox: Lose e-, lose hydrogen, gain oxygen, + OS – Re: Gain e-, gain hydrogen, lose oxygen, - OS • RED CAT, AN OX – Reduction Cathode, Anode Oxidation • VON POE – Voltaic cell Oxidation (Anode) Negative electrode – Positive electrode Oxidation (Anode) Electrolytic cell


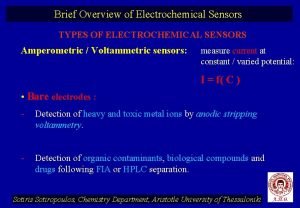
 Types of electrochemical sensors
Types of electrochemical sensors Types of electrochemical corrosion
Types of electrochemical corrosion Eukaryate
Eukaryate Strepto vs staphylo
Strepto vs staphylo Olfactory groove keros classification
Olfactory groove keros classification Proximal convoluted tubule
Proximal convoluted tubule Thyroid parafollicular cells
Thyroid parafollicular cells How are mitosis and meiosis similar
How are mitosis and meiosis similar Why dna is more stable than rna
Why dna is more stable than rna Red blood cells and white blood cells difference
Red blood cells and white blood cells difference Ghonarea
Ghonarea Plant vs animal cells venn diagram
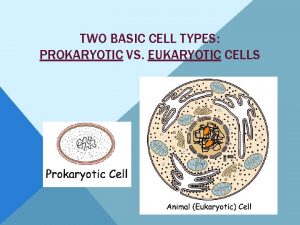
Plant vs animal cells venn diagram Prokaryotic cells
Prokaryotic cells Cell organelle jeopardy
Cell organelle jeopardy Masses of cells form and steal nutrients from healthy cells
Masses of cells form and steal nutrients from healthy cells Pseudostratified vs simple columnar
Pseudostratified vs simple columnar Cuál es la diferencia entre la célula animal y vegetal
Cuál es la diferencia entre la célula animal y vegetal Which compares prokaryotes and eukaryotes
Which compares prokaryotes and eukaryotes Nondisjunction in meiosis
Nondisjunction in meiosis Cell substance
Cell substance Cell chapter 20
Cell chapter 20 Electrochemical deposition
Electrochemical deposition Electrochemical theory of corrosion
Electrochemical theory of corrosion Electrochemical machining animation
Electrochemical machining animation Electrochemical gradient
Electrochemical gradient Electrochemical series
Electrochemical series Chloride half equation
Chloride half equation Diffusion limited
Diffusion limited Stress corrosion
Stress corrosion