ELECTROCHEMICAL CELLS GALVANIC CELLS EXPLAIN HOW A GALVANIC





- Slides: 10

ELECTROCHEMICAL CELLS (GALVANIC CELLS) EXPLAIN HOW A GALVANIC CELL IS USED TO PRODUCE ELECTRICAL ENERGY

• Galvani's frog united chemistry, physics • THE UNION of physics and chemistry began with the twitching of a frog's leg. In the 1790 s Luigi Galvani lay a dead frog on a table. Also on the table was a static electricity generator. An assistant accidentally touched the nerves of the frog with a scalpel and the muscles of the frog contracted convulsively. Galvani experimented afterward, fastening brass hooks in the legs of frogs and hanging them on an iron railing in his garden. He observed legs contracting "not only when the lightning flashed but even at times when the sky was quiet and serene. " • Galvani concluded that there was a new type of animal electricity inherent in the nerves and muscles of the frog and published the results of his experiments. • Galvani died in sorrow and poverty after being fired from his professorship for refusing to swear allegiance to Napoleon, but his work inspired physicist Alessandro Volta. • Volta interpreted the results differently. He attributed the muscle twitches to an electrical current flowing between two dissimilar metals. • The frog's leg was merely a conductor. • Volta became the first individual to produce electricity from chemistry in 1796 by making a Voltaic pile, a sandwich of brine -soaked felt between plates of silver and zinc. Today we call them batteries, which differ from Volta's original only in the material used. • Ironically, Napoleon declared Volta's work a triumph and awarded him a gold medal.


GALVANIC CELLS • Use a spontaneous redox reaction. • Two half equation reactions are separated into separate containers • The two half reactions are linked by a salt bridge • A half cell usually consists of a metal and an aqueous solution of metal ions called a redox couple eg: Cu 2+/Cu

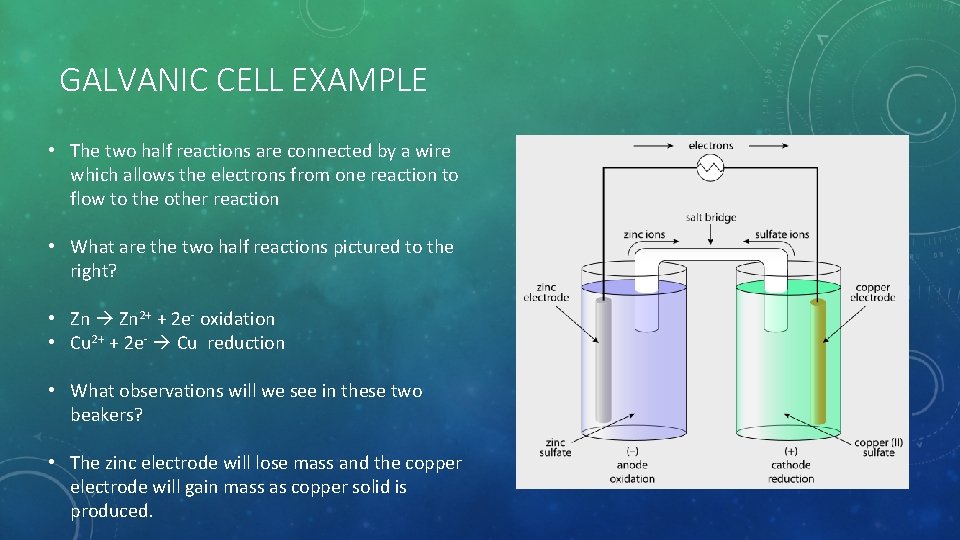
GALVANIC CELL EXAMPLE • The two half reactions are connected by a wire which allows the electrons from one reaction to flow to the other reaction • What are the two half reactions pictured to the right? • Zn 2+ + 2 e- oxidation • Cu 2+ + 2 e- Cu reduction • What observations will we see in these two beakers? • The zinc electrode will lose mass and the copper electrode will gain mass as copper solid is produced.

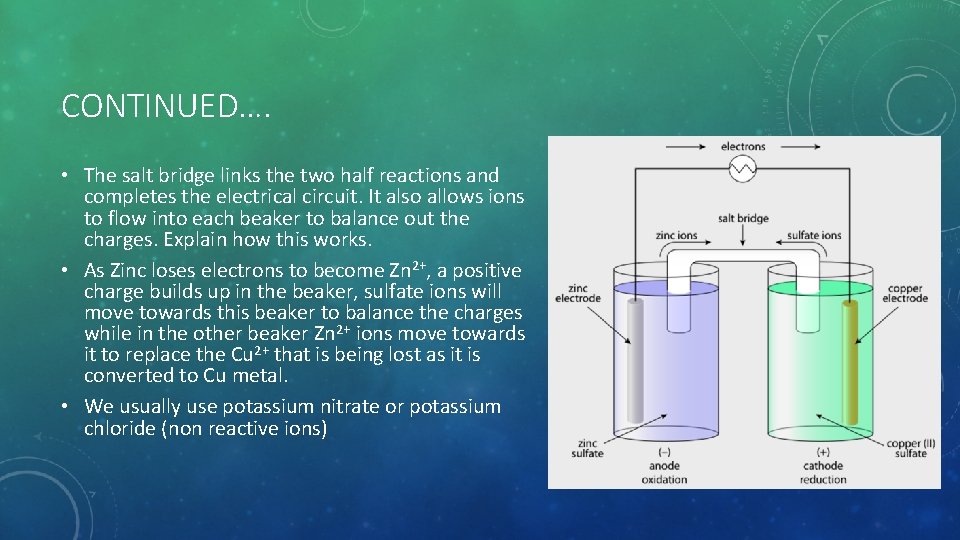
CONTINUED…. • The salt bridge links the two half reactions and completes the electrical circuit. It also allows ions to flow into each beaker to balance out the charges. Explain how this works. • As Zinc loses electrons to become Zn 2+, a positive charge builds up in the beaker, sulfate ions will move towards this beaker to balance the charges while in the other beaker Zn 2+ ions move towards it to replace the Cu 2+ that is being lost as it is converted to Cu metal. • We usually use potassium nitrate or potassium chloride (non reactive ions)

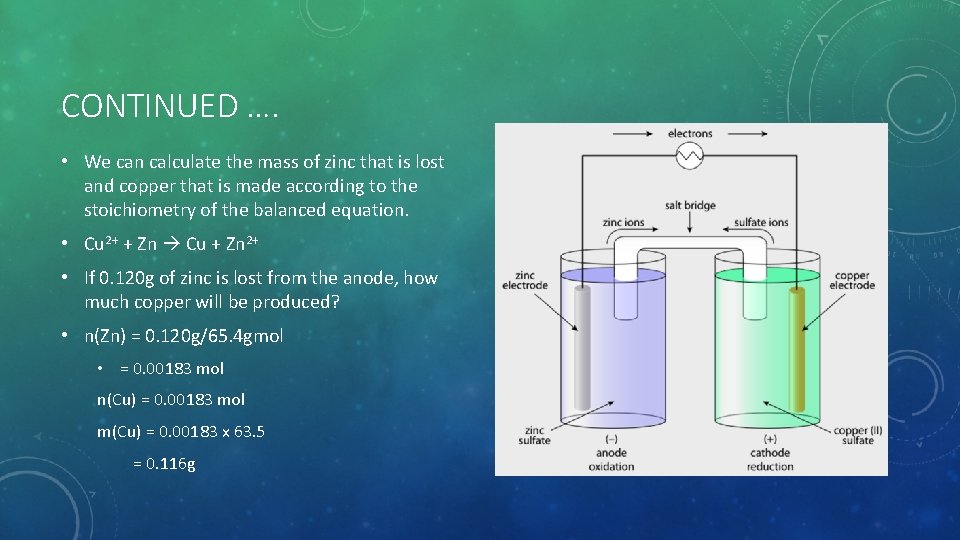
CONTINUED …. • We can calculate the mass of zinc that is lost and copper that is made according to the stoichiometry of the balanced equation. • Cu 2+ + Zn Cu + Zn 2+ • If 0. 120 g of zinc is lost from the anode, how much copper will be produced? • n(Zn) = 0. 120 g/65. 4 gmol • = 0. 00183 mol n(Cu) = 0. 00183 mol m(Cu) = 0. 00183 x 63. 5 = 0. 116 g

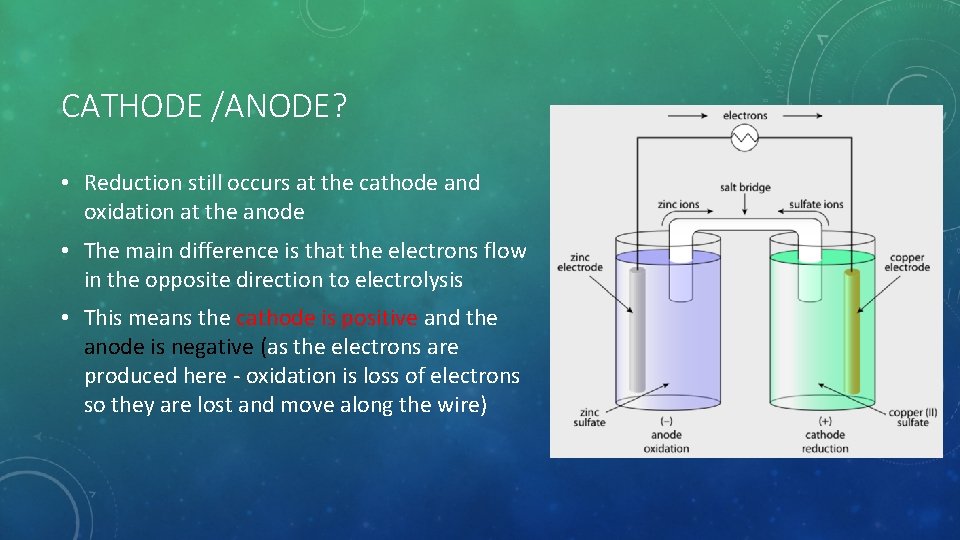
CATHODE /ANODE? • Reduction still occurs at the cathode and oxidation at the anode • The main difference is that the electrons flow in the opposite direction to electrolysis • This means the cathode is positive and the anode is negative (as the electrons are produced here - oxidation is loss of electrons so they are lost and move along the wire)

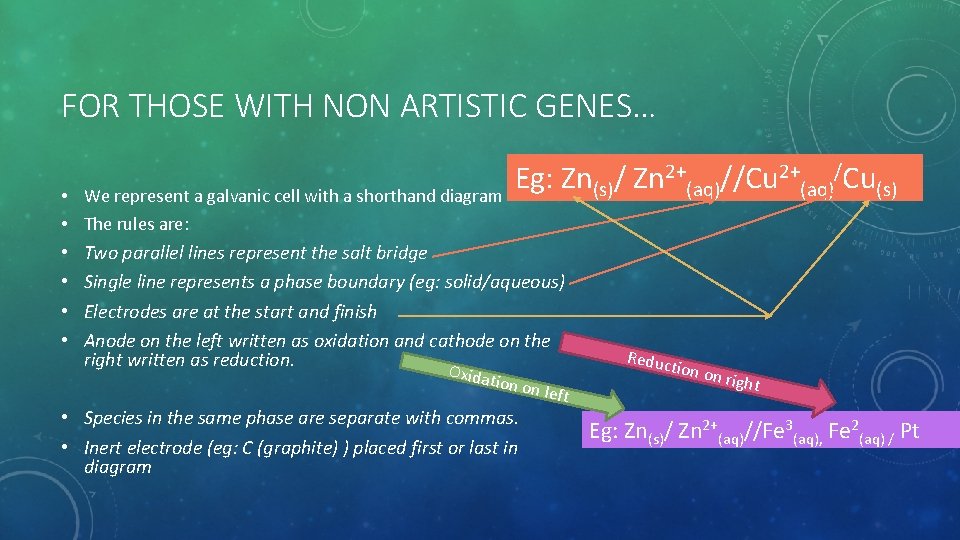
FOR THOSE WITH NON ARTISTIC GENES… Eg: Zn(s)/ Zn 2+(aq)//Cu 2+(aq)/Cu(s) • We represent a galvanic cell with a shorthand diagram • The rules are: • Two parallel lines represent the salt bridge • Single line represents a phase boundary (eg: solid/aqueous) • Electrodes are at the start and finish • Anode on the left written as oxidation and cathode on the right written as reduction. Oxidat ion on • Species in the same phase are separate with commas. • Inert electrode (eg: C (graphite) ) placed first or last in diagram left Reduc tion o n righ t Eg: Zn(s)/ Zn 2+(aq)//Fe 3(aq), Fe 2(aq) / Pt

ANIMATION AND WORK TIME • . . Animations25 Electrochemical cell. swf • Pg 307 in textbook Q 1 -4