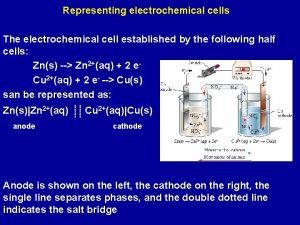
Electrochemical Cells The Electrochemical Series 1 A simple



![Redox Equilibrium [PRESS ZINC] Zn(s) Ý 2+ Zn (aq) Zn 2+ + 2 e Redox Equilibrium [PRESS ZINC] Zn(s) Ý 2+ Zn (aq) Zn 2+ + 2 e](https://slidetodoc.com/presentation_image_h2/a49f85fba7fabd527ee4e35e0c295fa2/image-7.jpg)





- Slides: 21

Electrochemical Cells & The Electrochemical Series 1. A simple cell from an exothermic redox reaction Zn (s) + Cu 2+ (aq) 2. Redox Equilibrium – zinc in water 3. Standard Hydrogen Reference Half Cell 4. Standard electrode potential – Zn/Zn 2+ 5. Standard electrode potential – Cu/Cu 2+ 6. Standard electrode potential – Fe 2+/Fe 3+ 7. The Electrochemical Series 8. Calculation of Cell e. m. f. 9. Questions 10. Useful Internet Link

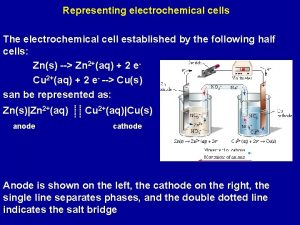
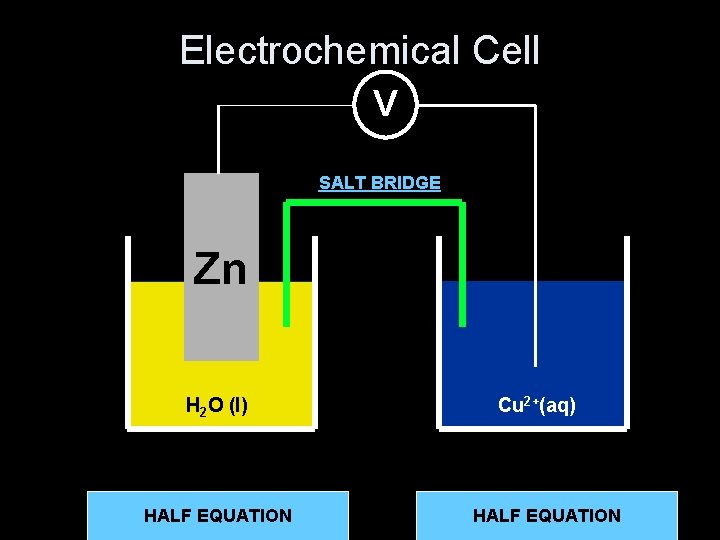
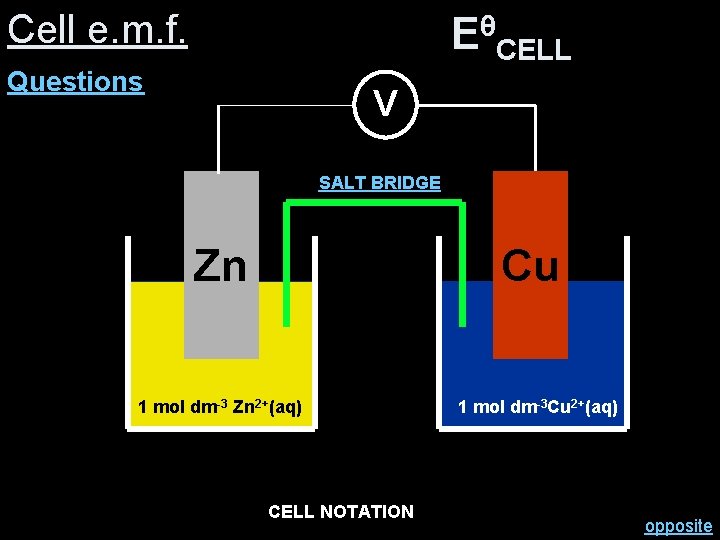
Electrochemical Cell V SALT BRIDGE Zn H 2 O (l) 2+(aq) + 2 e. Zn(s) Zn HALF EQUATION Cu 2+(aq) Cu 2+HALF (aq) +EQUATION 2 e- Cu(s)

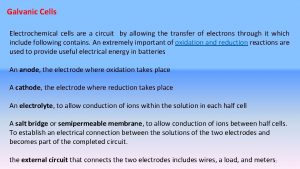
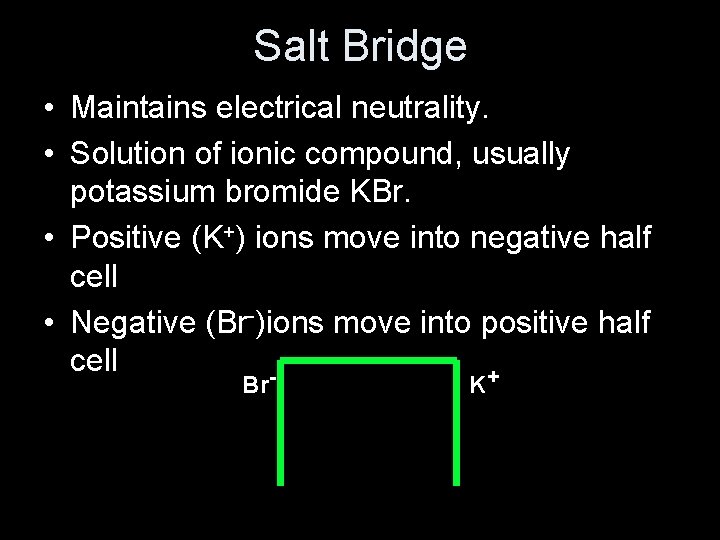
Salt Bridge • Maintains electrical neutrality. • Solution of ionic compound, usually potassium bromide KBr. • Positive (K+) ions move into negative half cell • Negative (Br-)ions move into positive half cell + Br K

Very High Resistance Voltmeter • No current drawn. • Measures the electromotive force [e. m. f. ] of the cell. • The potential for the cell to provide energy

Zn(s) Zn 2+(aq) + 2 e • OXIDATION • ANODE OXID ATION An anode is a place of oxidation

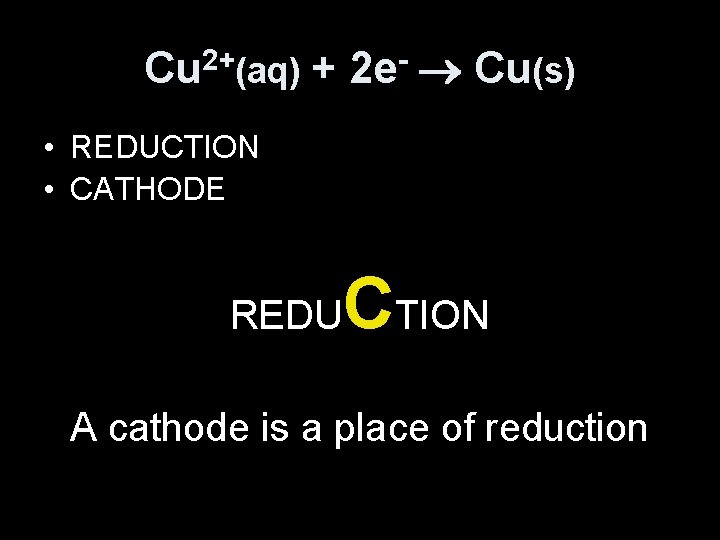
Cu 2+(aq) + 2 e- Cu(s) • REDUCTION • CATHODE REDU CTION A cathode is a place of reduction
![Redox Equilibrium PRESS ZINC Zns Ý 2 Zn aq Zn 2 2 e Redox Equilibrium [PRESS ZINC] Zn(s) Ý 2+ Zn (aq) Zn 2+ + 2 e](https://slidetodoc.com/presentation_image_h2/a49f85fba7fabd527ee4e35e0c295fa2/image-7.jpg)
Redox Equilibrium [PRESS ZINC] Zn(s) Ý 2+ Zn (aq) Zn 2+ + 2 e Zn 2+ - e- ee. Zn e- e- e. Zn Zinc e- e- Zn 2+ Add stronger oxidising agent Add stronger reducing agent ADDING REDOX AGENTS

Redox Equilibrium 2 MAGNESIUM Mg(s) Ý 2+ Mg (aq) Mg 2+ + 2 e Mg 2+ - e- ee. Mg e- e- Magnesium Mg 2+ Equilibrium for magnesium lies further to right than for zinc

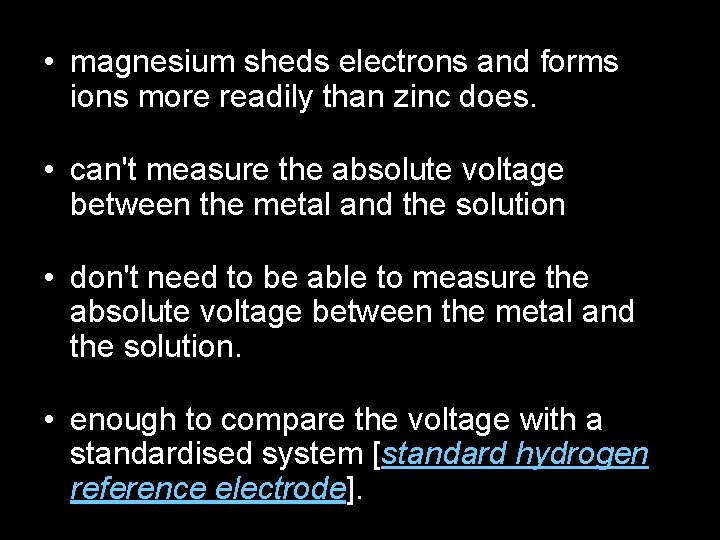
• magnesium sheds electrons and forms ions more readily than zinc does. • can't measure the absolute voltage between the metal and the solution • don't need to be able to measure the absolute voltage between the metal and the solution. • enough to compare the voltage with a standardised system [standard hydrogen reference electrode].

Oxidising agent added, e. g. Cu 2+: Cu 2+ Zn 2+ Cu e- e- Zn 2+ e- e- Zinc e- e- Zn 2+ e- e- EQUATION Zn(s) + Cu 2+(aq) Zn 2+(aq) + Cu(s)

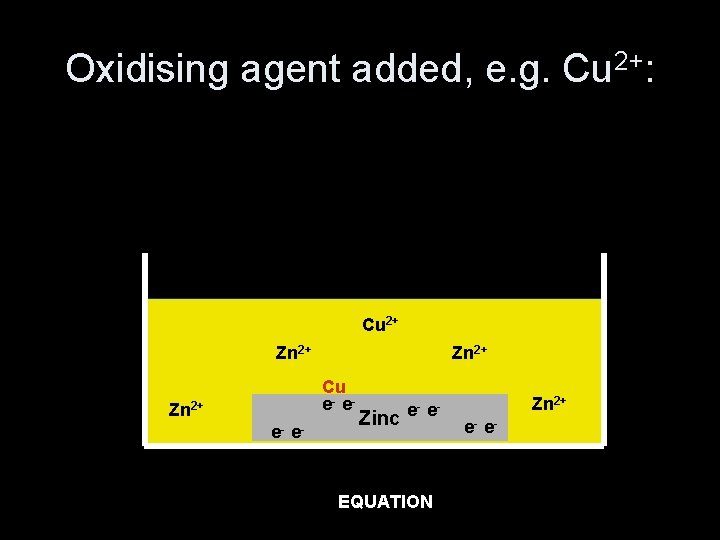
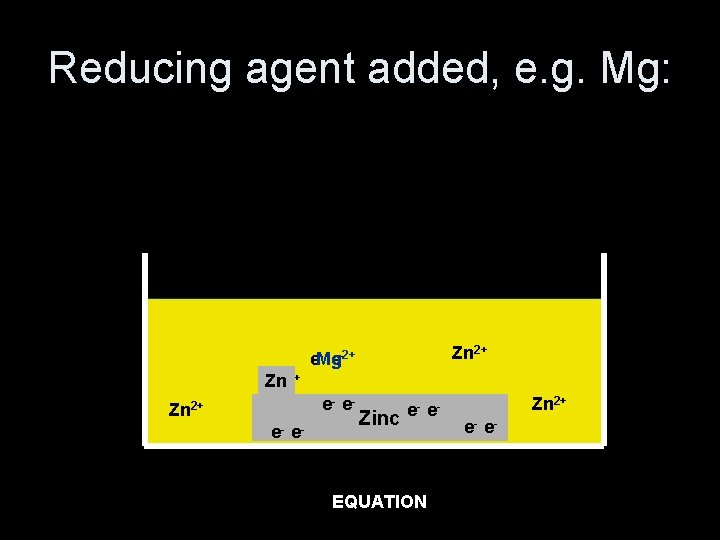
Reducing agent added, e. g. Mg: Zn 2+ - e-2+ e. Mg Zn 2+ e- e- Zinc e- e- Zn 2+ e- e- EQUATION Mg(s) + Zn 2+(aq) Mg 2+(aq) + Zn(s)

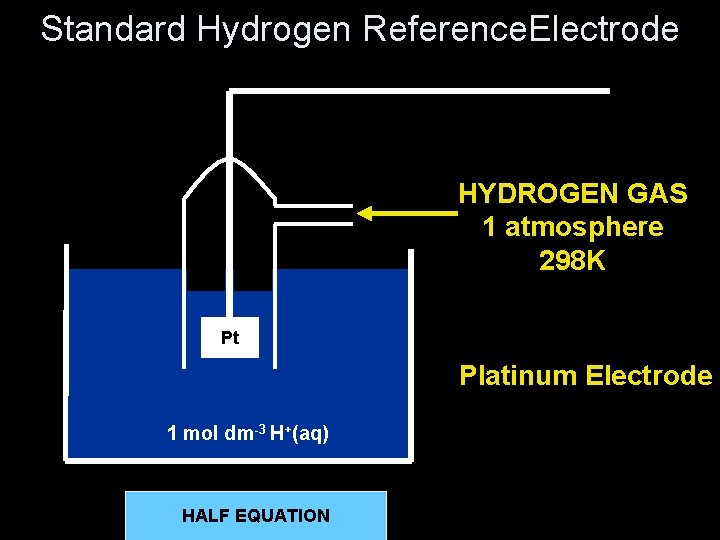
Standard Hydrogen Reference. Electrode HYDROGEN GAS 1 atmosphere 298 K Pt Platinum Electrode 1 mol dm-3 H+(aq) + 2 e- H (g) 2 HHALF EQUATION 2

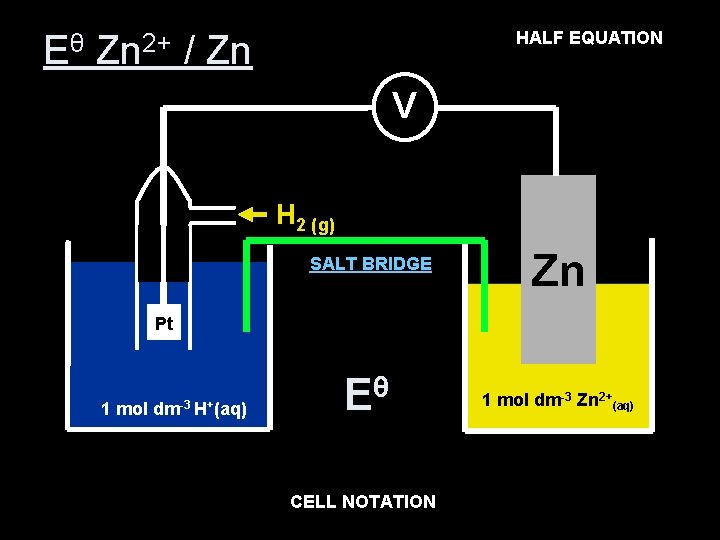
Zn 2+ (aq) EQUATION + 2 e- Zn(s) HALF Eθ Zn 2+ / Zn V H 2 (g) SALT BRIDGE Zn Pt 1 mol dm-3 H+(aq) θ E θ= E -0. 76 V 1 mol dm-3 Zn 2+(aq) NOTATION 2+(aq) Zn(s) Pt (s) H 2(g), CELL 2 H+(aq) Zn

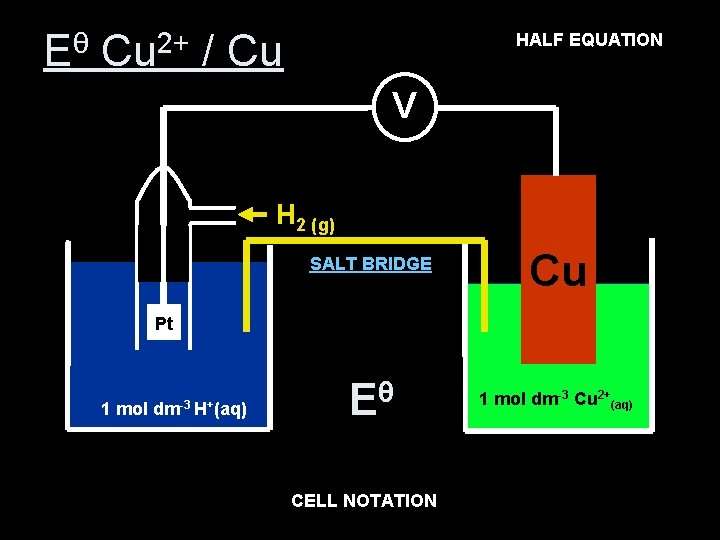
Cu 2+ (aq) EQUATION + 2 e- Cu(s) HALF Eθ Cu 2+ / Cu V H 2 (g) SALT BRIDGE Cu Pt 1 mol dm-3 H+(aq) Eθ= +0. 34 V Eθ 1 mol dm-3 Cu 2+(aq) CELL NOTATION 2+ +(aq) Cu Pt (s) H 2(g), 2 H (aq) Cu(s)

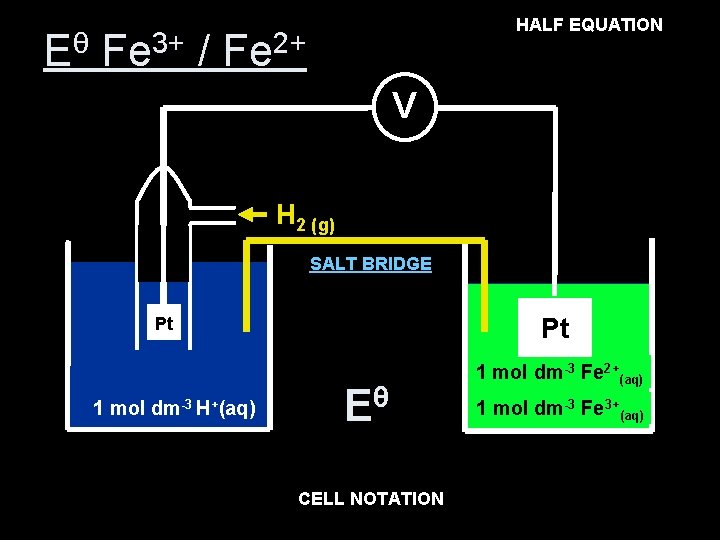
HALF EQUATION Fe 3+ (aq) + e- Fe 2+(aq) Eθ Fe 3+ / Fe 2+ V H 2 (g) SALT BRIDGE Pt Pt 1 mol dm-3 H+(aq) θ E θ= E +0. 77 V 1 mol dm-3 Fe 2+(aq) 1 mol dm-3 Fe 3+(aq) CELL NOTATION 3+(aq), Fe 2+(aq) Pt(s) Pt (s) H 2(g), 2 H+(aq) Fe

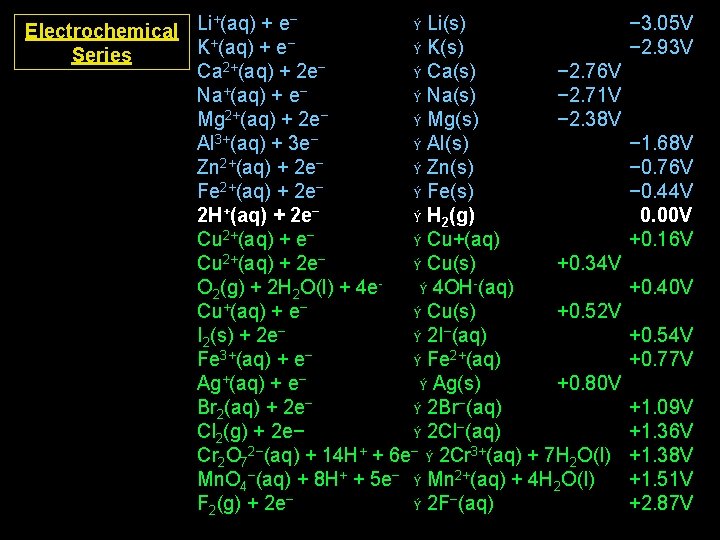
+ − Ý Li(s) Electrochemical Li (aq) + e K+(aq) + e− Ý K(s) Series Ca 2+(aq) + 2 e− Ý Ca(s) − 2. 76 V Na+(aq) + e− Ý Na(s) − 2. 71 V Mg 2+(aq) + 2 e− Ý Mg(s) − 2. 38 V Al 3+(aq) + 3 e− Ý Al(s) Zn 2+(aq) + 2 e− Ý Zn(s) Fe 2+(aq) + 2 e− Ý Fe(s) 2 H+(aq) + 2 e− Ý H 2(g) Cu 2+(aq) + e− Ý Cu+(aq) Cu 2+(aq) + 2 e− Ý Cu(s) +0. 34 V O 2(g) + 2 H 2 O(l) + 4 eÝ 4 OH-(aq) Cu+(aq) + e− Ý Cu(s) +0. 52 V I 2(s) + 2 e− Ý 2 I−(aq) Fe 3+(aq) + e− Ý Fe 2+(aq) Ag+(aq) + e− Ý Ag(s) +0. 80 V Br 2(aq) + 2 e− Ý 2 Br−(aq) Cl 2(g) + 2 e− Ý 2 Cl−(aq) Cr 2 O 72−(aq) + 14 H+ + 6 e− Ý 2 Cr 3+(aq) + 7 H 2 O(l) Mn. O 4−(aq) + 8 H+ + 5 e− Ý Mn 2+(aq) + 4 H 2 O(l) F 2(g) + 2 e− Ý 2 F−(aq) − 3. 05 V − 2. 93 V − 1. 68 V − 0. 76 V − 0. 44 V 0. 00 V +0. 16 V +0. 40 V +0. 54 V +0. 77 V +1. 09 V +1. 36 V +1. 38 V +1. 51 V +2. 87 V

Cell e. m. f. θ EθCELL= +0. 34 (-0. 76) = +1. 10 V E –CELL Questions V SALT BRIDGE Zn 1 mol dm-3 Zn 2+(aq) Cu 1 mol dm-3 Cu 2+(aq) 2+ Zn (s) Zn 2+CELL (aq) Cu NOTATION (aq) Cu(s) opposite

Cell e. m. f. θ EθCELL= -0. 76 (+0. 34) = -1. 10 V E –CELL Questions V SALT BRIDGE Cu 1 mol dm-3 Cu 2+(aq) Zn 1 mol dm-3 Zn 2+(aq) 2+ Cu (s) Cu 2+CELL (aq) Zn NOTATION (aq) Zn(s)

QUESTIONS Feasibility Of Reactions Electrochemical Cells

QUESTIONS Feasibility of Reactions • For each of the following mixtures predict whether a reaction is or isn’t feasible at s. t. p. If a reaction is feasible then write the balanced ionic equation. 1. Li(s) and Cu 2+(aq) 2. Zn(s) and Al 3+(aq) 3. O 2(g), H 2 O(l) and Mg(s) 4. Br 2(aq) and Fe(s) 5. Acidified Cr O 2 - and I-(aq)

• QUESTIONS electrochemical cells For each of the following redox reactions write the cell notation and calculate the e. m. f. of the cell at s. t. p. 1. 2. 3. 4. 5. Li(s) + Ag+(aq) Li+(aq) + Ag(s) 3 Na(s) + Al 3+(aq) 3 Na+(aq) + Al(s) Cr 2+(aq) + Fe 3+(aq) Fe 2+(aq) + Cr 3+(aq) O 2(g) + 2 H 2 O(l) + 2 Cu(s) 4 OH-(aq) + 2 Cu 2+(aq) Ca(s) + 2 K+(aq) Ca 2+(aq) + 2 K(s)
 Electrochemical series
Electrochemical series Chloride half equation
Chloride half equation What are electrochemical series
What are electrochemical series Electrochemical series order
Electrochemical series order Olfactory groove keros classification
Olfactory groove keros classification Medullary portion of collecting duct
Medullary portion of collecting duct Pineal gland
Pineal gland Gamete vs somatic cell
Gamete vs somatic cell Why dna is more stable than rna?
Why dna is more stable than rna? Red blood cells and white blood cells difference
Red blood cells and white blood cells difference Prokaryotic vs eukaryotic transcription worksheet
Prokaryotic vs eukaryotic transcription worksheet Animal cell venn diagram
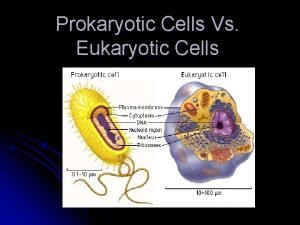
Animal cell venn diagram Prokaryotic cells vs eukaryotic cells
Prokaryotic cells vs eukaryotic cells Organelle trail
Organelle trail Masses of cells form and steal nutrients from healthy cells
Masses of cells form and steal nutrients from healthy cells Younger cells cuboidal older cells flattened
Younger cells cuboidal older cells flattened Cuál es la diferencia entre la célula animal y vegetal
Cuál es la diferencia entre la célula animal y vegetal Which compares prokaryotes and eukaryotes
Which compares prokaryotes and eukaryotes Nondisjunction in meiosis
Nondisjunction in meiosis Cells cells they're made of organelles meme
Cells cells they're made of organelles meme Cathode vs anode equation
Cathode vs anode equation Electrochemical deposition
Electrochemical deposition