Galvanic Replacement Deposition Galvanic replacement deposition processes in




- Slides: 58

Galvanic Replacement / Deposition Galvanic replacement / deposition processes in electrocatalysis and metal ion removal Sotiris Sotiropoulos, ISE 2016, The Hague Aristotle University

Galvanic Replacement / Deposition Outline • Principle • Historical backround • Approaches, deposition methods and substrates • Possibilities and advantages • Applications: electrocatalysis, electrosynthesis, metal ion removal Sotiropoulos, ISE 2016, The Hague Aristotle University

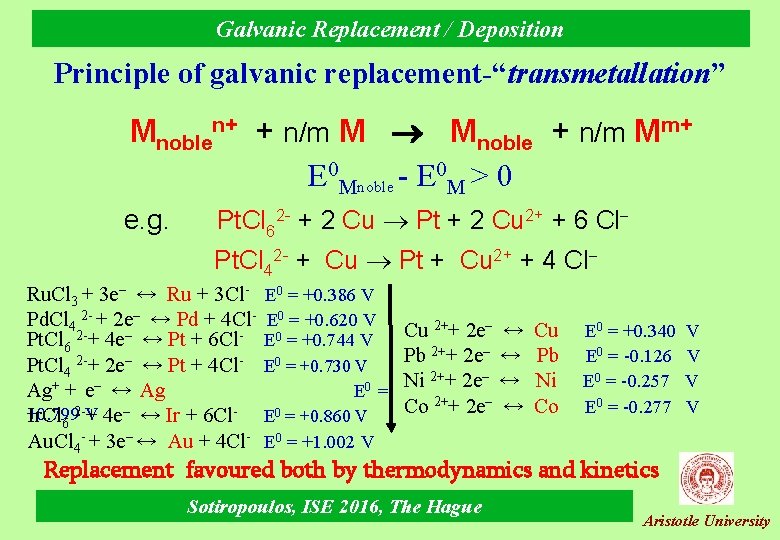
Galvanic Replacement / Deposition Principle of galvanic replacement-“transmetallation” Mnoblen+ + n/m M Mnoble + n/m Mm+ E 0 Mnoble - E 0 M > 0 e. g. Pt. Cl 62 - + 2 Cu Pt + 2 Cu 2+ + 6 Cl Pt. Cl 42 - + Cu Pt + Cu 2+ + 4 Cl Ru. Cl 3 + 3 e– ↔ Ru + 3 Cl. Pd. Cl 4 2 - + 2 e– ↔ Pd + 4 Cl. Pt. Cl 6 2 -+ 4 e– ↔ Pt + 6 Cl. Pt. Cl 4 2 -+ 2 e– ↔ Pt + 4 Cl. Ag+ + e– ↔ Ag +0. 799 Ir. Cl 6 2 -V + 4 e– ↔ Ir + 6 Cl. Au. Cl 4 - + 3 e– ↔ Au + 4 Cl- E 0 = +0. 386 V E 0 = +0. 620 V E 0 = +0. 744 V Cu 2++ 2 e– Pb 0 E = +0. 730 V 2++ 2 e– Ni 0 E = 2++ 2 e– Co 0 E = +0. 860 V ↔ ↔ Cu Pb Ni Co E 0 = +0. 340 E 0 = -0. 126 E 0 = -0. 257 E 0 = -0. 277 V V E 0 = +1. 002 V Replacement favoured both by thermodynamics and kinetics Sotiropoulos, ISE 2016, The Hague Aristotle University

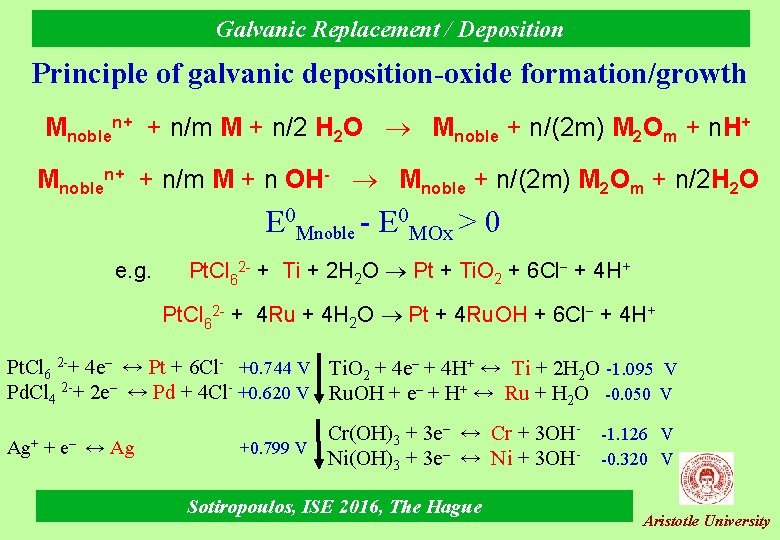
Galvanic Replacement / Deposition Principle of galvanic deposition-oxide formation/growth Mnoblen+ + n/m M + n/2 H 2 O Mnoble + n/(2 m) M 2 Om + n. H+ Mnoblen+ + n/m M + n OH- Mnoble + n/(2 m) M 2 Om + n/2 H 2 O E 0 Mnoble - E 0 MOx > 0 e. g. Pt. Cl 62 - + Ti + 2 H 2 O Pt + Ti. O 2 + 6 Cl + 4 H+ Pt. Cl 62 - + 4 Ru + 4 H 2 O Pt + 4 Ru. OH + 6 Cl + 4 H+ Pt. Cl 6 2 -+ 4 e– ↔ Pt + 6 Cl- +0. 744 V Ti. O 2 + 4 e– + 4 H+ ↔ Ti + 2 H 2 O -1. 095 V Pd. Cl 4 2 -+ 2 e– ↔ Pd + 4 Cl- +0. 620 V Ru. OH + e– + H+ ↔ Ru + H 2 O -0. 050 V Ag+ + e– ↔ Ag Cr(OH)3 + 3 e– ↔ Cr + 3 OH+0. 799 V Ni(OH)3 + 3 e– ↔ Ni + 3 OHSotiropoulos, ISE 2016, The Hague -1. 126 V -0. 320 V Aristotle University

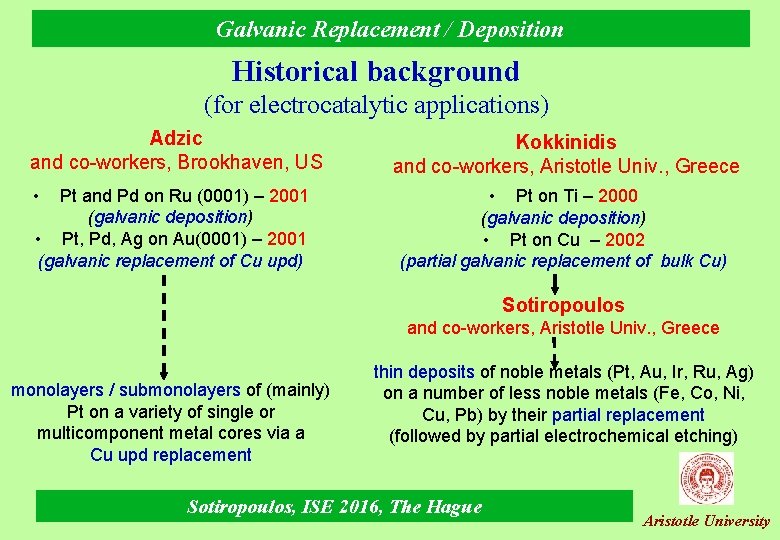
Galvanic Replacement / Deposition Historical background (for electrocatalytic applications) Adzic and co-workers, Brookhaven, US • Pt and Pd on Ru (0001) – 2001 (galvanic deposition) • Pt, Pd, Ag on Au(0001) – 2001 (galvanic replacement of Cu upd) Kokkinidis and co-workers, Aristotle Univ. , Greece • Pt on Ti – 2000 (galvanic deposition) • Pt on Cu – 2002 (partial galvanic replacement of bulk Cu) Sotiropoulos and co-workers, Aristotle Univ. , Greece monolayers / submonolayers of (mainly) Pt on a variety of single or multicomponent metal cores via a Cu upd replacement thin deposits of noble metals (Pt, Au, Ir, Ru, Ag) on a number of less noble metals (Fe, Co, Ni, Cu, Pb) by their partial replacement (followed by partial electrochemical etching) Sotiropoulos, ISE 2016, The Hague Aristotle University

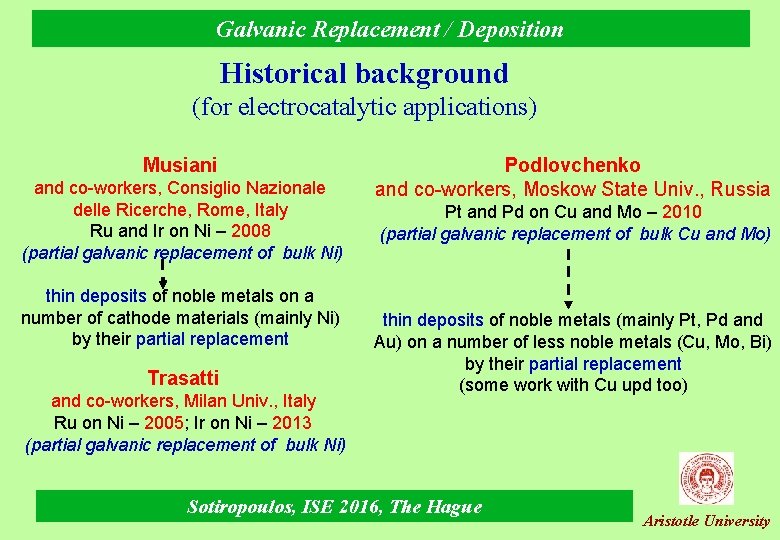
Galvanic Replacement / Deposition Historical background (for electrocatalytic applications) Musiani and co-workers, Consiglio Nazionale delle Ricerche, Rome, Italy Ru and Ir on Ni – 2008 (partial galvanic replacement of bulk Ni) thin deposits of noble metals on a number of cathode materials (mainly Ni) by their partial replacement Trasatti and co-workers, Milan Univ. , Italy Ru on Ni – 2005; Ir on Ni – 2013 (partial galvanic replacement of bulk Ni) Podlovchenko and co-workers, Moskow State Univ. , Russia Pt and Pd on Cu and Mo – 2010 (partial galvanic replacement of bulk Cu and Mo) thin deposits of noble metals (mainly Pt, Pd and Au) on a number of less noble metals (Cu, Mo, Bi) by their partial replacement (some work with Cu upd too) Sotiropoulos, ISE 2016, The Hague Aristotle University

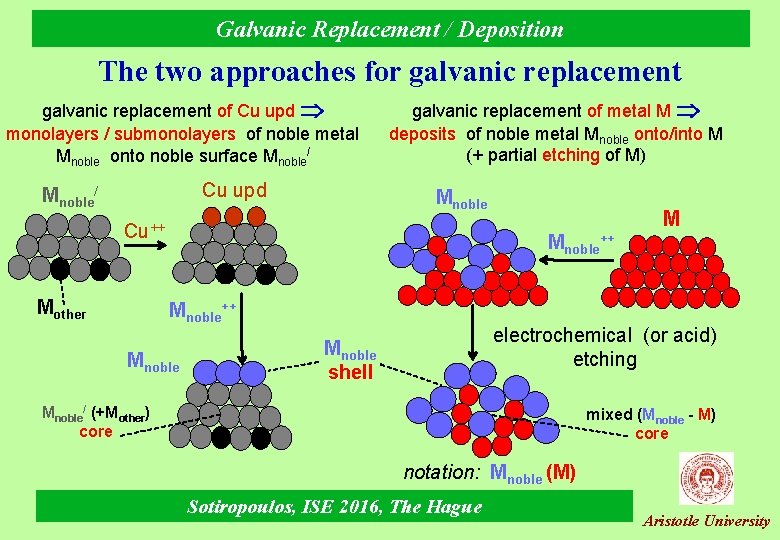
Galvanic Replacement / Deposition The two approaches for galvanic replacement of Cu upd monolayers / submonolayers of noble metal Mnoble onto noble surface Mnoble/ Cu upd Mnoble/ galvanic replacement of metal M deposits of noble metal Mnoble onto/into M (+ partial etching of M) Mnoble Cu++ Mother M Mnoble++ Mnoble electrochemical (or acid) etching Mnoble shell Mnoble/ (+Mother) core mixed (Mnoble - M) core notation: Mnoble (M) Sotiropoulos, ISE 2016, The Hague Aristotle University

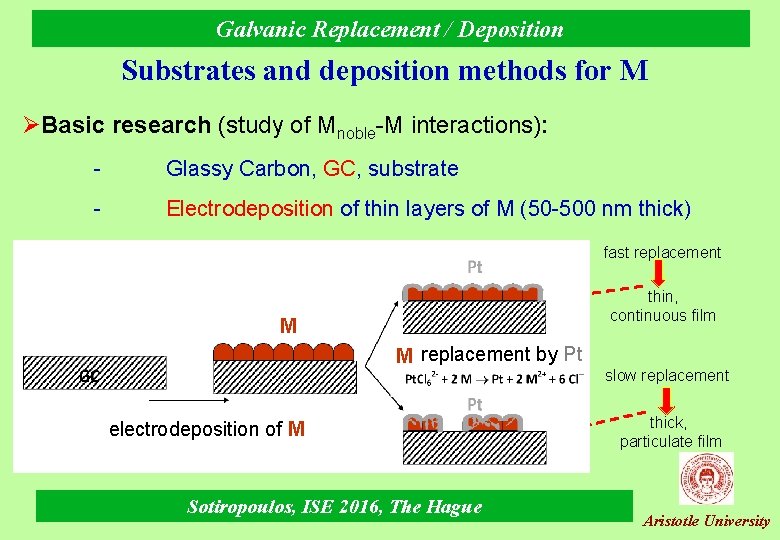
Galvanic Replacement / Deposition Substrates and deposition methods for M ØBasic research (study of Mnoble-M interactions): - Glassy Carbon, GC, substrate - Electrodeposition of thin layers of Μ (50 -500 nm thick) fast replacement thin, continuous film M Cu M replacement by Pt electrodeposition of M Sotiropoulos, ISE 2016, The Hague slow replacement thick, particulate film Aristotle University

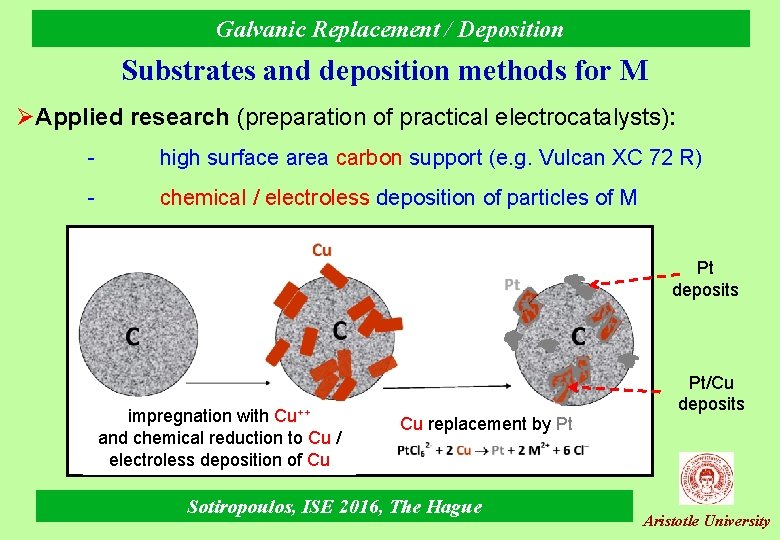
Galvanic Replacement / Deposition Substrates and deposition methods for M ØApplied research (preparation of practical electrocatalysts): - high surface area carbon support (e. g. Vulcan XC 72 R) - chemical / electroless deposition of particles of M Pt deposits impregnation with Cu++ and chemical reduction to Cu / electroless deposition of Cu Cu replacement by Pt Sotiropoulos, ISE 2016, The Hague Pt/Cu deposits Aristotle University

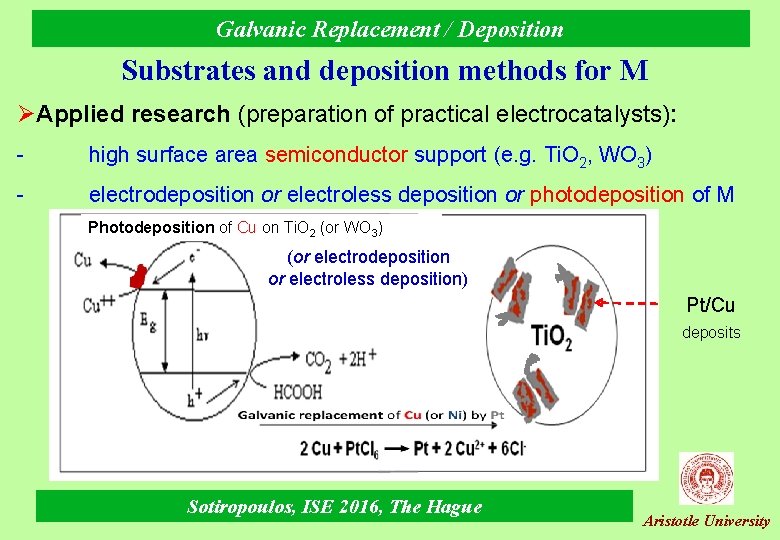
Galvanic Replacement / Deposition Substrates and deposition methods for M ØApplied research (preparation of practical electrocatalysts): - high surface area semiconductor support (e. g. Ti. O 2, WO 3) - electrodeposition or electroless deposition or photodeposition of M Photodeposition of Cu on Ti. O 2 (or WO 3) (or electrodeposition or electroless deposition) Pt/Cu deposits Sotiropoulos, ISE 2016, The Hague Aristotle University

Galvanic Replacement / Deposition Possibilities offered by the method Ø Modification of catalytic properties of Mnoble (applied research) Ø Decrease of the amount of the precious metal catalyst (applied research) Ø Binary Mnoble-M system formation at room temperature Ø (basic and applied research) Study of the electronic effect of M-rich core on the Mnoble shell (basic research) Advantage: low energy- and chemistry- intensive method Sotiropoulos, ISE 2016, The Hague Aristotle University

Galvanic Replacement / Deposition Applications in Electrocatalysis (Aristotle University) Ø Methanol oxidation reaction (MOR) and CO oxidation enhancement at Pt(Cu)/C, Pt. Ru(Ni)/C, Pt(Cu)/Ti. O 2, Pt(Ni)/WO 3 electrodes. Ø Oxygen reduction reaction (ORR) ehnancement at Pt(Co)/C, Pt(Ni)/C and Pt(Cu)/C electrodes. Ø Borohydride oxidation reaction (BOR) enhancement/optimization at Pt(Ni)/C and Pt. Au(Ni)/C electrodes. Ø Oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) enhancement at Ir. O 2/Ir(Ni) and Ir(Ni) electrodes. Ø Benzylbromide reduction enhancement at Ag(Ni) electrodes. Ø Practical Mnoble(M) catalysts on high area supports (carbon or semiconductor powders). Sotiropoulos, ISE 2016, The Hague Aristotle University

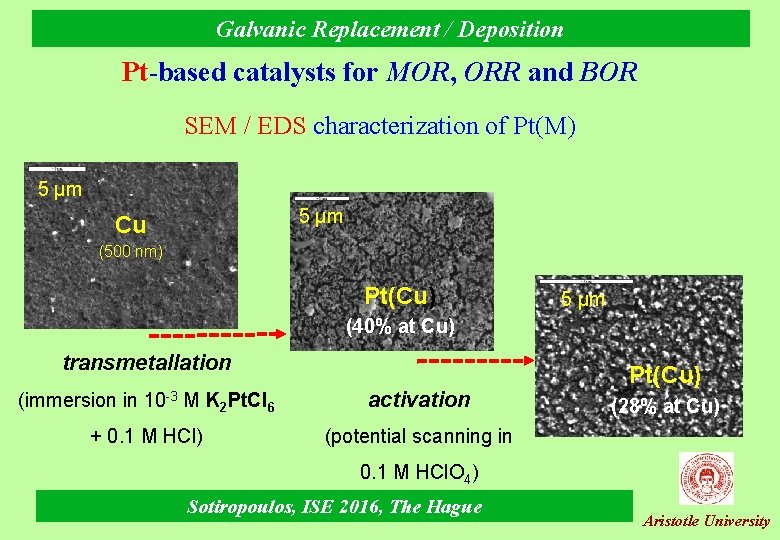
Galvanic Replacement / Deposition Pt-based catalysts for MOR, ORR and BOR SEM / EDS characterization of Pt(M) 5 μm Cu (500 nm) Pt(Cu) 5 μm (40% at Cu) transmetallation (immersion in 10 -3 Μ K 2 Pt. Cl 6 activation + 0. 1 M HCl) (potential scanning in Pt(Cu) (28% at Cu) 0. 1 Μ HCl. O 4) Sotiropoulos, ISE 2016, The Hague Aristotle University

Galvanic Replacement / Deposition SEM micrographs of samples from a “library”of Pt(Cu), Pt(Pb), Pt(Ni), Pt(Co) deposits Pt (Cu 28%) 5 μm Pt (Pb 27%) 5 μm Pt (Co 27%) Pt (Ni 23%) Sotiropoulos, ISE 2016, The Hague Aristotle University

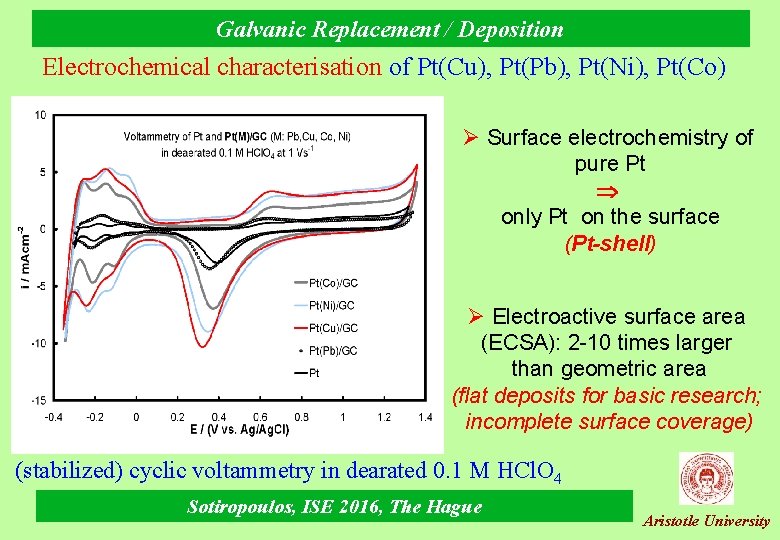
Galvanic Replacement / Deposition Electrochemical characterisation of Pt(Cu), Pt(Pb), Pt(Ni), Pt(Co) Ø Surface electrochemistry of pure Pt only Pt on the surface (Pt-shell) Ø Electroactive surface area (ECSA): 2 -10 times larger than geometric area (flat deposits for basic research; incomplete surface coverage) (stabilized) cyclic voltammetry in dearated 0. 1 Μ ΗCl. O 4 Sotiropoulos, ISE 2016, The Hague Aristotle University

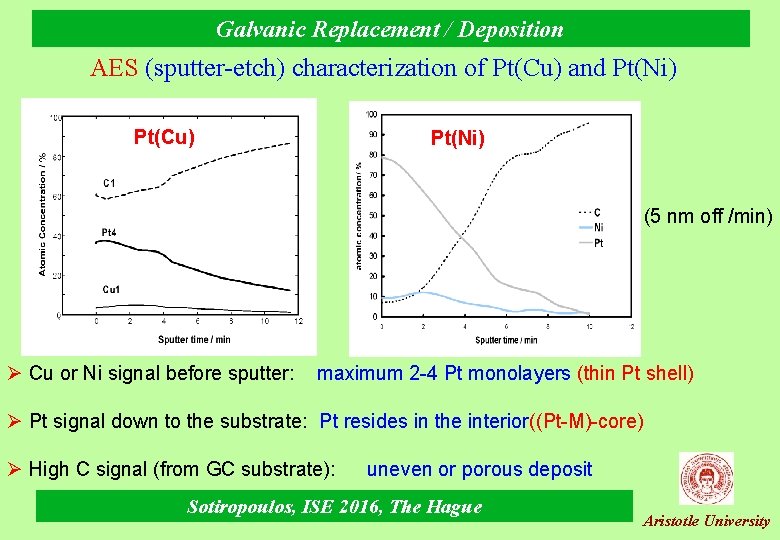
Galvanic Replacement / Deposition AES (sputter-etch) characterization of Pt(Cu) and Pt(Ni) Pt(Cu) Pt(Ni) (5 nm off /min) Ø Cu or Ni signal before sputter: maximum 2 -4 Pt monolayers (thin Pt shell) Ø Pt signal down to the substrate: Pt resides in the interior((Pt-M)-core) Ø High C signal (from GC substrate): uneven or porous deposit Sotiropoulos, ISE 2016, The Hague Aristotle University

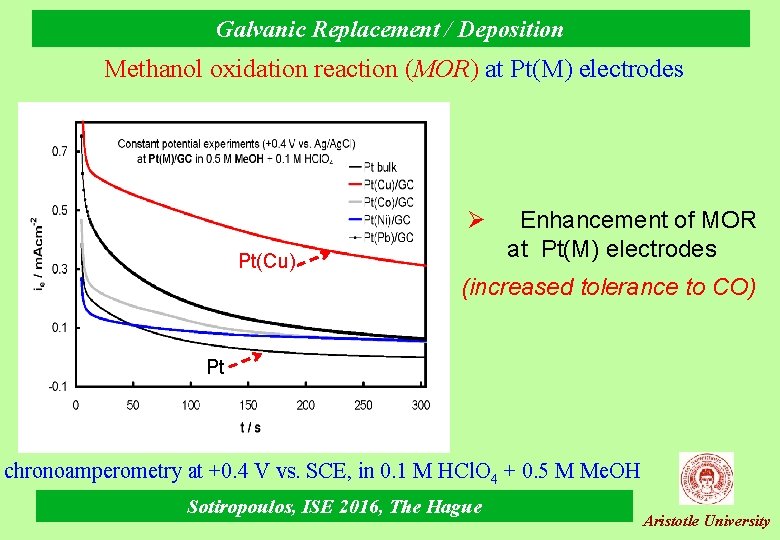
Galvanic Replacement / Deposition Methanol oxidation reaction (MOR) at Pt(M) electrodes Ø Pt(Cu) Enhancement of MOR at Pt(Μ) electrodes (increased tolerance to CO) Pt chronoamperometry at +0. 4 V vs. SCE, in 0. 1 Μ ΗCl. O 4 + 0. 5 M Me. OH Sotiropoulos, ISE 2016, The Hague Aristotle University

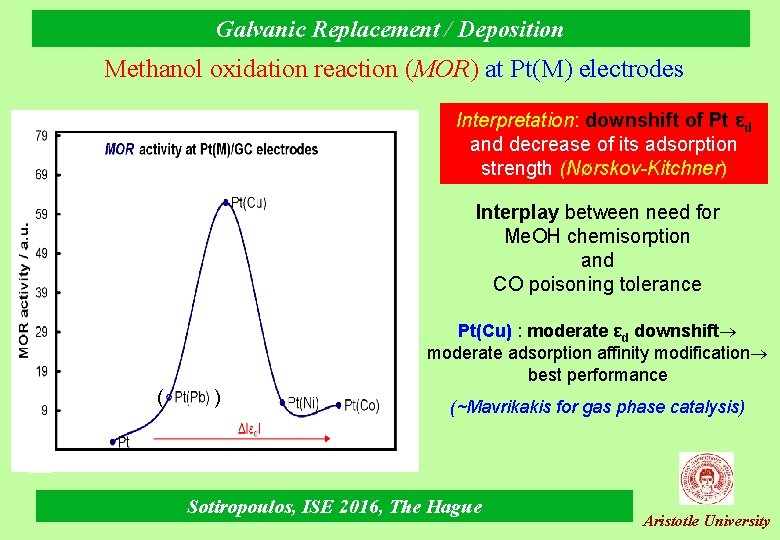
Galvanic Replacement / Deposition Methanol oxidation reaction (MOR) at Pt(M) electrodes Interpretation: downshift of Pt εd and decrease of its adsorption strength (Νørskov-Kitchner) Interplay between need for Me. OH chemisorption and CO poisoning tolerance Pt(Cu) : moderate εd downshift moderate adsorption affinity modification best performance ( ) (~Mavrikakis for gas phase catalysis) Sotiropoulos, ISE 2016, The Hague Aristotle University

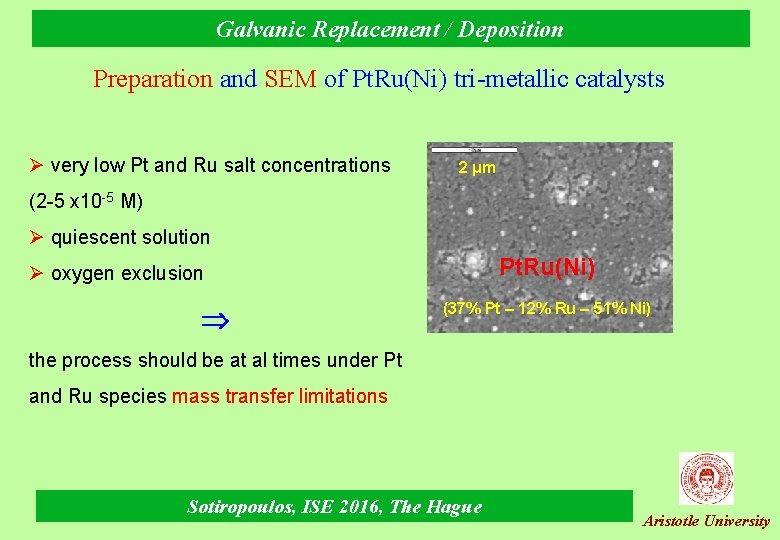
Galvanic Replacement / Deposition Preparation and SEM of Pt. Ru(Ni) tri-metallic catalysts Ø very low Pt and Ru salt concentrations 2 μm (2 -5 x 10 -5 M) Ø quiescent solution Pt. Ru(Ni) Ø oxygen exclusion (37% Pt – 12% Ru – 51% Ni) the process should be at al times under Pt and Ru species mass transfer limitations Sotiropoulos, ISE 2016, The Hague Aristotle University

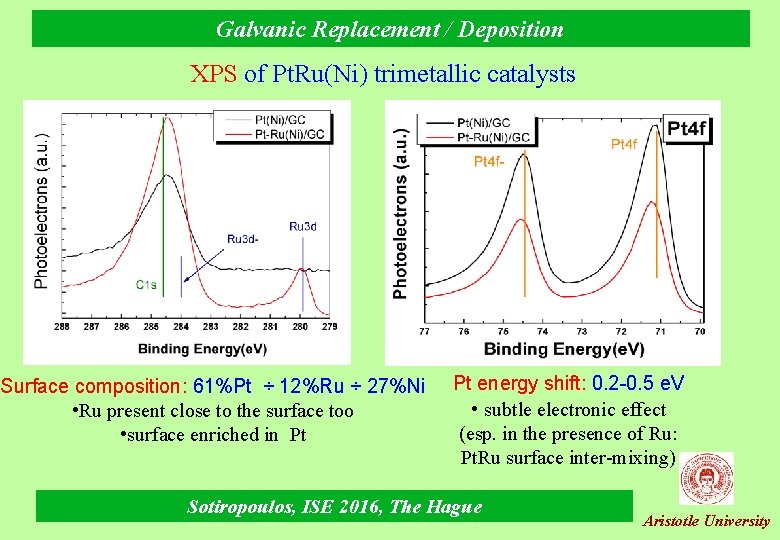
Galvanic Replacement / Deposition XPS of Pt. Ru(Ni) trimetallic catalysts Surface composition: 61%Pt ÷ 12%Ru ÷ 27%Ni • Ru present close to the surface too • surface enriched in Pt Pt energy shift: 0. 2 -0. 5 e. V • subtle electronic effect (esp. in the presence of Ru: Pt. Ru surface inter-mixing) Sotiropoulos, ISE 2016, The Hague Aristotle University

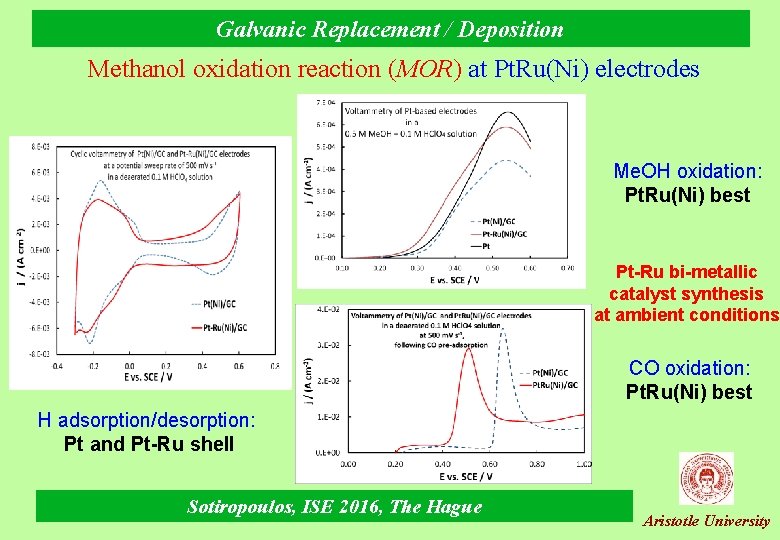
Galvanic Replacement / Deposition Methanol oxidation reaction (MOR) at Pt. Ru(Ni) electrodes Me. OH oxidation: Pt. Ru(Ni) best Pt-Ru bi-metallic catalyst synthesis at ambient conditions CO oxidation: Pt. Ru(Ni) best H adsorption/desorption: Pt and Pt-Ru shell Sotiropoulos, ISE 2016, The Hague Aristotle University

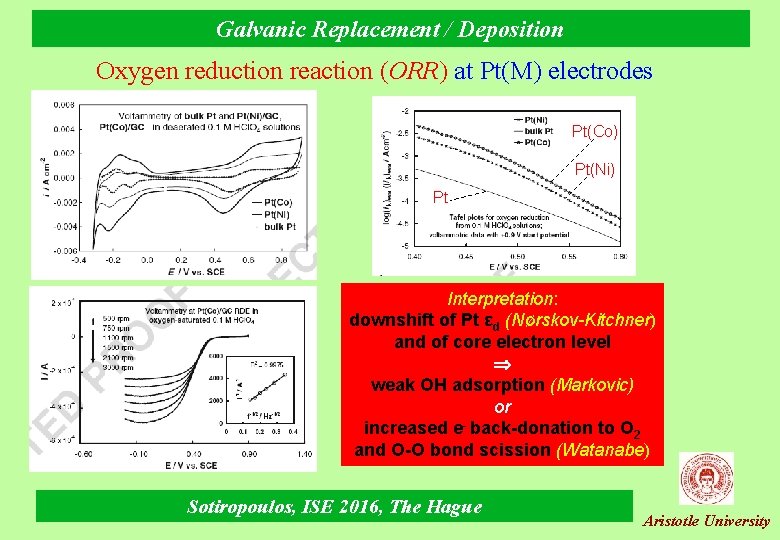
Galvanic Replacement / Deposition Oxygen reduction reaction (ORR) at Pt(M) electrodes Pt(Co) Pt(Ni) Pt Interpretation: downshift of Pt εd (Νørskov-Kitchner) and of core electron level weak OH adsorption (Markovic) or increased e- back-donation to O 2 and O-O bond scission (Watanabe) Sotiropoulos, ISE 2016, The Hague Aristotle University

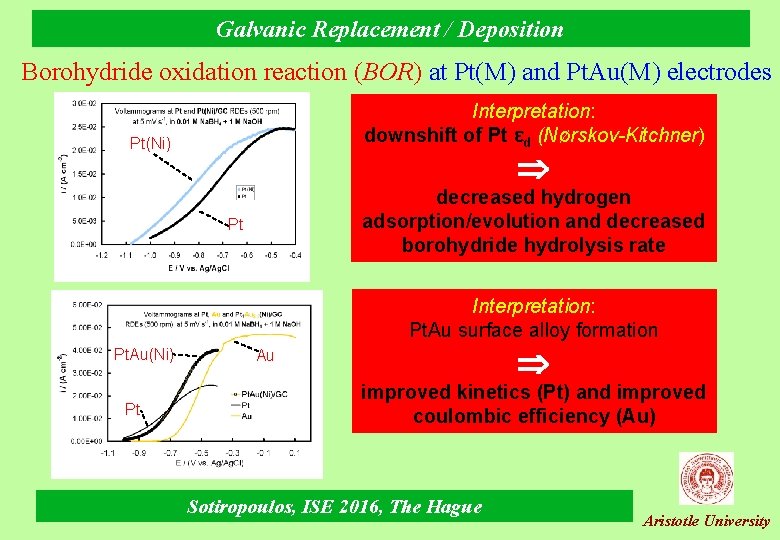
Galvanic Replacement / Deposition Borohydride oxidation reaction (BOR) at Pt(M) and Pt. Au(M) electrodes Interpretation: downshift of Pt εd (Νørskov-Kitchner) Pt(Ni) decreased hydrogen adsorption/evolution and decreased borohydride hydrolysis rate Pt Interpretation: Pt. Au surface alloy formation Pt. Au(Ni) Pt Au improved kinetics (Pt) and improved coulombic efficiency (Au) Sotiropoulos, ISE 2016, The Hague Aristotle University

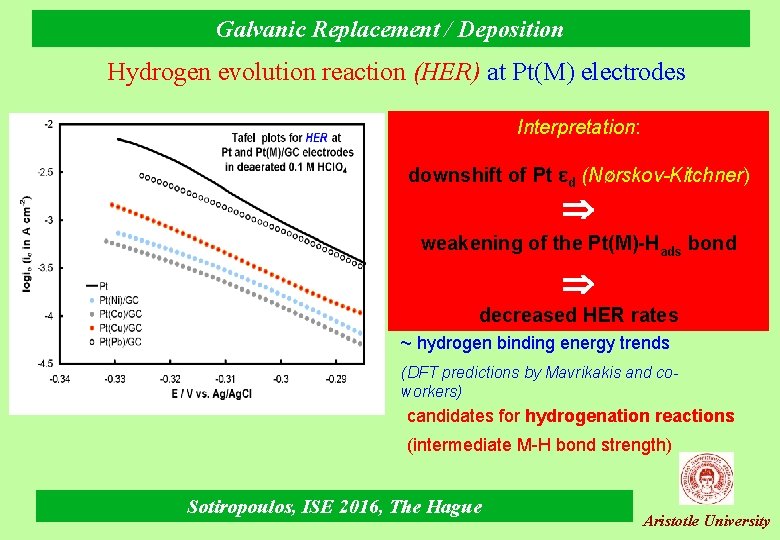
Galvanic Replacement / Deposition Hydrogen evolution reaction (HER) at Pt(M) electrodes Interpretation: downshift of Pt εd (Νørskov-Kitchner) weakening of the Pt(M)-Hads bond decreased HER rates ~ hydrogen binding energy trends (DFT predictions by Mavrikakis and coworkers) candidates for hydrogenation reactions (intermediate M-H bond strength) Sotiropoulos, ISE 2016, The Hague Aristotle University

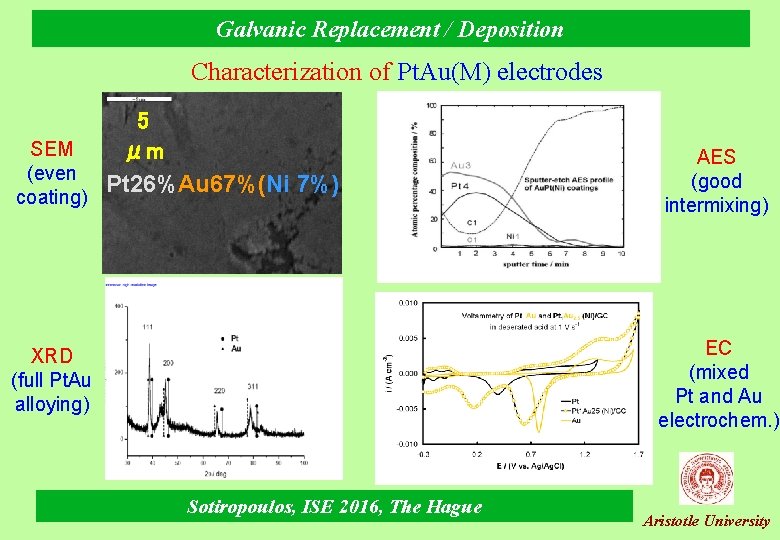
Galvanic Replacement / Deposition Characterization of Pt. Au(M) electrodes 5 SEM μm (even Pt 26%Au 67%(Ni 7%) coating) AES (good intermixing) EC (mixed Pt and Au electrochem. ) XRD (full Pt. Au alloying) Sotiropoulos, ISE 2016, The Hague Aristotle University

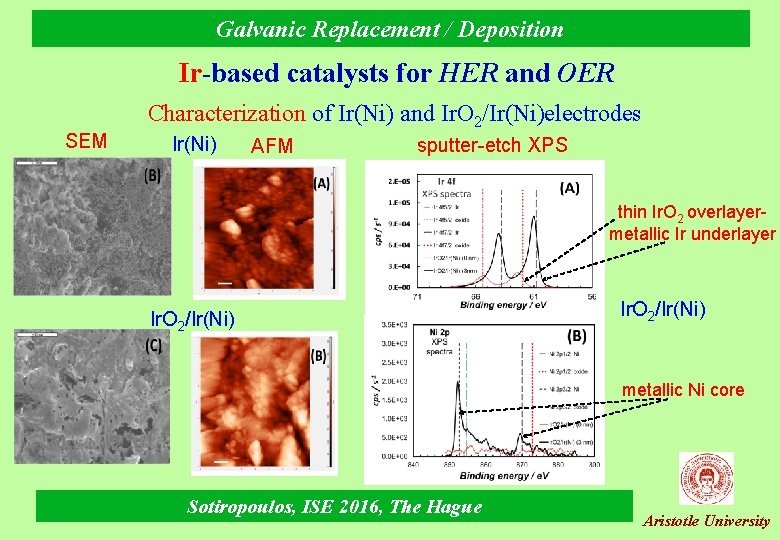
Galvanic Replacement / Deposition Ir-based catalysts for HER and OER SEM Characterization of Ir(Ni) and Ir. O 2/Ir(Ni)electrodes Ir(Ni) AFM sputter-etch XPS thin Ir. O 2 overlayermetallic Ir underlayer Ir. O 2/Ir(Ni) metallic Ni core Sotiropoulos, ISE 2016, The Hague Aristotle University

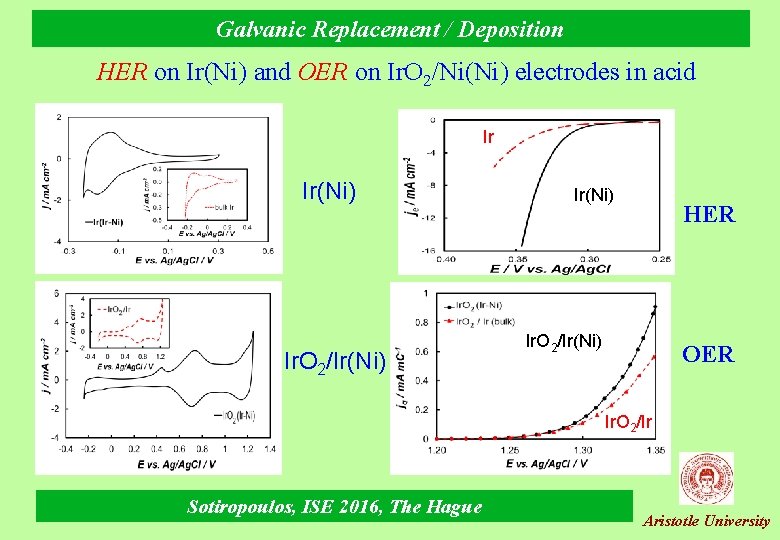
Galvanic Replacement / Deposition HER on Ir(Ni) and OER on Ir. O 2/Ni(Ni) electrodes in acid Ir Ir(Ni) Ir. O 2/Ir(Ni) HER Ir. O 2/Ir(Ni) OER Ir. O 2/Ir Sotiropoulos, ISE 2016, The Hague Aristotle University

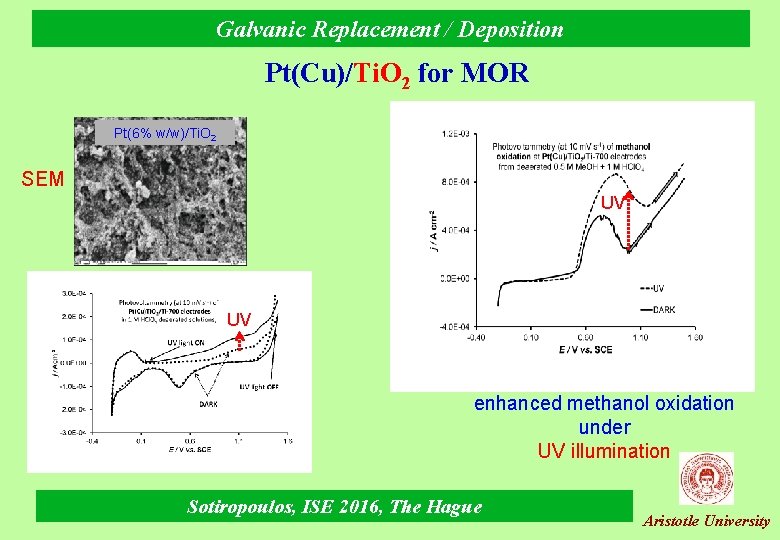
Galvanic Replacement / Deposition Pt(Cu)/Ti. O 2 for MOR Pt(6% w/w)/Ti. O 2 SEM UV UV enhanced methanol oxidation under UV illumination Sotiropoulos, ISE 2016, The Hague Aristotle University

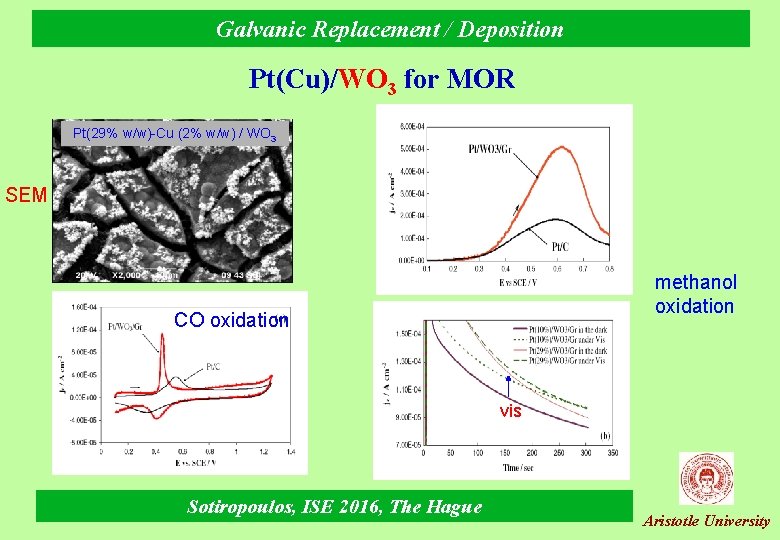
Galvanic Replacement / Deposition Pt(Cu)/WO 3 for MOR Pt(29% w/w)-Cu (2% w/w) / WO 3 SEM methanol oxidation CO oxidation vis Sotiropoulos, ISE 2016, The Hague Aristotle University

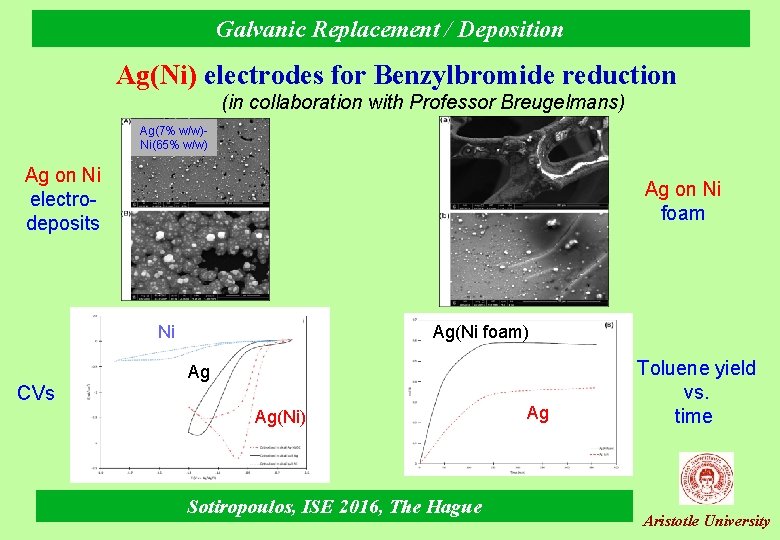
Galvanic Replacement / Deposition Ag(Ni) electrodes for Benzylbromide reduction (in collaboration with Professor Breugelmans) Ag(7% w/w)Ni(65% w/w) Ag on Ni electrodeposits Ag on Ni foam Ag(Ni foam) Ni Ag CVs Ag(Ni) Sotiropoulos, ISE 2016, The Hague Ag Toluene yield vs. time Aristotle University

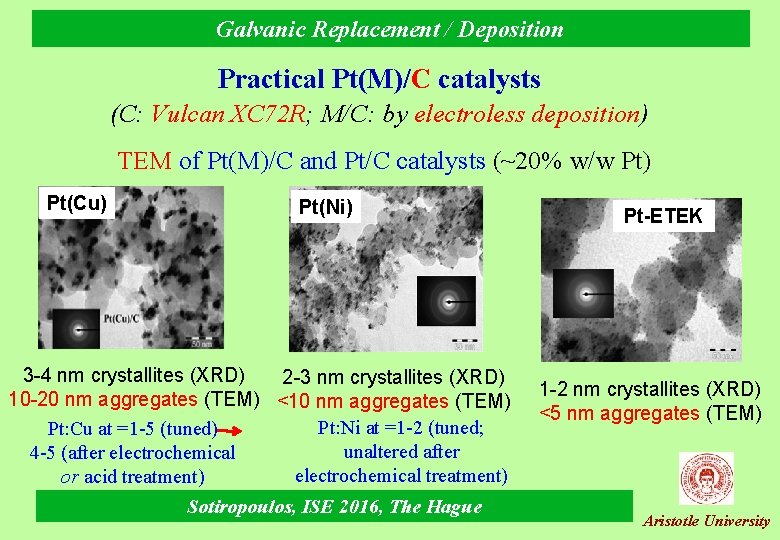
Galvanic Replacement / Deposition Practical Pt(M)/C catalysts (C: Vulcan XC 72 R; M/C: by electroless deposition) TEM of Pt(M)/C and Pt/C catalysts (~20% w/w Pt) Pt(Cu) Pt(Ni) 3 -4 nm crystallites (XRD) 2 -3 nm crystallites (XRD) 10 -20 nm aggregates (TEM) <10 nm aggregates (TEM) Pt: Ni at =1 -2 (tuned; Pt: Cu at =1 -5 (tuned) unaltered after 4 -5 (after electrochemical treatment) or acid treatment) Sotiropoulos, ISE 2016, The Hague Pt-ETEK 1 -2 nm crystallites (XRD) <5 nm aggregates (TEM) Aristotle University

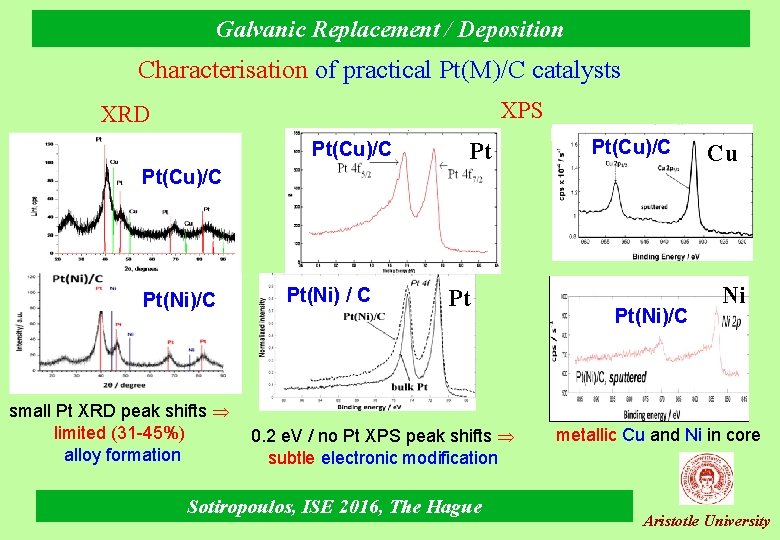
Galvanic Replacement / Deposition Characterisation of practical Pt(M)/C catalysts XPS XRD Pt(Cu)/C Pt(Ni)/C small Pt XRD peak shifts limited (31 -45%) alloy formation Pt(Ni) / C Pt 0. 2 e. V / no Pt XPS peak shifts subtle electronic modification Sotiropoulos, ISE 2016, The Hague Pt(Ni)/C Cu Ni metallic Cu and Ni in core Aristotle University

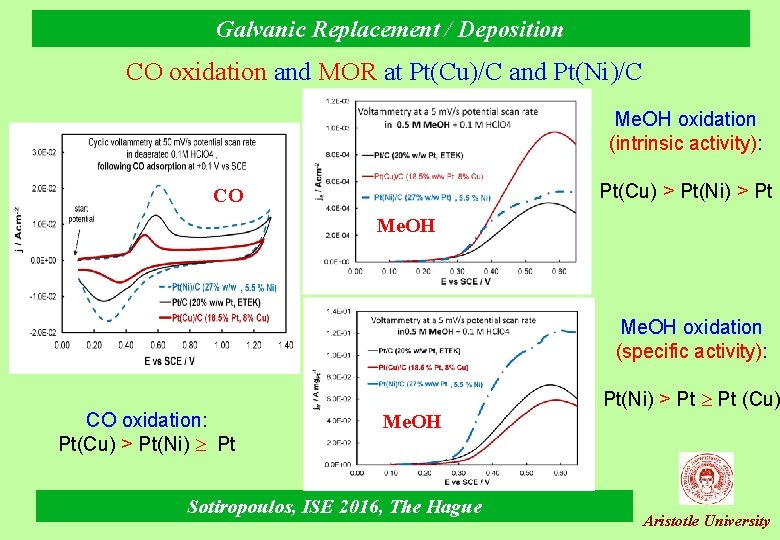
Galvanic Replacement / Deposition CO oxidation and MOR at Pt(Cu)/C and Pt(Ni)/C Me. OH oxidation (intrinsic activity): Pt(Cu) > Pt(Ni) > Pt CO Me. OH oxidation (specific activity): CO oxidation: Pt(Cu) > Pt(Ni) Pt Me. OH Sotiropoulos, ISE 2016, The Hague Pt(Νi) > Pt (Cu) Aristotle University

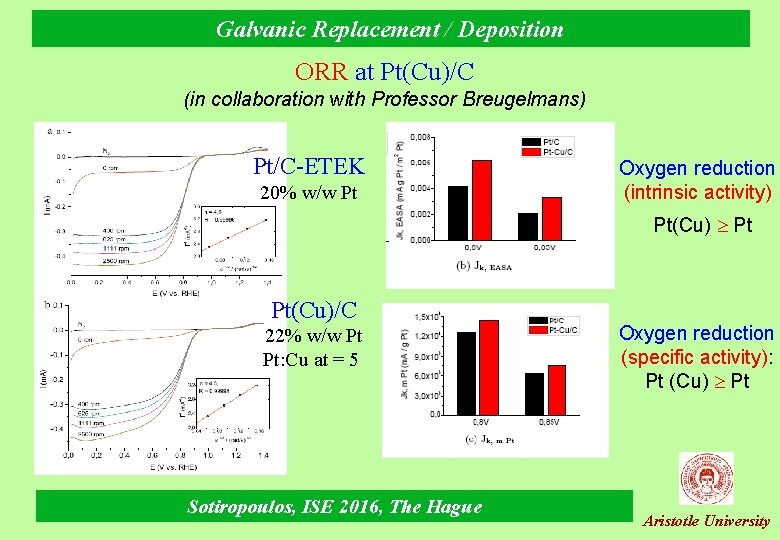
Galvanic Replacement / Deposition ORR at Pt(Cu)/C (in collaboration with Professor Breugelmans) Pt/C-ETEK 20% w/w Pt Oxygen reduction (intrinsic activity) Pt(Cu) Pt Pt(Cu)/C 22% w/w Pt Pt: Cu at = 5 Sotiropoulos, ISE 2016, The Hague Oxygen reduction (specific activity): Pt (Cu) Pt Aristotle University

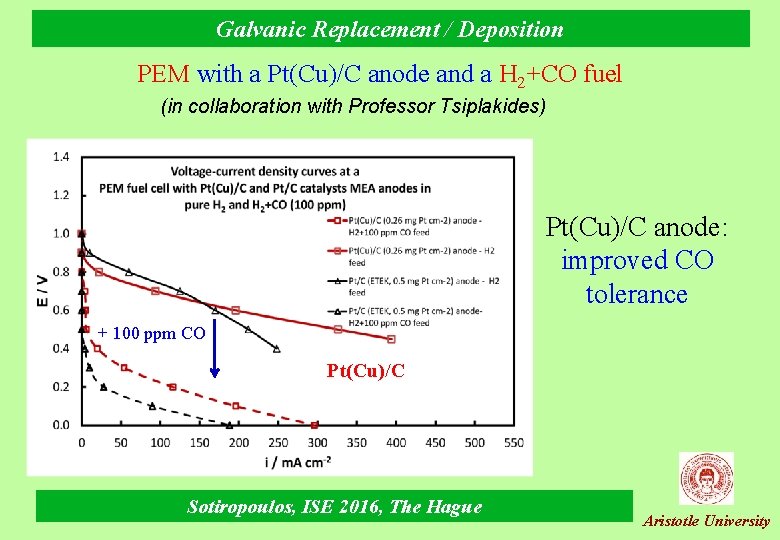
Galvanic Replacement / Deposition PEM with a Pt(Cu)/C anode and a H 2+CO fuel (in collaboration with Professor Tsiplakides) Pt(Cu)/C anode: improved CO tolerance + 100 ppm CO Pt(Cu)/C Sotiropoulos, ISE 2016, The Hague Aristotle University

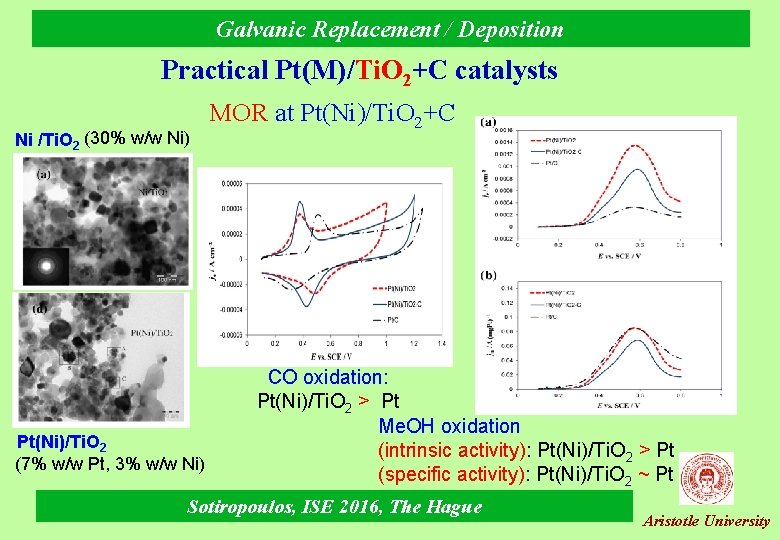
Galvanic Replacement / Deposition Practical Pt(M)/Ti. O 2+C catalysts Ni /Ti. O 2 (30% w/w Ni) Pt(Ni)/Ti. O 2 (7% w/w Pt, 3% w/w Ni) MOR at Pt(Ni)/Ti. O 2+C CO oxidation: Pt(Ni)/Ti. O 2 > Pt Me. OH oxidation (intrinsic activity): Pt(Ni)/Ti. O 2 > Pt (specific activity): Pt(Ni)/Ti. O 2 ~ Pt Sotiropoulos, ISE 2016, The Hague Aristotle University

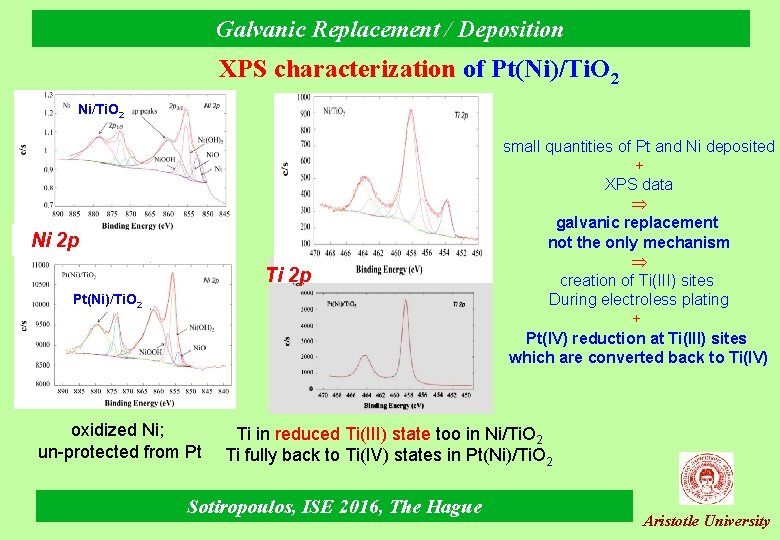
Galvanic Replacement / Deposition XPS characterization of Pt(Ni)/Ti. O 2 Ni 2 p Ti 2 p Pt(Ni)/Ti. O 2 oxidized Ni; un-protected from Pt small quantities of Pt and Ni deposited + XPS data galvanic replacement not the only mechanism creation of Ti(III) sites During electroless plating + Pt(IV) reduction at Ti(III) sites which are converted back to Ti(IV) Ti in reduced Ti(III) state too in Ni/Ti. O 2 Ti fully back to Ti(IV) states in Pt(Ni)/Ti. O 2 Sotiropoulos, ISE 2016, The Hague Aristotle University

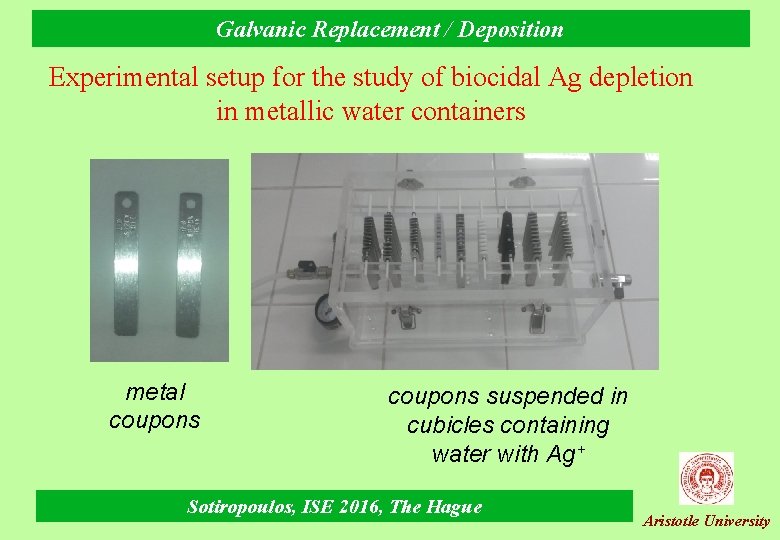
Galvanic Replacement / Deposition Galvanic deposition in metal ion removal Biocidal Ag depletion in metallic water containers (in collaboration with Professor Karapantsios) Ø The problem (ESA for ISS missions): Ag+ used as a biocide in space mission potable water is depleted from 0. 5 ppm to below 0. 2 ppm levels when stored and transferred in stainless steel tanks. Ø The task (Aristotle University): - identify the mechanism (colloidal silver/oxide/hydroxide deposition, adsorption, other) - propose changes (process, solution chemistry and/or materials) Ø The methodology (Aristotle University): - accelerated tests (high S/V) of various materials specimens at both disinfection and potable water Ag+ ion levels (10 and 0. 5 ppm) - quantitative chemical analysis of Ag (and other ions) in solution and from etched surfaces. - XPS to probe the chemical state of Ag Sotiropoulos, ISE 2016, The Hague Aristotle University

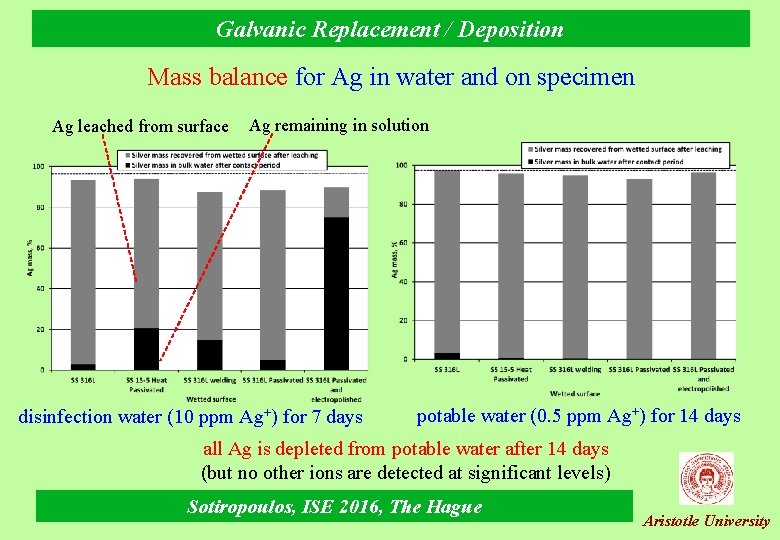
Galvanic Replacement / Deposition Mass balance for Ag in water and on specimen Ag leached from surface Ag remaining in solution disinfection water (10 ppm Ag+) for 7 days potable water (0. 5 ppm Ag+) for 14 days all Ag is depleted from potable water after 14 days (but no other ions are detected at significant levels) Sotiropoulos, ISE 2016, The Hague Aristotle University

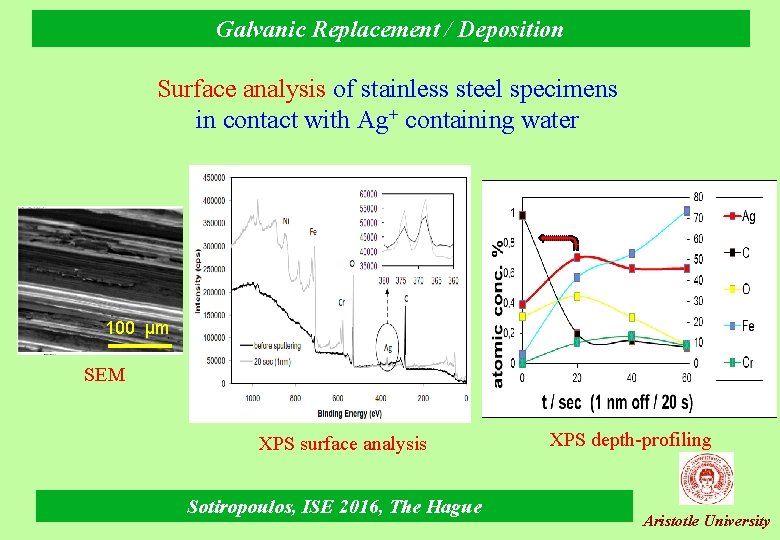
Galvanic Replacement / Deposition Surface analysis of stainless steel specimens in contact with Ag+ containing water 100 μm SEM XPS surface analysis Sotiropoulos, ISE 2016, The Hague XPS depth-profiling Aristotle University

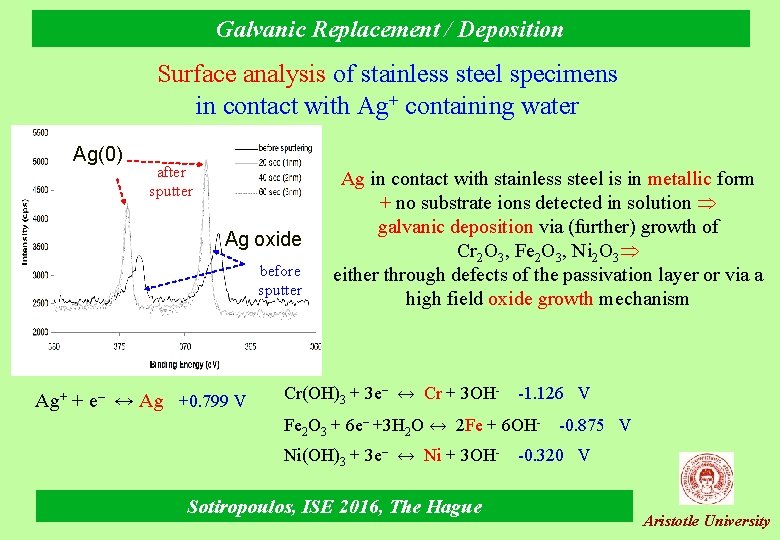
Galvanic Replacement / Deposition Surface analysis of stainless steel specimens in contact with Ag+ containing water Ag(0) after sputter Ag oxide before sputter Ag+ + e– ↔ Ag +0. 799 V Ag in contact with stainless steel is in metallic form + no substrate ions detected in solution galvanic deposition via (further) growth of Cr 2 O 3, Fe 2 O 3, Ni 2 O 3 either through defects of the passivation layer or via a high field oxide growth mechanism Cr(OH)3 + 3 e– ↔ Cr + 3 OH- -1. 126 V Fe 2 O 3 + 6 e– +3 H 2 O ↔ 2 Fe + 6 OHNi(OH)3 + 3 e– ↔ Ni + 3 OH- Sotiropoulos, ISE 2016, The Hague -0. 875 V -0. 320 V Aristotle University

Galvanic Replacement / Deposition Systems with further research and development potential Ø Pt(Cu) and Pt(Ni) particle anodes in impure H 2+CO fuel streams. Ø Pt(Cu), Pt(Ni), Pt(Co) particle ORR cathodes. Ø Modified (e. g. by Ir, Ag) Ni, Cu, steel cathodes for HER or electrosynthesis. Ø Pt-modified Ti. O 2 and WO 3 anodes for photoelectrocatalysis. Ø Materials research into metal containers that cannot/can deplete traces of noble metals. Sotiropoulos, ISE 2016, The Hague Aristotle University

Galvanic Replacement / Deposition Acknowledgements / Collaborations • G. Georgieva, S. Armyanov, E. Valova and co-workers Bulgarian Academy of Sciences • A. Hubin and co-workers Vrije Universiteit Brussels • T. Breugelmans and co-workers University of Antwerp • D. Tsiplakides, S. Balomenou and co-workers CPERI, Thessaloniki • Th. Karapantsios, M. Petala and co-workers • Electrochemistry Group (G. Kokkinidis, A. Papaderakis, I. Mintsouli, S. Papadimitriou, A. Tegou, E. Kolotha) Aristotle Univ. , Thessaloniki Sotiropoulos, ISE 2016, The Hague Aristotle Univ. , Thessaloniki Aristotle University

Galvanic Replacement / Deposition SUPPLEMENTS Sotiropoulos, ISE 2016, The Hague Aristotle University

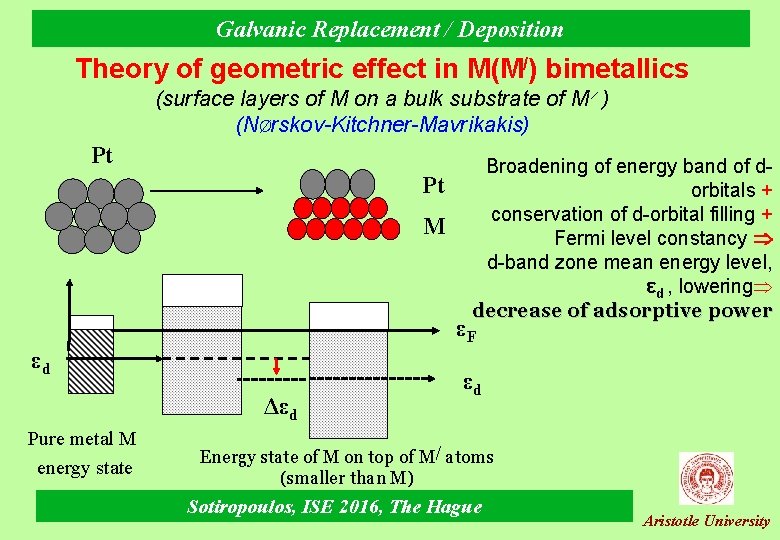
Galvanic Replacement / Deposition Theory of properties modification in Μ(Μ/) bi-metallics Geometric effect: different size (Wigner-Seitz radius) of M and M/ atoms surface atoms of M under (tensile or compressive) strain from the M/ atoms of the substrate change of electronic properties of M Electronic (“ligand”) effect: M-M/ atom interaction due to differences in electronegativity Sotiropoulos, ISE 2016, The Hague Aristotle University

Galvanic Replacement / Deposition Theory of geometric effect in Μ(Μ/) bimetallics (surface layers of Μ on a bulk substrate of Μ ) (NØrskov-Kitchner-Mavrikakis) Pt Pt Μ Broadening of energy band of dorbitals + conservation of d-orbital filling + Fermi level constancy d-band zone mean energy level, εd , lowering decrease of adsorptive power εF εd Δεd Pure metal M energy state εd Energy state of M on top of M/ atoms (smaller than M) Sotiropoulos, ISE 2016, The Hague Aristotle University

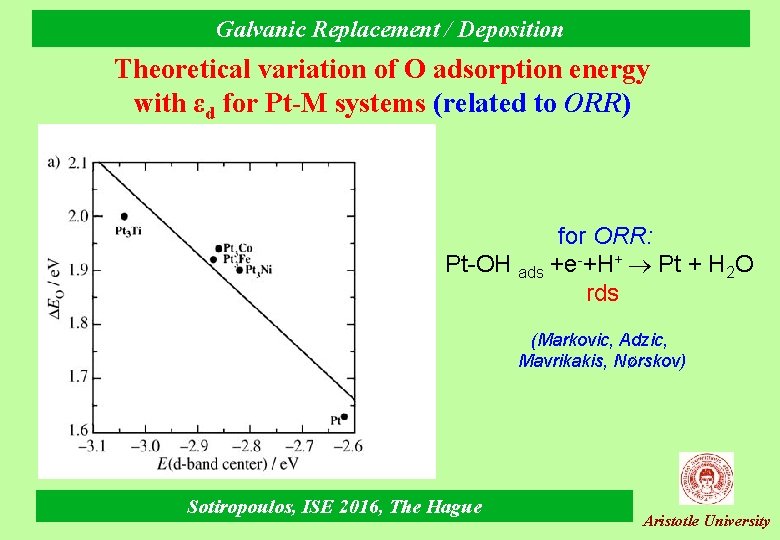
Galvanic Replacement / Deposition Theoretical variation of O adsorption energy with εd for Pt-M systems (related to ORR) for ORR: Pt-OH ads +e-+H+ Pt + Η 2Ο rds (Markovic, Adzic, Mavrikakis, Nørskov) Sotiropoulos, ISE 2016, The Hague Aristotle University

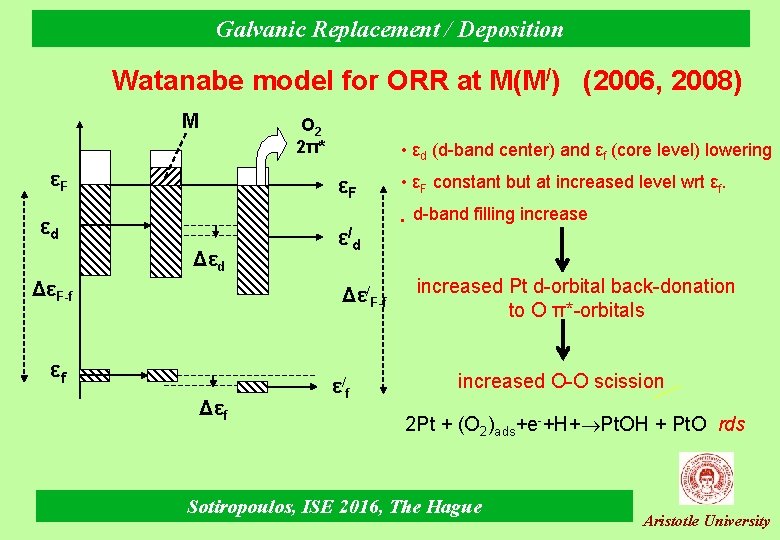
Galvanic Replacement / Deposition Watanabe model for ORR at M(M/) (2006, 2008) M Ο 2 2π* εF • εd (d-band center) and εf (core level) lowering εF εd ΔεF-f ε/d Δε/F-f εf Δεf ε/f • εF constant but at increased level wrt εf. • d-band filling increased Pt d-orbital back-donation to O π*-orbitals increased O-O scission 2 Pt + (O 2)ads+e-+H+ Pt. OH + Pt. O rds Sotiropoulos, ISE 2016, The Hague Aristotle University

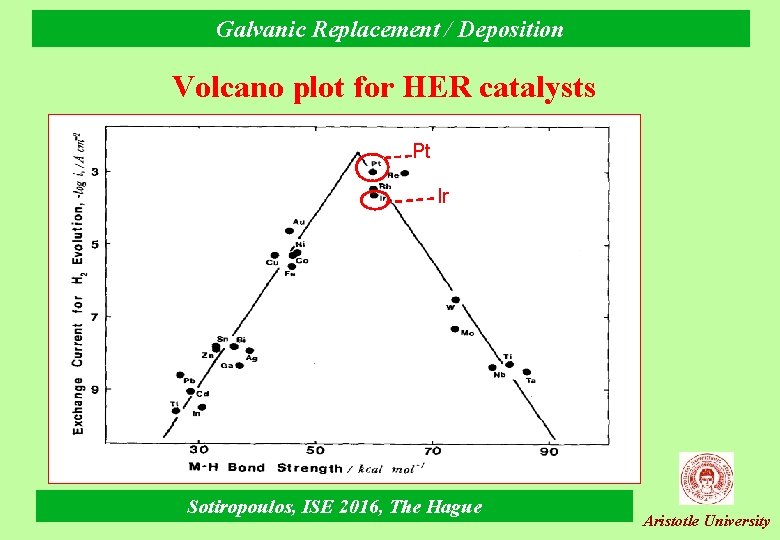
Galvanic Replacement / Deposition Volcano plot for HER catalysts Pt Ir Sotiropoulos, ISE 2016, The Hague Aristotle University

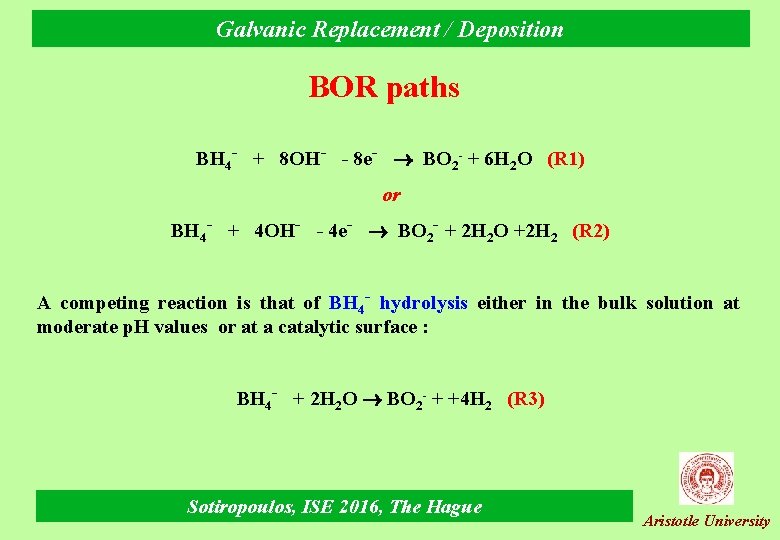
Galvanic Replacement / Deposition BOR paths BH 4 - + 8 OH- - 8 e- BO 2 - + 6 H 2 O (R 1) or BH 4 - + 4 OH- - 4 e- BO 2 - + 2 H 2 O +2 H 2 (R 2) A competing reaction is that of BH 4 - hydrolysis either in the bulk solution at moderate p. H values or at a catalytic surface : BH 4 - + 2 H 2 O BO 2 - + +4 H 2 (R 3) Sotiropoulos, ISE 2016, The Hague Aristotle University

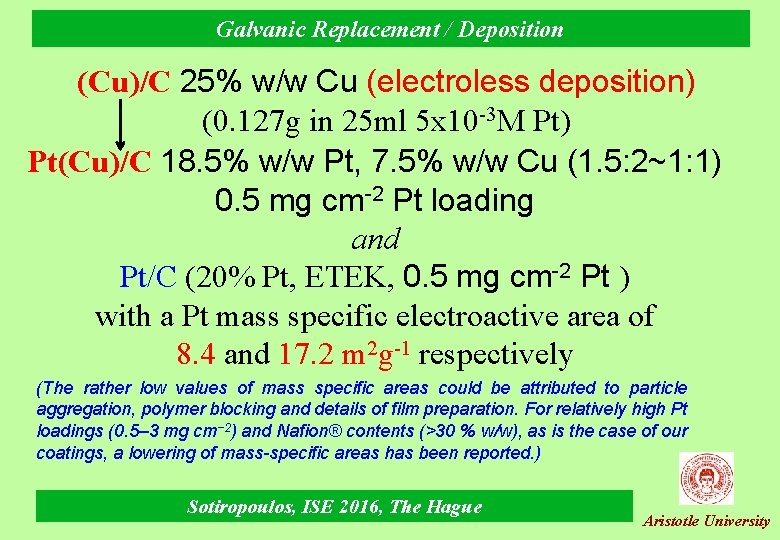
Galvanic Replacement / Deposition (Cu)/C 25% w/w Cu (electroless deposition) (0. 127 g in 25 ml 5 x 10 -3 M Pt) Pt(Cu)/C 18. 5% w/w Pt, 7. 5% w/w Cu (1. 5: 2~1: 1) 0. 5 mg cm-2 Pt loading and Pt/C (20% Pt, ETEK, 0. 5 mg cm-2 Pt ) with a Pt mass specific electroactive area of 8. 4 and 17. 2 m 2 g-1 respectively (The rather low values of mass specific areas could be attributed to particle aggregation, polymer blocking and details of film preparation. For relatively high Pt loadings (0. 5– 3 mg cm− 2) and Nafion® contents (>30 % w/w), as is the case of our coatings, a lowering of mass-specific areas has been reported. ) Sotiropoulos, ISE 2016, The Hague Aristotle University

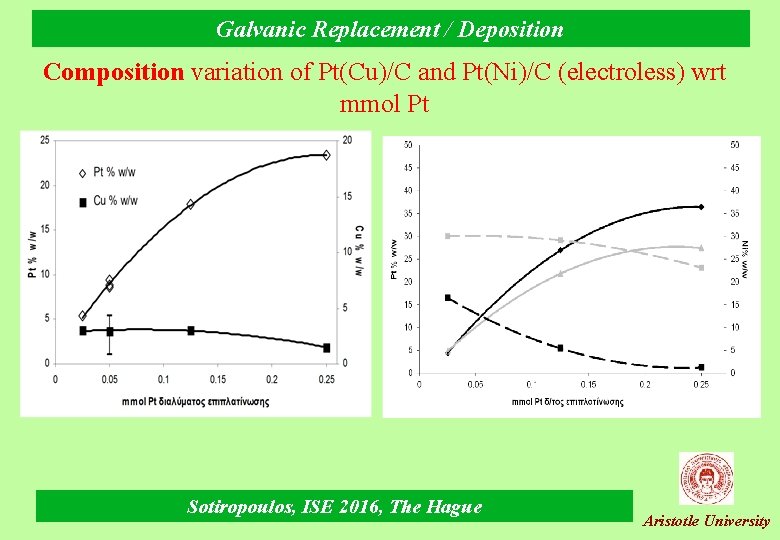
Galvanic Replacement / Deposition Composition variation of Pt(Cu)/C and Pt(Ni)/C (electroless) wrt mmol Pt Sotiropoulos, ISE 2016, The Hague Aristotle University

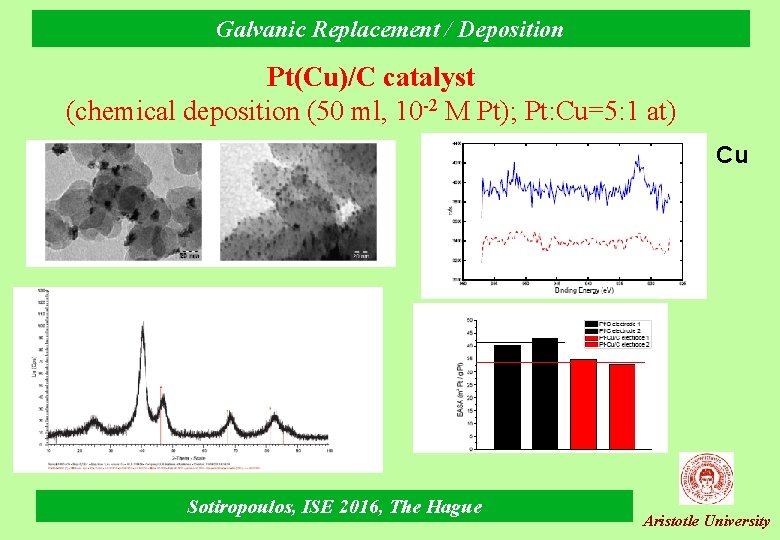
Galvanic Replacement / Deposition Pt(Cu)/C catalyst (chemical deposition (50 ml, 10 -2 M Pt); Pt: Cu=5: 1 at) Cu Sotiropoulos, ISE 2016, The Hague Aristotle University

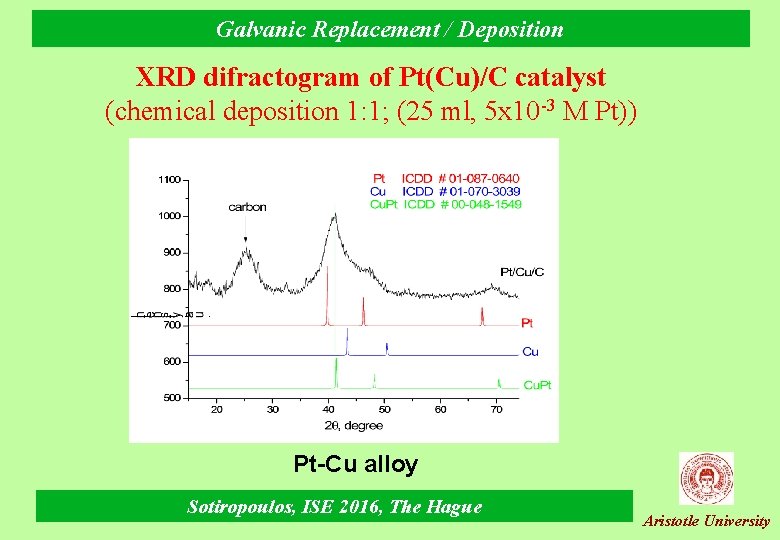
Galvanic Replacement / Deposition XRD difractogram of Pt(Cu)/C catalyst (chemical deposition 1: 1; (25 ml, 5 x 10 -3 M Pt)) Pt-Cu alloy Sotiropoulos, ISE 2016, The Hague Aristotle University

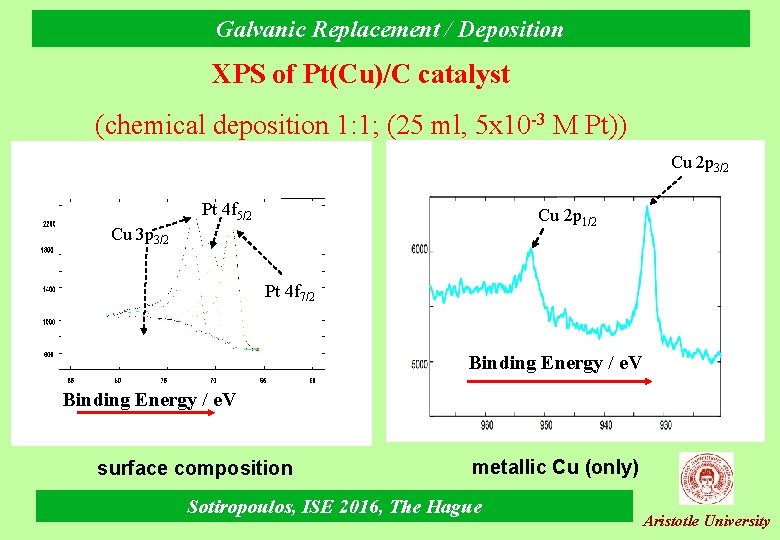
Galvanic Replacement / Deposition ΧPS of Pt(Cu)/C catalyst (chemical deposition 1: 1; (25 ml, 5 x 10 -3 M Pt)) Cu 2 p 3/2 Pt 4 f 5/2 Cu 2 p 1/2 Cu 3 p 3/2 Pt 4 f 7/2 Binding Energy / e. V surface composition metallic Cu (only) Sotiropoulos, ISE 2016, The Hague Aristotle University

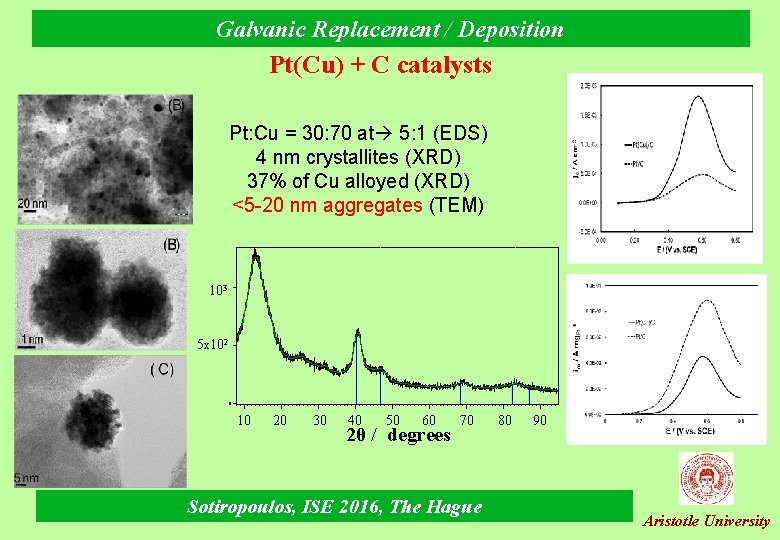
Galvanic Replacement / Deposition Pt(Cu) + C catalysts Pt: Cu = 30: 70 at 5: 1 (EDS) 4 nm crystallites (XRD) 37% of Cu alloyed (XRD) <5 -20 nm aggregates (TEM) 103 5 x 102 0 10 20 30 40 50 60 2θ / degrees 70 Sotiropoulos, ISE 2016, The Hague 80 90 Aristotle University

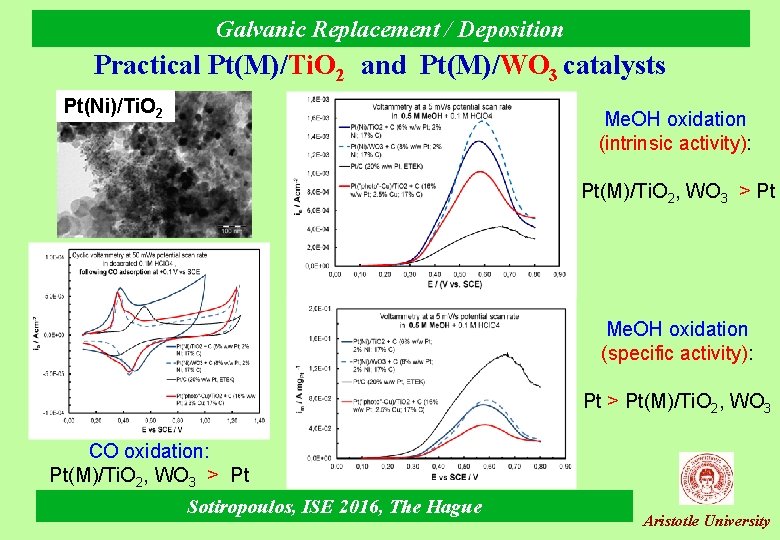
Galvanic Replacement / Deposition Practical Pt(M)/Ti. O 2 and Pt(M)/WO 3 catalysts Pt(Ni)/Ti. O 2 Me. OH oxidation (intrinsic activity): Pt(M)/Ti. O 2, WO 3 > Pt Me. OH oxidation (specific activity): Pt > Pt(M)/Ti. O 2, WO 3 CO oxidation: Pt(M)/Ti. O 2, WO 3 > Pt Sotiropoulos, ISE 2016, The Hague Aristotle University

Galvanic Replacement / Deposition Experimental setup for the study of biocidal Ag depletion in metallic water containers metal coupons suspended in cubicles containing water with Ag+ Sotiropoulos, ISE 2016, The Hague Aristotle University