Chapter 20 Electrochemistry 20 1 Introduction to Electrochemistry











- Slides: 25

Chapter 20 Electrochemistry 20. 1 Introduction to Electrochemistry

Electrochemistry • The branch of chemistry that deals with electricity-related applications of oxidationreduction reactions. • Electrochemical Cells: A system of electrodes and electrolytes in which either chemical reactions produce energy or an electrical current produces chemical change

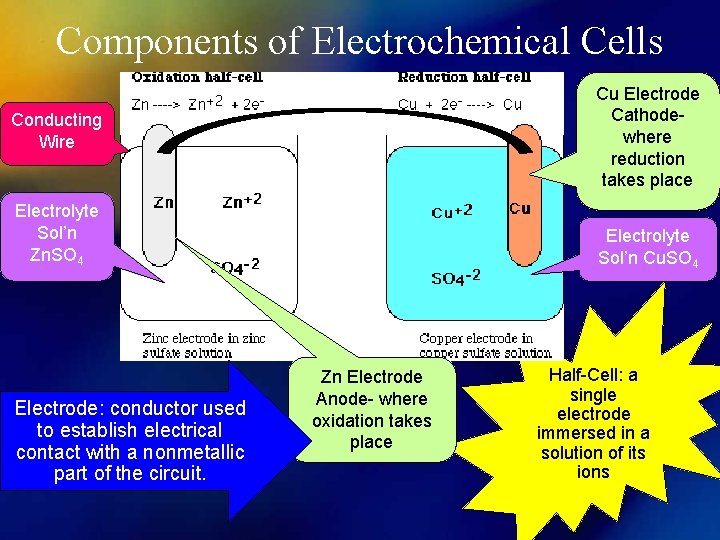
Components of Electrochemical Cells Cu Electrode Cathodewhere reduction takes place Conducting Wire Electrolyte Sol’n Zn. SO 4 Electrode: conductor used to establish electrical contact with a nonmetallic part of the circuit. Electrolyte Sol’n Cu. SO 4 Zn Electrode Anode- where oxidation takes place Half-Cell: a single electrode immersed in a solution of its ions

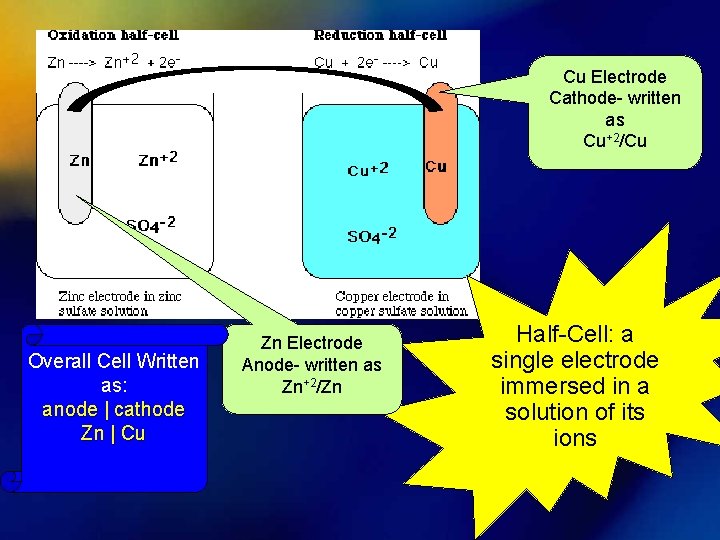
Cu Electrode Cathode- written as Cu+2/Cu Overall Cell Written as: anode | cathode Zn | Cu Zn Electrode Anode- written as Zn+2/Zn Half-Cell: a single electrode immersed in a solution of its ions


Chapter 20 Electrochemistry 20. 2 Voltaic Cells

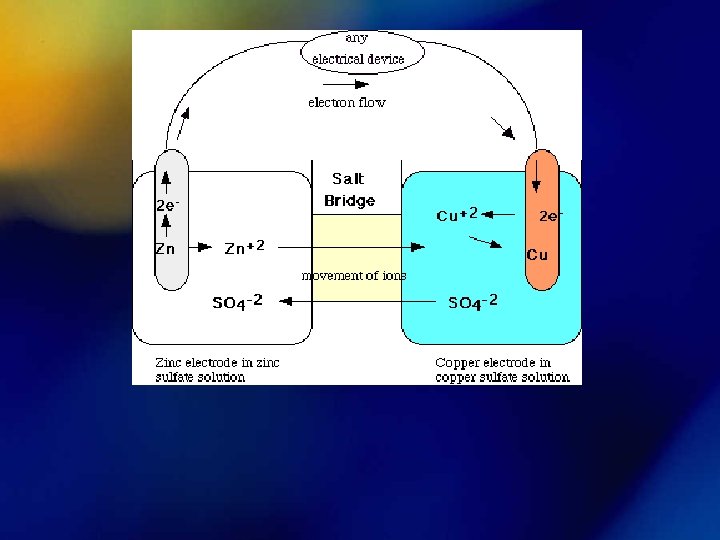
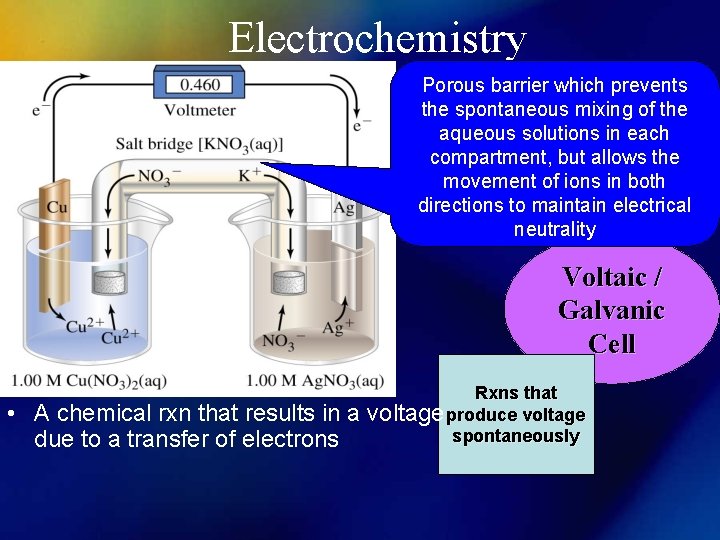
Electrochemistry Porous barrier which prevents the spontaneous mixing of the aqueous solutions in each compartment, but allows the movement of ions in both directions to maintain electrical neutrality Voltaic / Galvanic Cell • A chemical rxn that results in a due to a transfer of electrons Rxns that voltage produce voltage spontaneously

Batteries Zn → Zn+2 + 2 e 2 Mn. O 2 + H 2 O + 2 e- → Mn 2 O 3 + 2 OH - • Two or more dry voltaic cells • Zinc-Carbon Battery

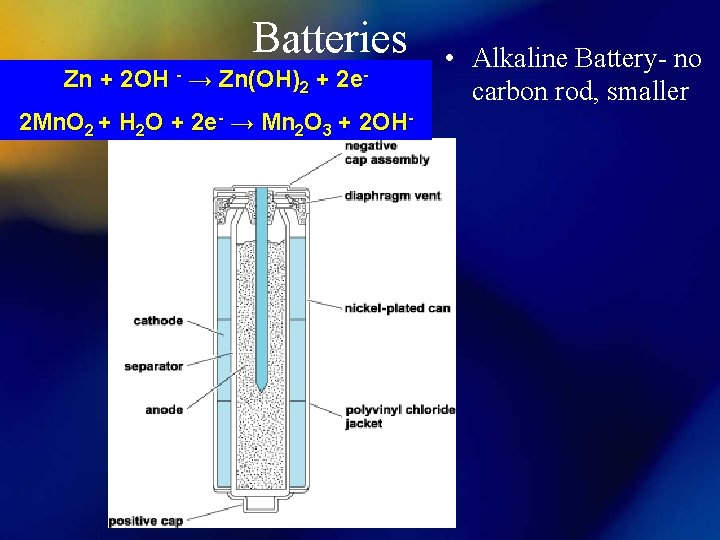
Batteries Zn + 2 OH → Zn(OH)2 + - 2 e- 2 Mn. O 2 + H 2 O + 2 e- → Mn 2 O 3 + 2 OH- • Alkaline Battery- no carbon rod, smaller

Batteries • Mercury Battery- no Zn + 2 OH - → Zn(OH)2 + 2 e- Hg. O + H 2 O + 2 e- → Hg + 2 OH - carbon rod, smallest

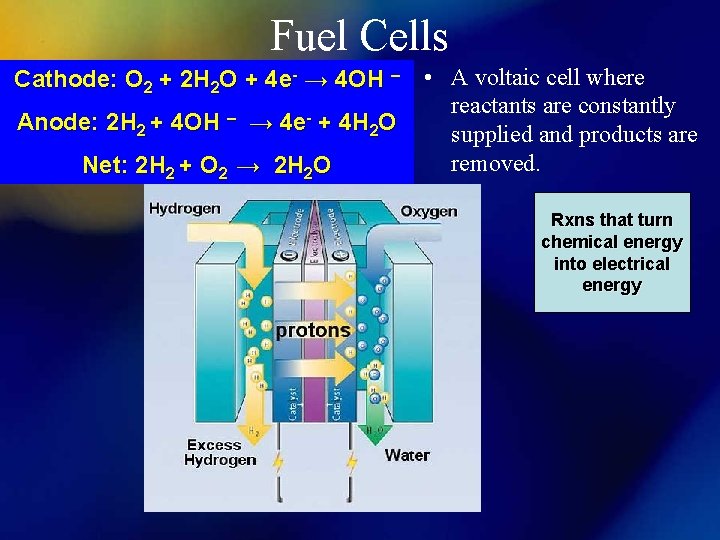
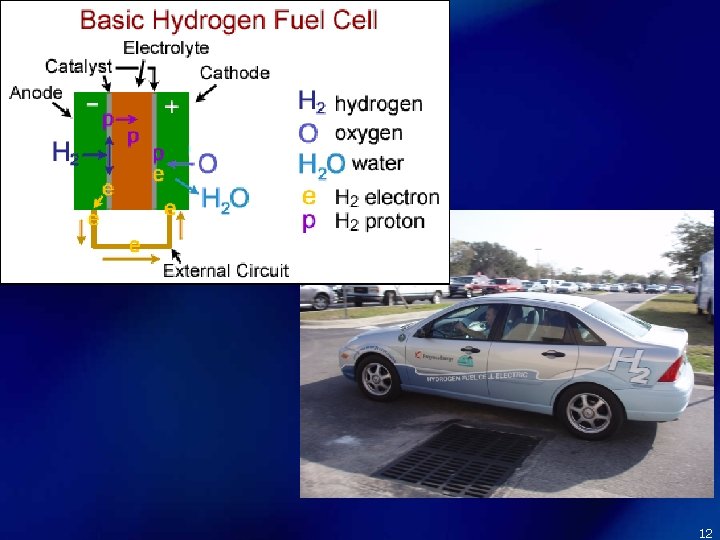
Fuel Cells Cathode: O 2 + 2 H 2 O + 4 e- → 4 OH – Anode: 2 H 2 + 4 OH – → 4 e- + 4 H 2 O Net: 2 H 2 + O 2 → 2 H 2 O • A voltaic cell where reactants are constantly supplied and products are removed. Rxns that turn chemical energy into electrical energy

12

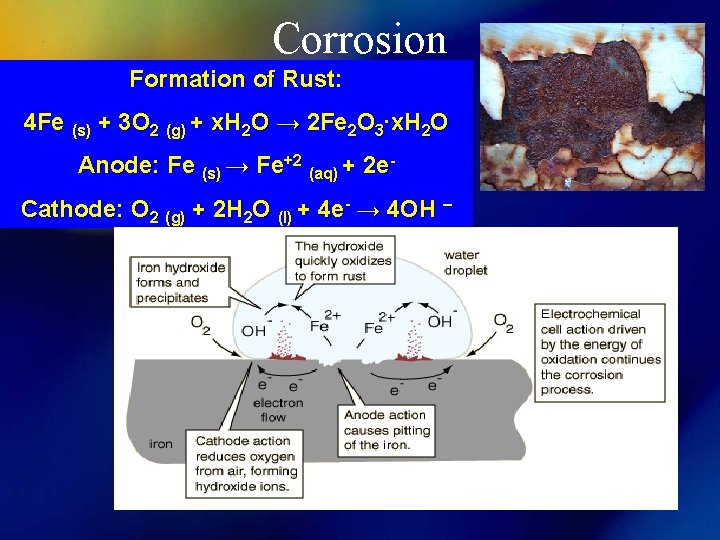
Corrosion Formation of Rust: 4 Fe (s) + 3 O 2 (g) + x. H 2 O → 2 Fe 2 O 3∙x. H 2 O Anode: Fe (s) → Fe+2 (aq) + 2 e. Cathode: O 2 (g) + 2 H 2 O (l) + 4 e- → 4 OH –

Prevention of Corrosion Galvanizing Process by which iron or any metal is coated with zinc. Cathodic Protection Since zinc is more easily oxidized, it is a sacrificial anode.

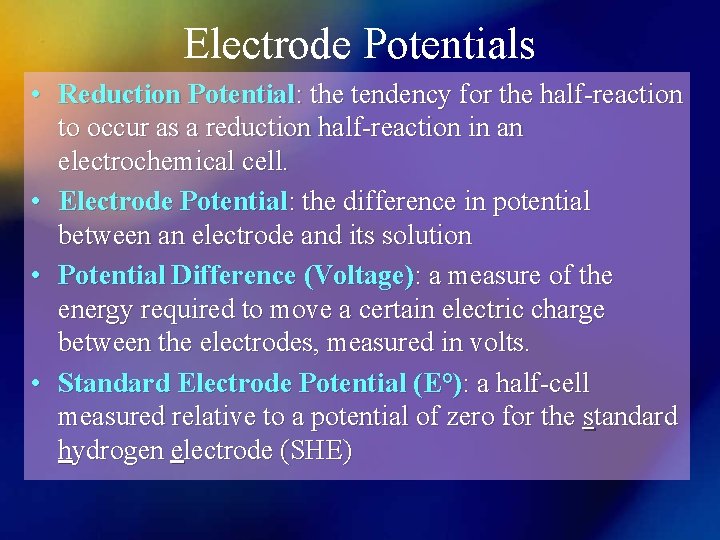
Electrode Potentials • Reduction Potential: the tendency for the half-reaction to occur as a reduction half-reaction in an electrochemical cell. • Electrode Potential: the difference in potential between an electrode and its solution • Potential Difference (Voltage): a measure of the energy required to move a certain electric charge between the electrodes, measured in volts. • Standard Electrode Potential (E°): a half-cell measured relative to a potential of zero for the standard hydrogen electrode (SHE)

Standard Electrode Potential, E° • Positive E° means hydrogen is more willing to give up its electron, so positive reduction potentials are favored. Naturally occurring rxns have a positive value. E° cell = E° cathode - E° anode • Negative E° means the metal electrode is more willing to give up its electron, this is not favored. These rxns prefer oxidation over reduction.

Standard Electrode Potential, E° • When a half-cell is multiplied by a constant (for balancing) the E° value is NOT multiplied! • When a rxn is reversed (flipped) the sign of the E° value switches. • In a voltaic cell, the half-rxn with the more negative standard electrode potential is the anode, where oxidation occurs.

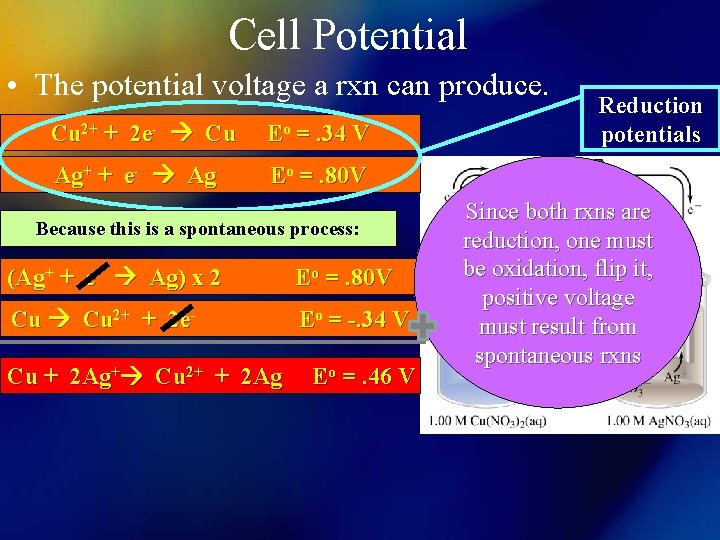
Cell Potential • The potential voltage a rxn can produce. Cu 2+ + 2 e- Cu Eo =. 34 V Ag+ + e- Ag Eo =. 80 V Because this is a spontaneous process: (Ag+ + e- Ag) x 2 Eo =. 80 V Cu 2+ + 2 e- Eo = -. 34 V Cu + 2 Ag+ Cu 2+ + 2 Ag Eo =. 46 V Reduction potentials Since both rxns are reduction, one must be oxidation, flip it, positive voltage must result from spontaneous rxns

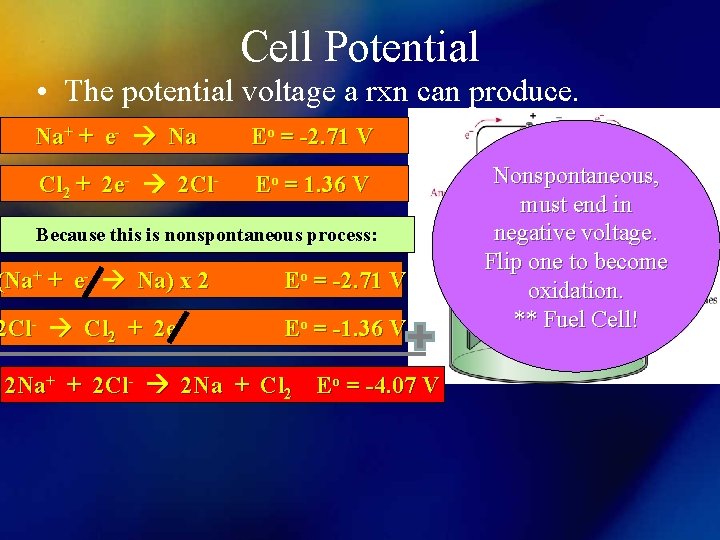
Cell Potential • The potential voltage a rxn can produce. Na+ + e- Na Eo = -2. 71 V Cl 2 + 2 e- 2 Cl- Eo = 1. 36 V Because this is nonspontaneous process: (Na+ + e- Na) x 2 Eo = -2. 71 V 2 Cl- Cl 2 + 2 e- Eo = -1. 36 V 2 Na+ + 2 Cl- 2 Na + Cl 2 Eo = -4. 07 V Nonspontaneous, must end in negative voltage. Flip one to become oxidation. ** Fuel Cell!

Chapter 20 Electrochemistry 20. 3 Electrolytic Cells

Electrochemistry • When electric voltage is used to produce a redox reaction, it is called electrolysis Electrolytic Cell Rxns that require an energy source to react

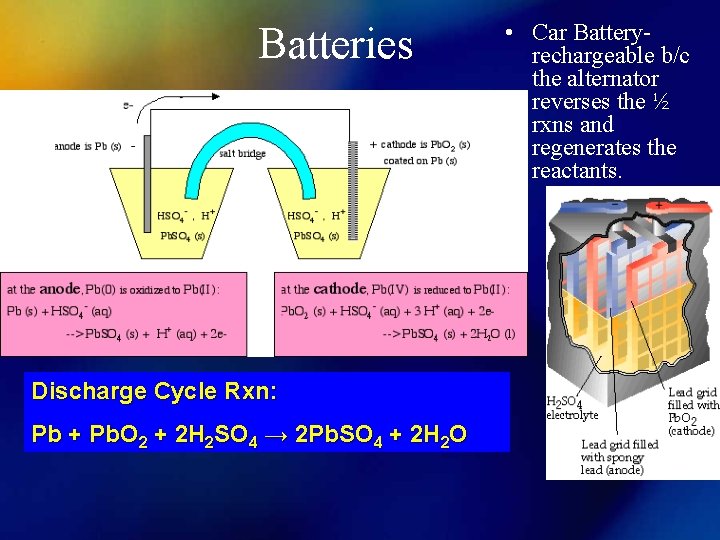
Batteries Discharge Cycle Rxn: Pb + Pb. O 2 + 2 H 2 SO 4 → 2 Pb. SO 4 + 2 H 2 O • Car Batteryrechargeable b/c the alternator reverses the ½ rxns and regenerates the reactants.

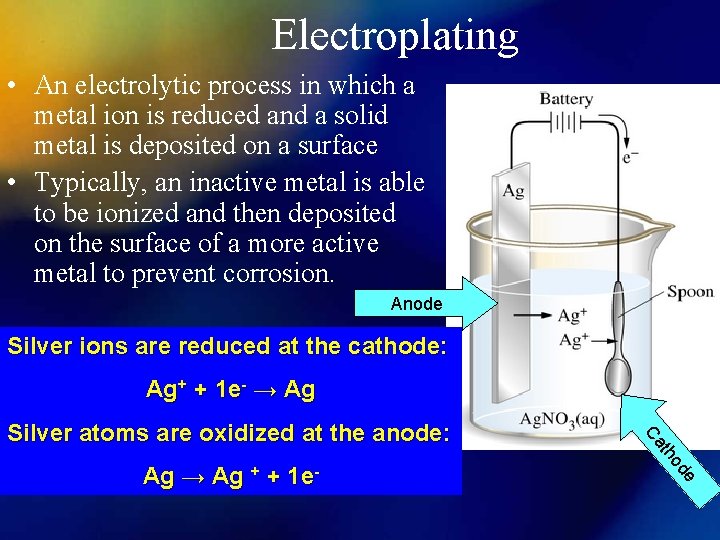
Electroplating • An electrolytic process in which a metal ion is reduced and a solid metal is deposited on a surface • Typically, an inactive metal is able to be ionized and then deposited on the surface of a more active metal to prevent corrosion. Anode Silver ions are reduced at the cathode: Ag+ + 1 e- → Ag e Ag → Ag + + 1 e- od th Ca Silver atoms are oxidized at the anode:

Voltaic vs. Electrolytic • If the positive battery terminal is attached to the cathode of a voltaic cell, and the negative terminal is attached to the anode, the flow of electrons will change directions. • Electrolytic cells need the electrodes attached to a battery, where voltaic is its own source of electrical power. Voltaic = spontaneous chemical energy → electrical energy Electrolytic = non-spontaneous electrical energy → chemical energy

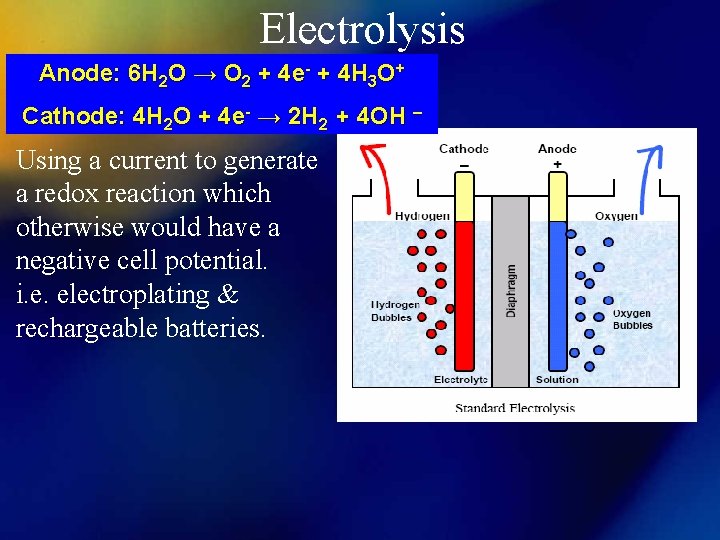
Electrolysis Anode: 6 H 2 O → O 2 + 4 e- + 4 H 3 O+ Cathode: 4 H 2 O + 4 e- → 2 H 2 + 4 OH – Using a current to generate a redox reaction which otherwise would have a negative cell potential. i. e. electroplating & rechargeable batteries.