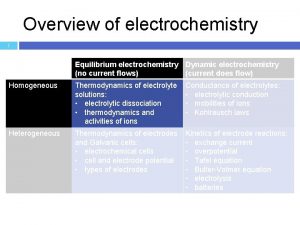
Intersection 13 Electrochemistry Outline Eds Demos hydrolysis of







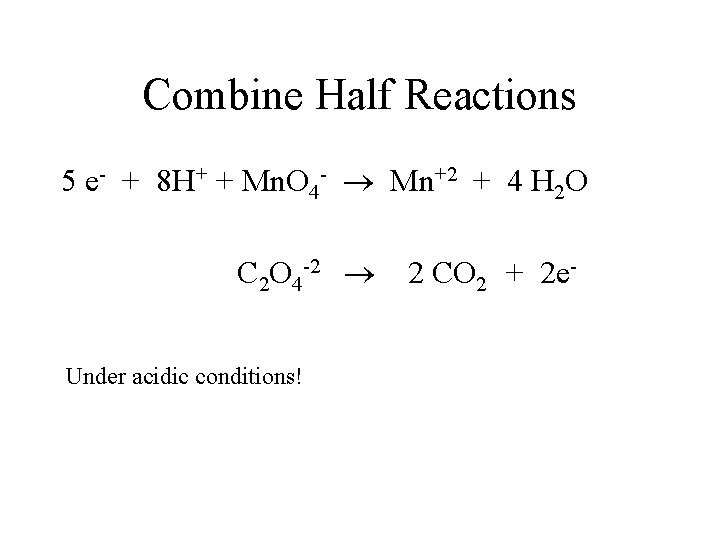












- Slides: 43

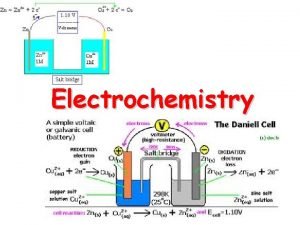
Intersection 13 Electrochemistry

Outline • Ed’s Demos: hydrolysis of water, oxidation states of V, potato clock, Zn/Cu electrochemical cell • Electrochemistry – Balancing redox equations – Electrochemical cells and Standard Hydrogen Electrodes – Nernst – Quantifying current

Ed’s Demos

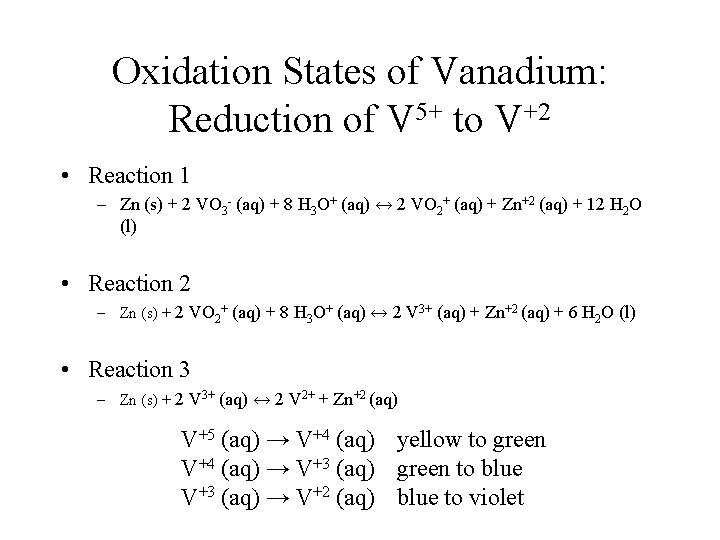
Oxidation States of Vanadium: Reduction of V 5+ to V+2 • Reaction 1 – Zn (s) + 2 VO 3 - (aq) + 8 H 3 O+ (aq) ↔ 2 VO 2+ (aq) + Zn+2 (aq) + 12 H 2 O (l) • Reaction 2 – Zn (s) + 2 VO 2+ (aq) + 8 H 3 O+ (aq) ↔ 2 V 3+ (aq) + Zn+2 (aq) + 6 H 2 O (l) • Reaction 3 – Zn (s) + 2 V 3+ (aq) ↔ 2 V 2+ + Zn+2 (aq) V+5 (aq) → V+4 (aq) yellow to green V+4 (aq) → V+3 (aq) green to blue V+3 (aq) → V+2 (aq) blue to violet

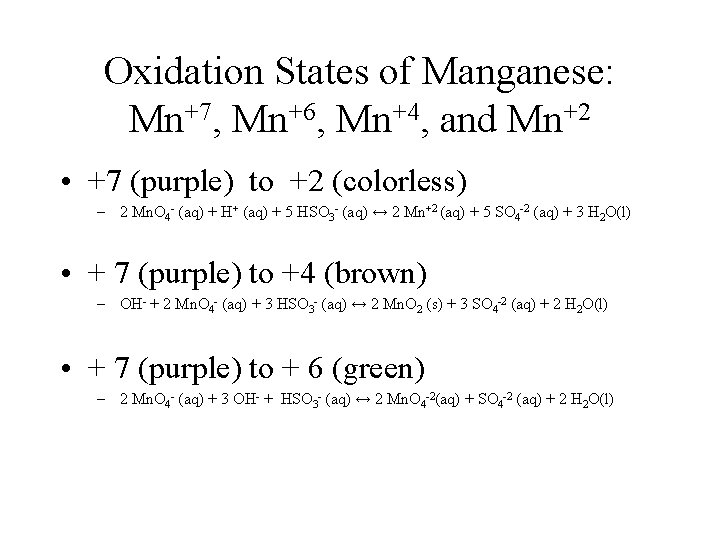
Oxidation States of Manganese: Mn+7, Mn+6, Mn+4, and Mn+2 • +7 (purple) to +2 (colorless) – 2 Mn. O 4 - (aq) + H+ (aq) + 5 HSO 3 - (aq) ↔ 2 Mn+2 (aq) + 5 SO 4 -2 (aq) + 3 H 2 O(l) • + 7 (purple) to +4 (brown) – OH- + 2 Mn. O 4 - (aq) + 3 HSO 3 - (aq) ↔ 2 Mn. O 2 (s) + 3 SO 4 -2 (aq) + 2 H 2 O(l) • + 7 (purple) to + 6 (green) – 2 Mn. O 4 - (aq) + 3 OH- + HSO 3 - (aq) ↔ 2 Mn. O 4 -2(aq) + SO 4 -2 (aq) + 2 H 2 O(l)

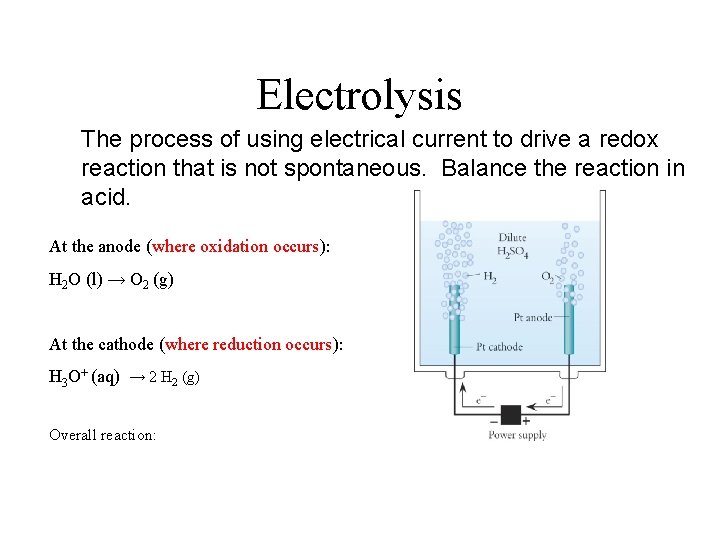
Electrolysis The process of using electrical current to drive a redox reaction that is not spontaneous. Balance the reaction in acid. At the anode (where oxidation occurs): H 2 O (l) → O 2 (g) At the cathode (where reduction occurs): H 3 O+ (aq) → 2 H 2 (g) Overall reaction:

Overall reaction: 2 H 2 O (l) → 2 H 2(g) + O 2 (g) p. 951

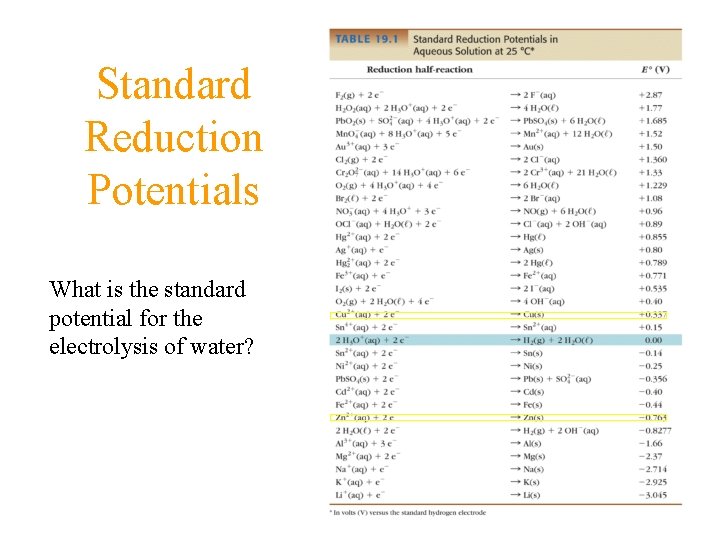
Standard Reduction Potentials What is the standard potential for the electrolysis of water?

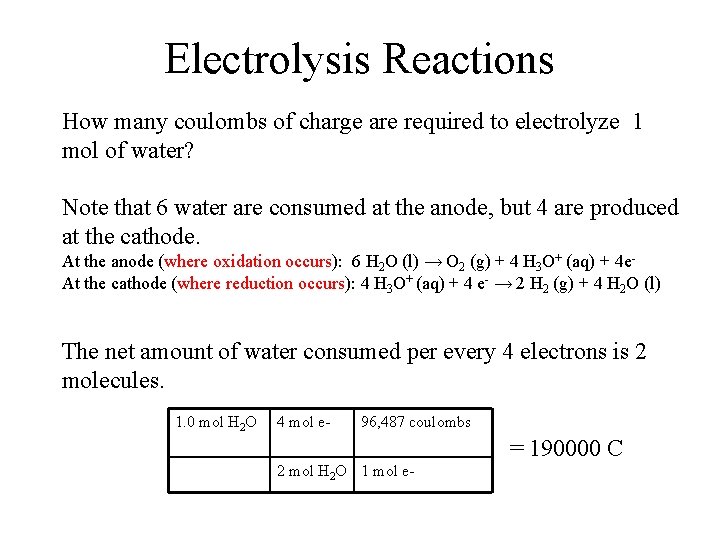
Electrolysis Reactions How many coulombs of charge are required to electrolyze 1 mol of water? Note that 6 water are consumed at the anode, but 4 are produced at the cathode. At the anode (where oxidation occurs): 6 H 2 O (l) → O 2 (g) + 4 H 3 O+ (aq) + 4 e. At the cathode (where reduction occurs): 4 H 3 O+ (aq) + 4 e- → 2 H 2 (g) + 4 H 2 O (l) The net amount of water consumed per every 4 electrons is 2 molecules. 1. 0 mol H 2 O 4 mol e- 96, 487 coulombs = 190000 C 2 mol H 2 O 1 mol e-

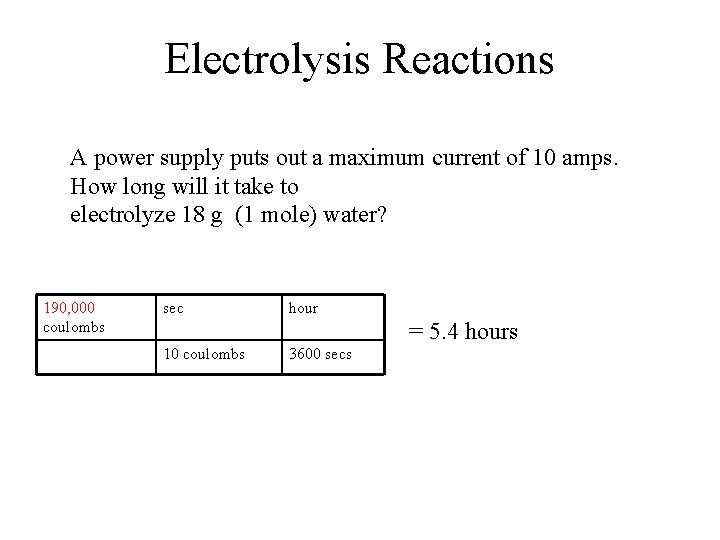
Electrolysis Reactions A power supply puts out a maximum current of 10 amps. How long will it take to electrolyze 18 g (1 mole) water? 190, 000 coulombs sec hour = 5. 4 hours 10 coulombs 3600 secs

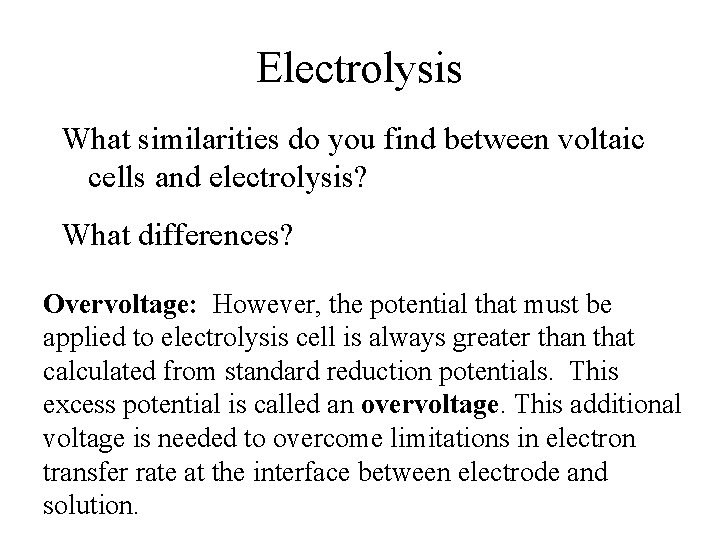
Electrolysis What similarities do you find between voltaic cells and electrolysis? What differences? Overvoltage: However, the potential that must be applied to electrolysis cell is always greater than that calculated from standard reduction potentials. This excess potential is called an overvoltage. This additional voltage is needed to overcome limitations in electron transfer rate at the interface between electrode and solution.


Balancing Redox Reactions When KMn. O 4 (potassium permanganate) is mixed with Na 2 C 2 O 4 (sodium oxalate) under acidic conditions, Mn+2(aq) ions and CO 2(g) form. The unbalanced chemical equation is: KMn. O 4(aq) + Na 2 C 2 O 4(aq) Mn+2(aq) + CO 2(g) + K+(aq) + Na+(aq) K+ and Na+ are spectator ions, so we can ignore them at this point. Mn. O 4 - (aq) + C 2 O 4 -2(aq) Mn+2(aq) + CO 2(g)

Half-Reactions Mn. O 4 - (aq) + C 2 O 4 -2(aq) Mn+2(aq) + CO 2(g) • Reduction reaction • Oxidation reaction

Reduction reaction Mn. O 4 - Mn+2 Step 1: Balance all elements other than oxygen and hydrogen. Step 2: Balance the oxygens by adding water. Step 3: Balance the hydrogens using H+ Step 4: Balance the electrons Mn+7 on reactants side Mn+2 on products side Step 5: Check charge balance and elemental balance

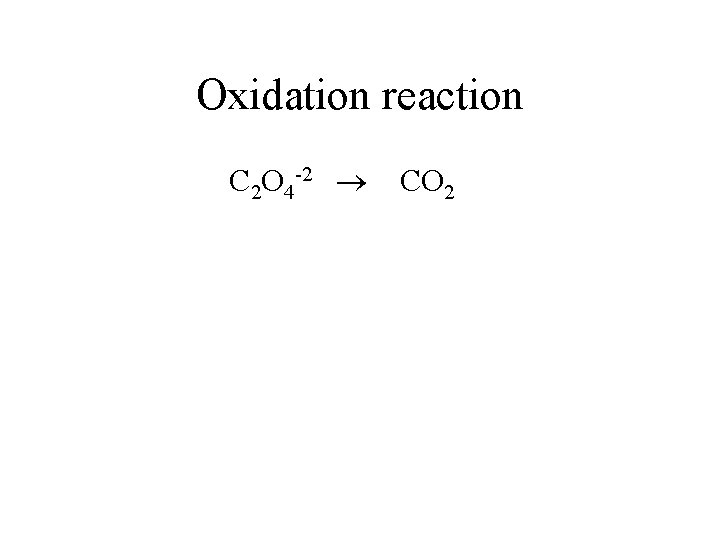
Oxidation reaction C 2 O 4 -2 CO 2
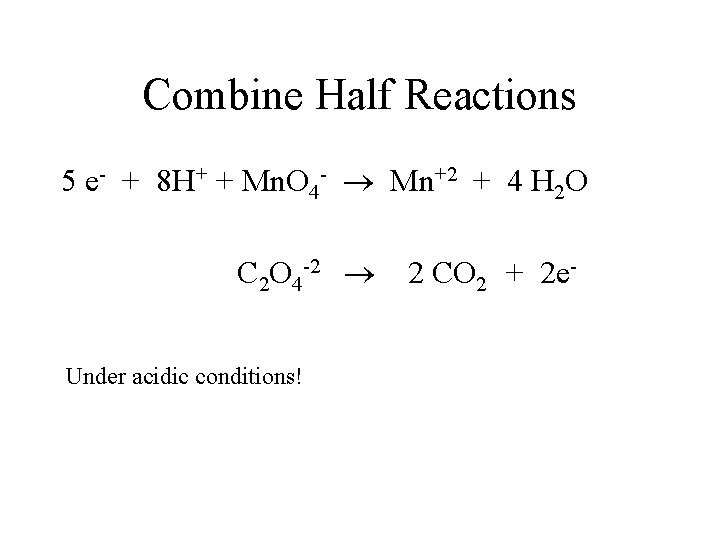
Combine Half Reactions 5 e- + 8 H+ + Mn. O 4 - Mn+2 + 4 H 2 O C 2 O 4 -2 2 CO 2 + 2 e. Under acidic conditions!

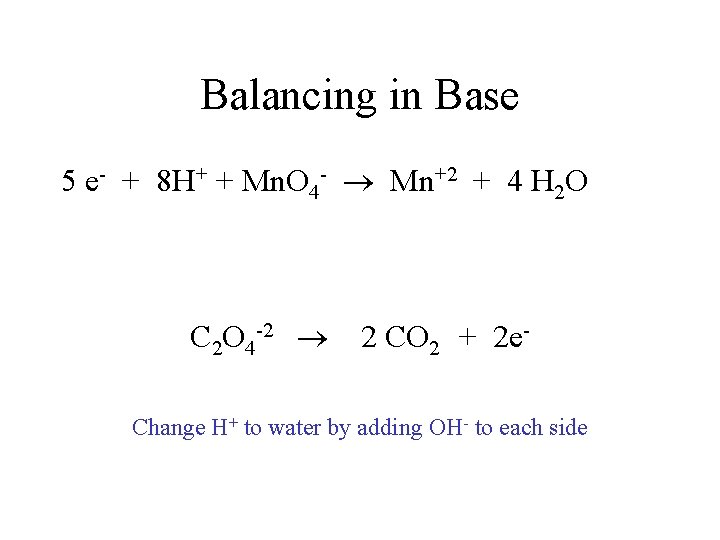
Balancing in Base 5 e- + 8 H+ + Mn. O 4 - Mn+2 + 4 H 2 O C 2 O 4 -2 2 CO 2 + 2 e. Change H+ to water by adding OH- to each side



Definitions • Voltaic cell (battery): An electrochemical cell or group of cells in which a product-favored redox reaction is used to produce an electric current. • Electrochemical cell: A combination of anode, cathode, and other materials arranged so that a product-favored redox reaction cause a current to flow or an electric current can cause a reactantfavored redox reaction to occur • Galvanic cell: A cell in which an irreversible chemical reaction produces electrical current • Electrolytic cell: electrochemical reactions are produced by applying electrical energy

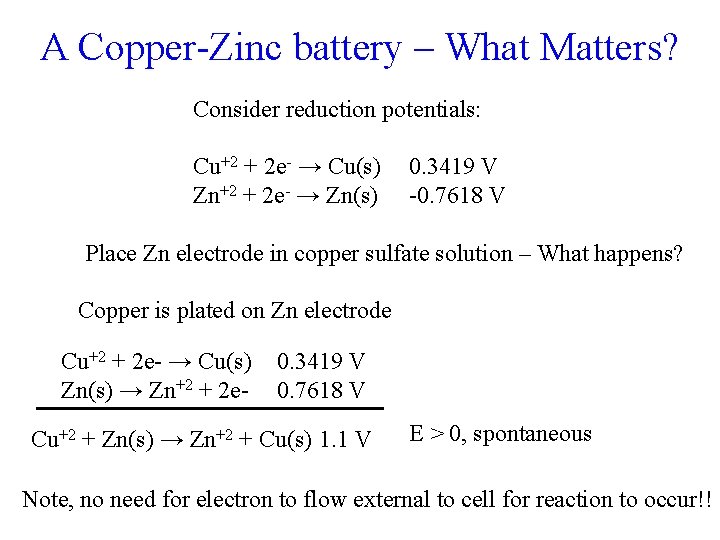
A Copper-Zinc battery – What Matters? Consider reduction potentials: Cu+2 + 2 e- → Cu(s) Zn+2 + 2 e- → Zn(s) 0. 3419 V -0. 7618 V Place Zn electrode in copper sulfate solution – What happens? Copper is plated on Zn electrode Cu+2 + 2 e- → Cu(s) Zn(s) → Zn+2 + 2 e- 0. 3419 V 0. 7618 V Cu+2 + Zn(s) → Zn+2 + Cu(s) 1. 1 V E > 0, spontaneous Note, no need for electron to flow external to cell for reaction to occur!!

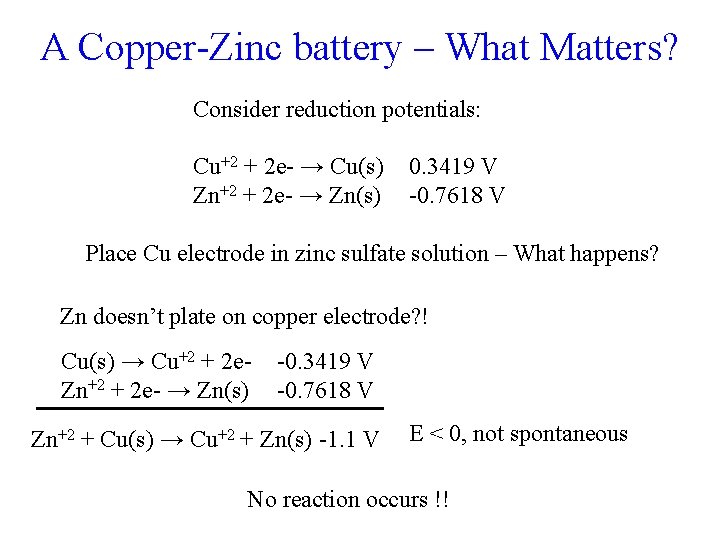
A Copper-Zinc battery – What Matters? Consider reduction potentials: Cu+2 + 2 e- → Cu(s) Zn+2 + 2 e- → Zn(s) 0. 3419 V -0. 7618 V Place Cu electrode in zinc sulfate solution – What happens? Zn doesn’t plate on copper electrode? ! Cu(s) → Cu+2 + 2 e- -0. 3419 V Zn+2 + 2 e- → Zn(s) -0. 7618 V Zn+2 + Cu(s) → Cu+2 + Zn(s) -1. 1 V E < 0, not spontaneous No reaction occurs !!

Fig. 19 -3, p. 918

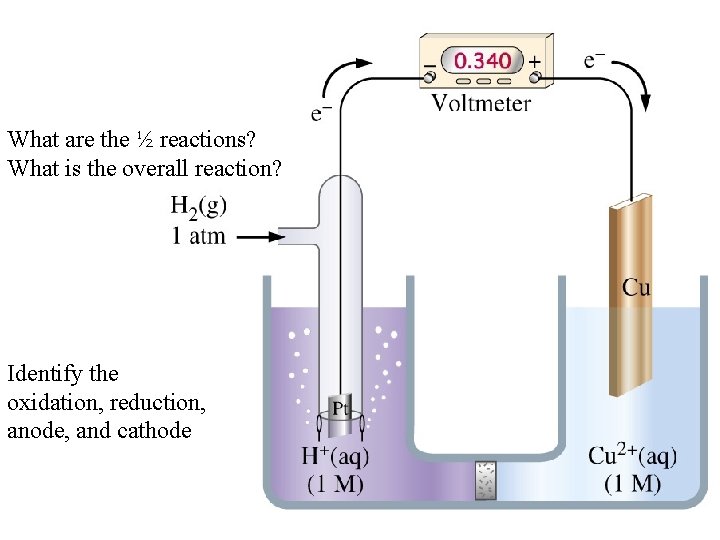
What are the ½ reactions? What is the overall reaction? Identify the oxidation, reduction, anode, and cathode

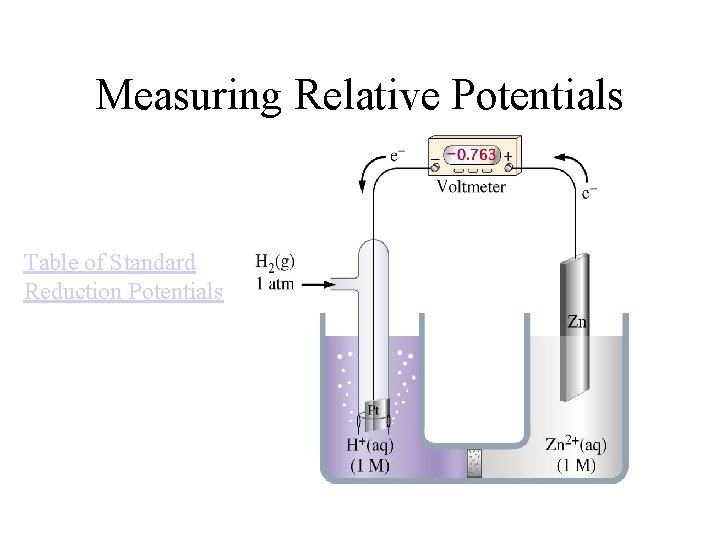
SHE: Standard Hydrogen Electrode 2 H 3 O+(aq, 1. 00 M) + 2 e- <-> H 2(g, 1 atm) + 2 H 2 O(l) Eo = 0 V Standard conditions: 1 M, 1 atm, 25 o. C Fig. 19 -7, p. 922

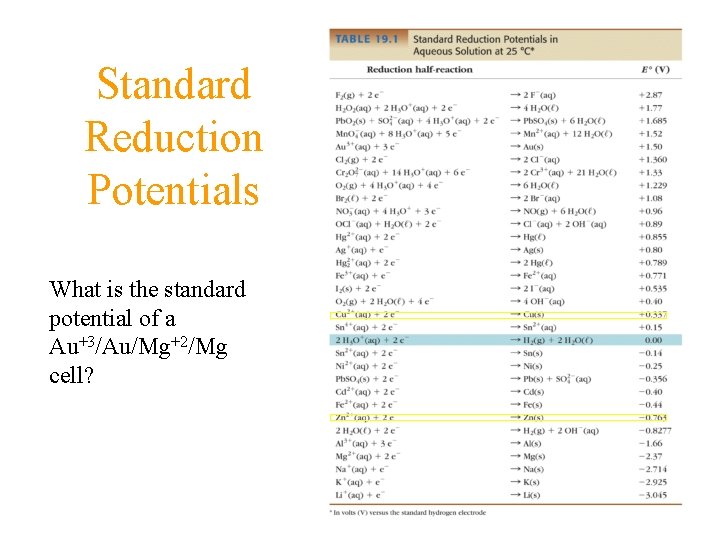
Measuring Relative Potentials Table of Standard Reduction Potentials

Standard Reduction Potentials What is the standard potential of a Au+3/Au/Mg+2/Mg cell?

The half-reaction with the more positive standard reduction potential occurs at the cathode as reduction. The half-reaction with the more negative standard reduction potential occurs at the anode as oxidation.


Nernst Picture from: www. corrosion-doctors. org/ Biographies/images/

Is potential always the same? Standard conditions: 1 atm, 25 o. C, 1 M What will influence the potential of a cell?

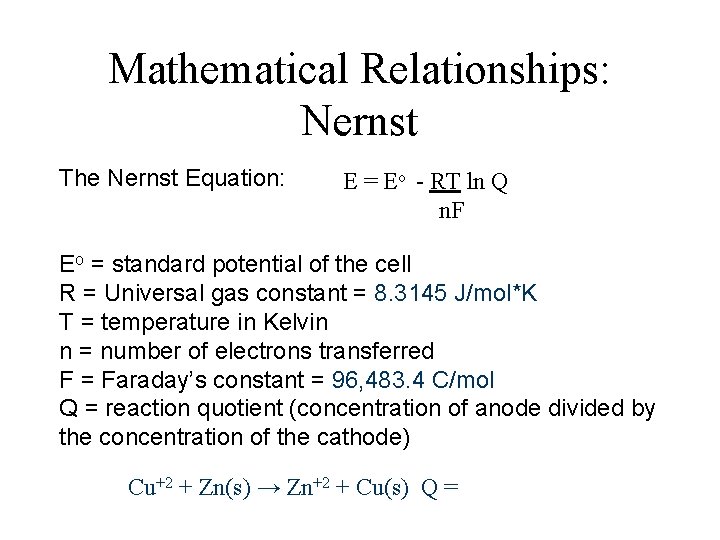
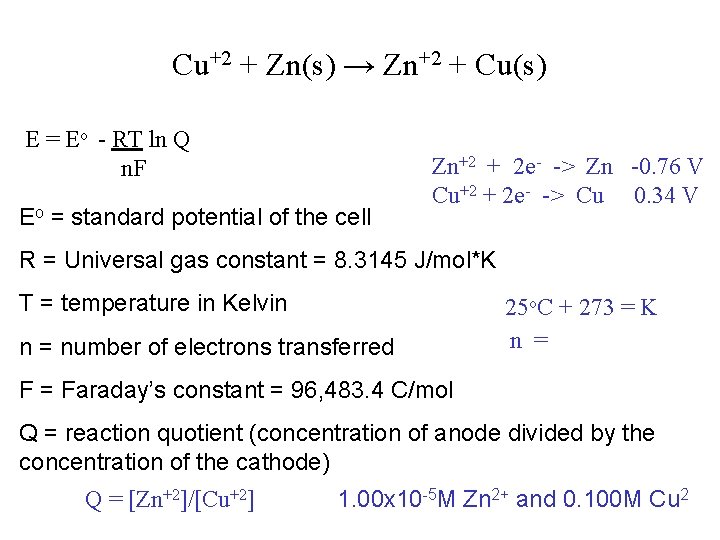
Mathematical Relationships: Nernst The Nernst Equation: E = Eo - RT ln Q n. F Eo = standard potential of the cell R = Universal gas constant = 8. 3145 J/mol*K T = temperature in Kelvin n = number of electrons transferred F = Faraday’s constant = 96, 483. 4 C/mol Q = reaction quotient (concentration of anode divided by the concentration of the cathode) Cu+2 + Zn(s) → Zn+2 + Cu(s) Q =

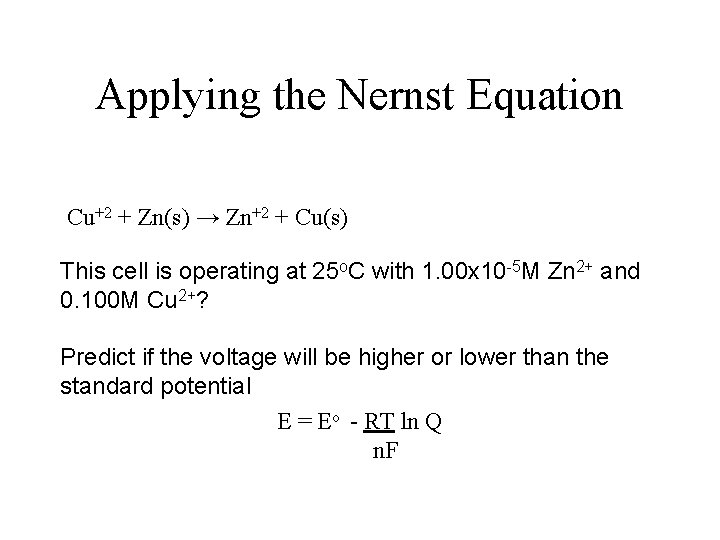
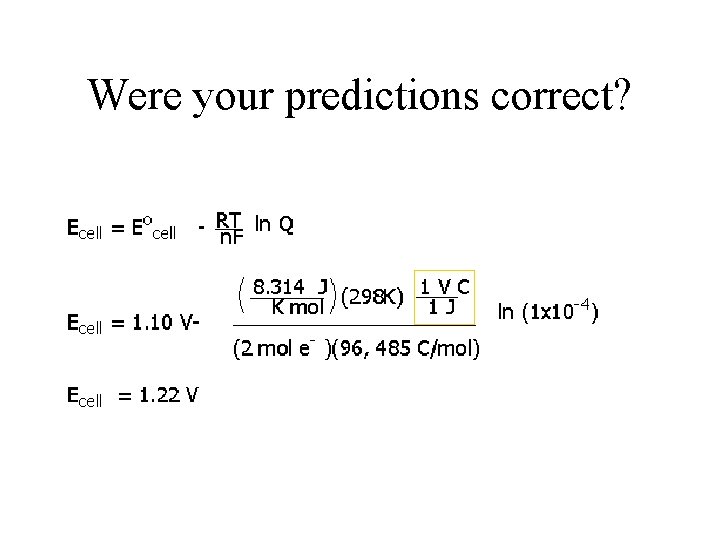
Applying the Nernst Equation Cu+2 + Zn(s) → Zn+2 + Cu(s) This cell is operating at 25 o. C with 1. 00 x 10 -5 M Zn 2+ and 0. 100 M Cu 2+? Predict if the voltage will be higher or lower than the standard potential E = Eo - RT ln Q n. F

Cu+2 + Zn(s) → Zn+2 + Cu(s) E = Eo - RT ln Q n. F Eo = standard potential of the cell Zn+2 + 2 e- -> Zn -0. 76 V Cu+2 + 2 e- -> Cu 0. 34 V R = Universal gas constant = 8. 3145 J/mol*K T = temperature in Kelvin n = number of electrons transferred 25 o. C + 273 = K n = F = Faraday’s constant = 96, 483. 4 C/mol Q = reaction quotient (concentration of anode divided by the concentration of the cathode) Q = [Zn+2]/[Cu+2] 1. 00 x 10 -5 M Zn 2+ and 0. 100 M Cu 2

Were your predictions correct?


Ampere Picture from: musee-ampere. univ-lyon 1. fr/

The Units of Electrochemistry • Coulomb – 1 coulomb equals 2. 998 x 109 electrostatic units (eu) – eu is amount of charge needed to repel an identical charge 1 cm away with unit force – Charge on one electron is -1. 602 x 10 -19 coulomb Problem: An aluminum ion has a +3 charge. What is this value in coulombs? magnitude of charge is same at that of e-, opposite sign 3 x 1. 602 x 10 -19 = 4. 806 x 10 -19 coulomb Key Point: electrons or ions charges can be measured in coloumbs

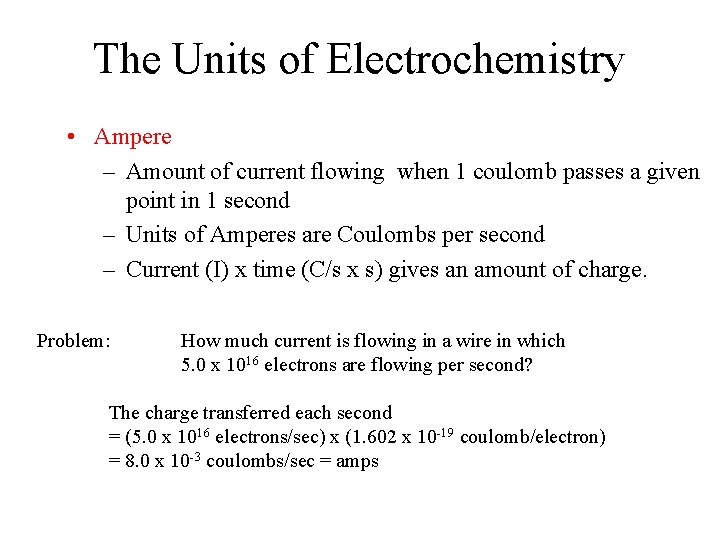
The Units of Electrochemistry • Ampere – Amount of current flowing when 1 coulomb passes a given point in 1 second – Units of Amperes are Coulombs per second – Current (I) x time (C/s x s) gives an amount of charge. Problem: How much current is flowing in a wire in which 5. 0 x 1016 electrons are flowing per second? The charge transferred each second = (5. 0 x 1016 electrons/sec) x (1. 602 x 10 -19 coulomb/electron) = 8. 0 x 10 -3 coulombs/sec = amps

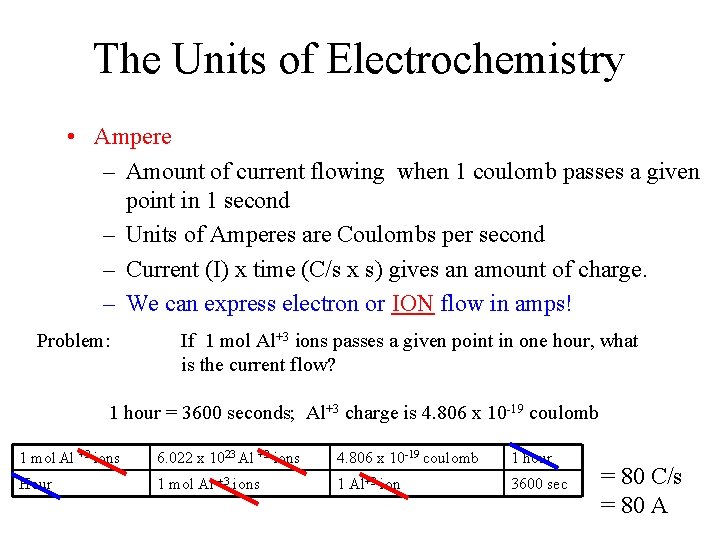
The Units of Electrochemistry • Ampere – Amount of current flowing when 1 coulomb passes a given point in 1 second – Units of Amperes are Coulombs per second – Current (I) x time (C/s x s) gives an amount of charge. – We can express electron or ION flow in amps! Problem: If 1 mol Al+3 ions passes a given point in one hour, what is the current flow? 1 hour = 3600 seconds; Al+3 charge is 4. 806 x 10 -19 coulomb 1 mol Al +3 ions 6. 022 x 1023 Al +3 ions 4. 806 x 10 -19 coulomb 1 hour Hour 1 mol Al +3 ions 1 Al+3 ion 3600 sec = 80 C/s = 80 A

Look Ahead • • Today: Watershed Thursday: Scurvy 12/6 Tuesday HW 12 due; polymers 12/7 Wednesday: Lecture, Poster session 12/8 Thursday: Watershed paper due; check out 12/13 Tuesday: Fluoridation Report Due 12/16 Friday: Final Exam 8 -10 am

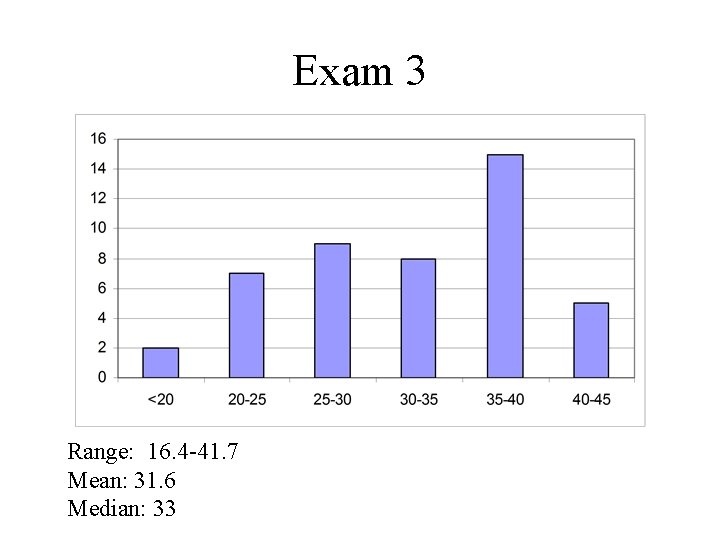
Exam 3 Range: 16. 4 -41. 7 Mean: 31. 6 Median: 33
 Electrochemistry eds
Electrochemistry eds 이중결합요소
이중결합요소 Demos kratos greek
Demos kratos greek Products and services
Products and services Epi demos logos
Epi demos logos Demos
Demos Biomotion lab demos
Biomotion lab demos Epi demos logos
Epi demos logos Demos and kratein
Demos and kratein Epi demos logos
Epi demos logos The mother of all demos
The mother of all demos Demos kratein
Demos kratein Atmosfer berasal dari
Atmosfer berasal dari Vertex form graph calculator
Vertex form graph calculator Quale fu il rapporto tra i tiranni è il demos
Quale fu il rapporto tra i tiranni è il demos Penn state gen eds
Penn state gen eds Myofascial release floyd county
Myofascial release floyd county Eds webmail
Eds webmail Eds.kedschool
Eds.kedschool Zespół ehlersa-danlosa zdjęcia
Zespół ehlersa-danlosa zdjęcia Eds çalışma prensibi
Eds çalışma prensibi Eds.a.ebscohost
Eds.a.ebscohost The ross sisters ehlers-danlos syndrome
The ross sisters ehlers-danlos syndrome Kā pievienot čekus eds
Kā pievienot čekus eds Eds
Eds Eds vid
Eds vid Eds.a.ebscohost
Eds.a.ebscohost Mccalls festoon
Mccalls festoon St eds vle
St eds vle St eds vle
St eds vle Eds/m
Eds/m Evidence sandwich example
Evidence sandwich example Electrons flow from anode to cathode
Electrons flow from anode to cathode Electrochemistry tutorial
Electrochemistry tutorial Electrochemistry balancing equations
Electrochemistry balancing equations What is polarization in electrochemistry
What is polarization in electrochemistry E cell formula
E cell formula Electrochemistry balancing equations
Electrochemistry balancing equations Define concentration cells
Define concentration cells Mass transport electrochemistry
Mass transport electrochemistry What is electrochemistry
What is electrochemistry What is electrochemistry in chemistry
What is electrochemistry in chemistry Ap chemistry electrochemistry
Ap chemistry electrochemistry Ir drop
Ir drop