Chemistry 30 Electrochemical Changes Cell Stoichiometry Half Cell




- Slides: 10

Chemistry 30 Electrochemical Changes Cell Stoichiometry

Half Cell Stoichiometry • Let’s see what you remember about Stoichiometry ▫ What do we need in order to use stoichiometry? • That’s right! MOLES! ▫ Then we just use the ratio to find whatever we need

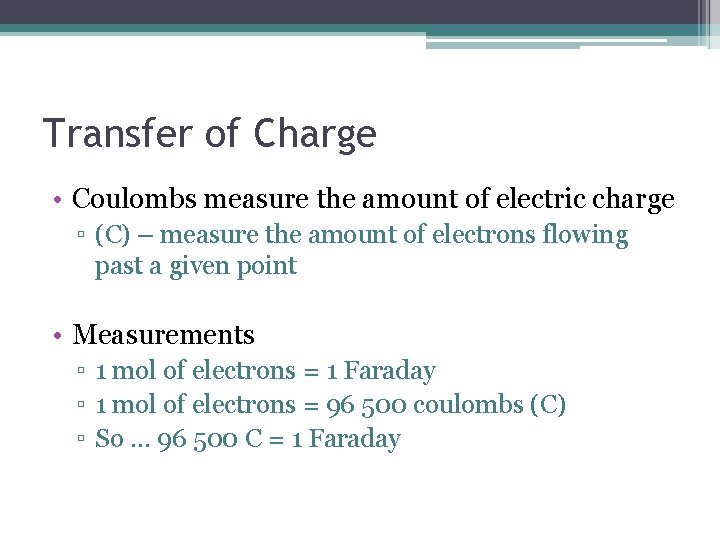
Transfer of Charge • Coulombs measure the amount of electric charge ▫ (C) – measure the amount of electrons flowing past a given point • Measurements ▫ 1 mol of electrons = 1 Faraday ▫ 1 mol of electrons = 96 500 coulombs (C) ▫ So … 96 500 C = 1 Faraday

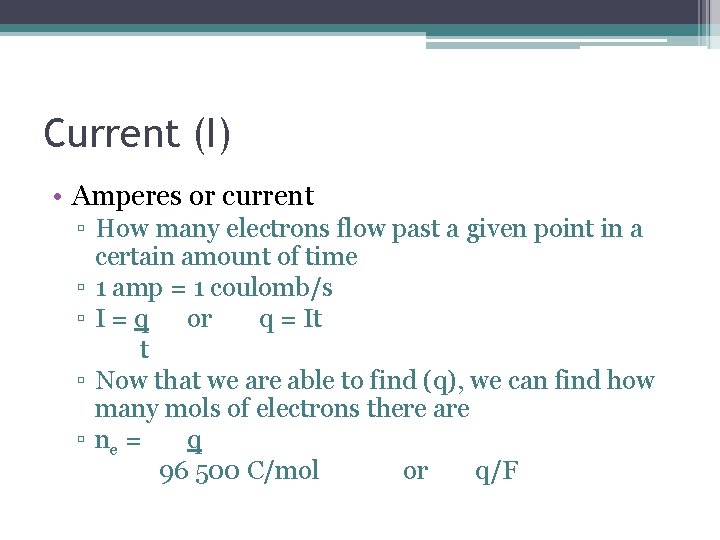
Current (I) • Amperes or current ▫ How many electrons flow past a given point in a certain amount of time ▫ 1 amp = 1 coulomb/s ▫ I = q or q = It t ▫ Now that we are able to find (q), we can find how many mols of electrons there are ▫ ne = q 96 500 C/mol or q/F

Example: Practice #3 Page 653 • Calculate the charge transferred by a current of 250 m. A in a time of 28. 5 s

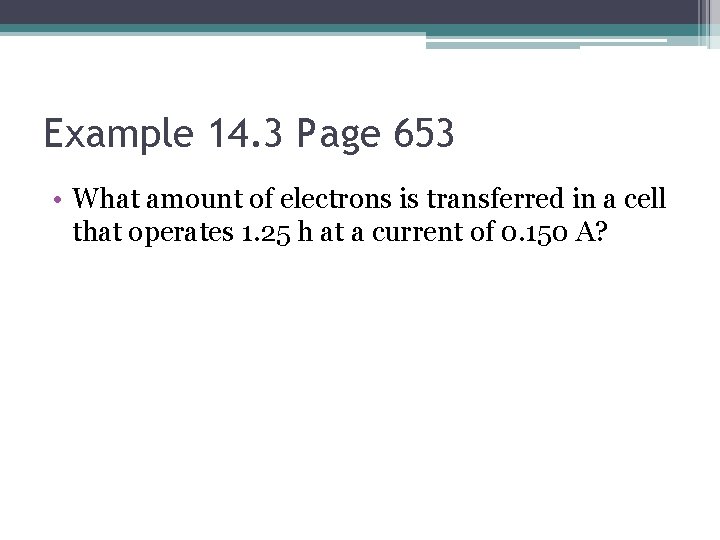
Example 14. 3 Page 653 • What amount of electrons is transferred in a cell that operates 1. 25 h at a current of 0. 150 A?

Faraday’s Law • Was able to predict the mass of elements formed at the cathode and lost at the anode based on charge transferred • Faraday’s Law ▫ The mass produced or consumed at an electrode is directly proportional to the time the cell operated as long as the current is constant ▫ Faraday’s Constant (F) 96 500 C/mol of electrons

Steps for Cell Stoichiometry 1) Write a balanced equation for the half cell reaction of the substance produced or consumed 2) Convert the given measurements to an amount in mols by using the appropriate formula 3) Calculate the amount of the required substance by using the mol ratio fro the half reaction equation 4) Convert the calculated amount to the final quantity by using the appropriate conversion factor (formula)

Example 3 Page 656 (Nelson) • Silver is deposited on objects in a silver electroplating cell. If 0. 175 g of silver is to be deposited from a silver cyanide solution in a tiome of 10. 0 min, predict the current required.

Assignment • Reading ▫ Nelson Pages 652 -657 • Assignment ▫ SNAP Pages 300 -301 #’s 1 -6 ▫ Nelson Page 657 #’s 5 -8