Chemistry 30 Electrochemical Changes Redox Stoichiometry Review of



- Slides: 9

Chemistry 30 Electrochemical Changes Redox Stoichiometry

Review of Titration Terminology • Titrant ▫ Solution contained in the buret • Endpoint ▫ The point in a titration when a sharp change in some measurable property occurs (often a colour change) • Equivalence Point ▫ The quantity of titrant added to reach the endpoint

Stoichiometry • For a successful chemical analysis to take place, the concentration of the titrant must be precisely known (standard solution)

Redox Stoichiometry • In acid/base tirations, the titrant is usually a strong acid or base • In redox titrations, the titrant is usually a SRA or SOA • Common oxidizing agents used: ▫ Permanganate Ion (Mn. O 4 -) ▫ Dichromate Ion (Cr 2 O 72 -) ▫ They are SOA’s and change colour upon oxidizing a reducing agent

Example 13. 14 Page 597 • The permanganate ion is commonly used as a titrant in redox titrations because it produces a very obvious colour change at the equivalence point. • A problem however … a solution of potassium permanganate cannot be directly prepared with a precisely known concentration because the permanganate ion reacts quickly with impurities in the water and with water itself.

Example 13. 14 Page 597 • Potassium permanganate must be standardized using a primary standard (a chemical used to prepare a standard solution) before it can be used as a titrant. • Problem: ▫ What is the concentration of the potassium permanganate solution? • Design ▫ Potassium permanganate is titrated against samples of acidic tin (2) chloride solution (the primary standard)

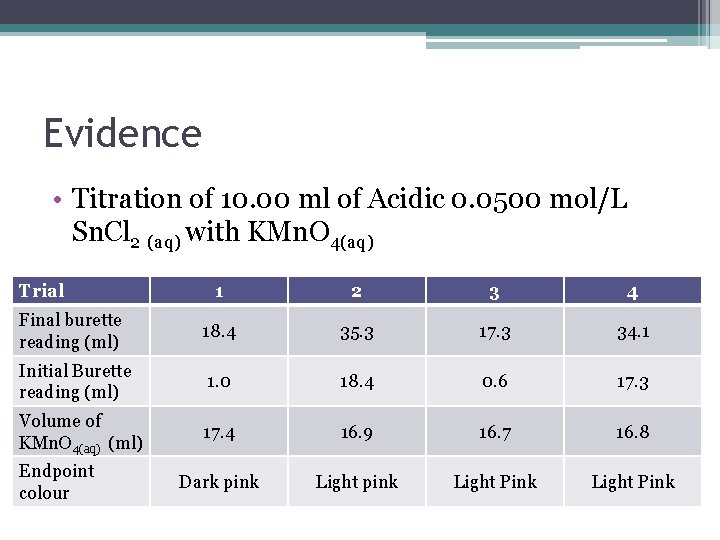
Evidence • Titration of 10. 00 ml of Acidic 0. 0500 mol/L Sn. Cl 2 (aq) with KMn. O 4(aq) Trial 1 2 3 4 Final burette reading (ml) 18. 4 35. 3 17. 3 34. 1 Initial Burette reading (ml) 1. 0 18. 4 0. 6 17. 3 Volume of KMn. O 4(aq) (ml) 17. 4 16. 9 16. 7 16. 8 Dark pink Light Pink Endpoint colour

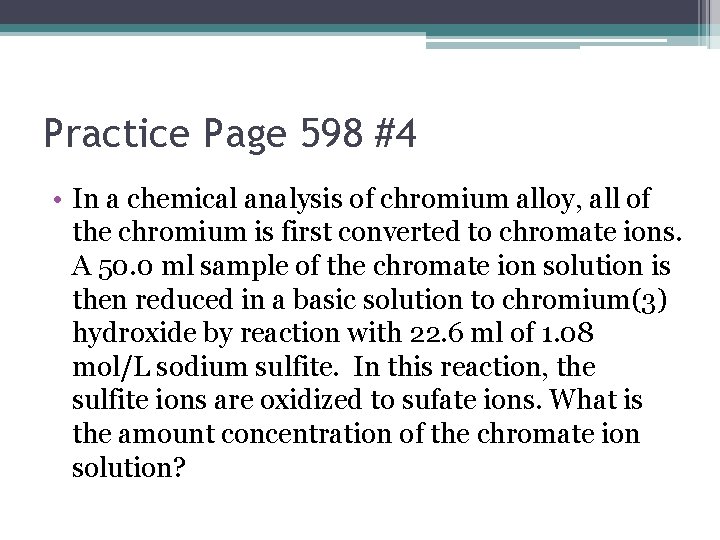
Practice Page 598 #4 • In a chemical analysis of chromium alloy, all of the chromium is first converted to chromate ions. A 50. 0 ml sample of the chromate ion solution is then reduced in a basic solution to chromium(3) hydroxide by reaction with 22. 6 ml of 1. 08 mol/L sodium sulfite. In this reaction, the sulfite ions are oxidized to sufate ions. What is the amount concentration of the chromate ion solution?

Assignment • Reading ▫ Nelson Pages 596 -600 • Questions ▫ Nelson Page 600 #’s 1 -5

















