6 C Electrochemical Cells Redox reactions Redox reaction







- Slides: 22

6 C: Electrochemical Cells

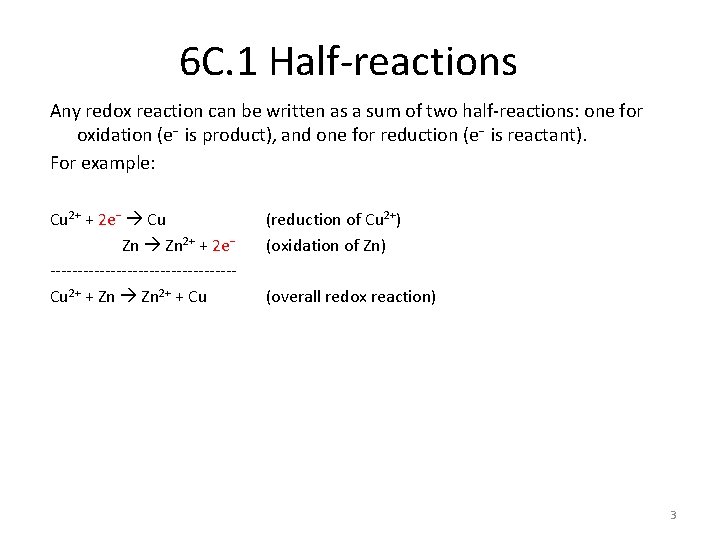
Redox reactions Redox reaction: a reaction involving a transfer of electrons from the reductant (losing electrons, getting oxidized) to the oxidant (gaining electrons, getting reduced) e. g. corrosion e. g. combustion e. g. photosynthesis e. g. 4 Fe(s) + 3 O 2(aq) 2 Fe 2 O 3 (s) e. g. Cn. H 2 n + n O 2 n CO 2 + n H 2 O e. g. n CO 2(g) + n H 2 O(l) (HCOH)n + n O 2(g) are all redox reactions. 2

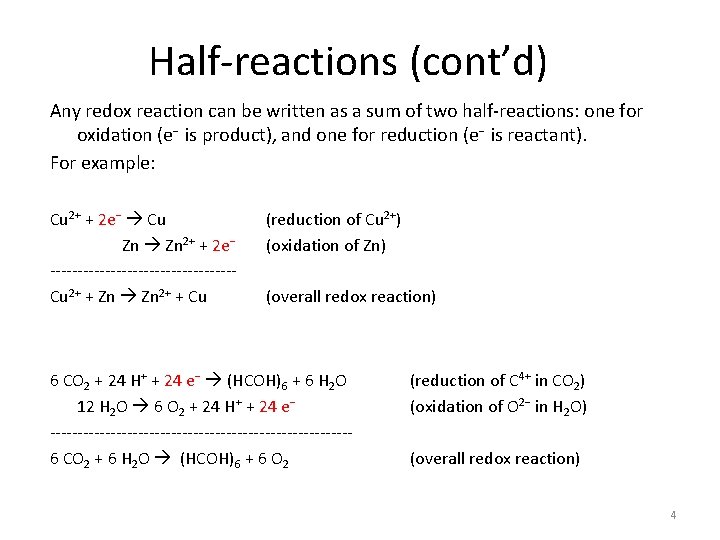
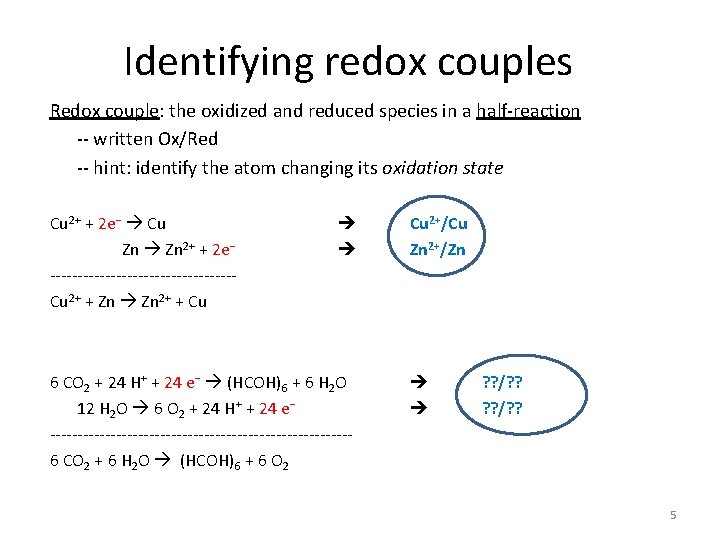
6 C. 1 Half-reactions Any redox reaction can be written as a sum of two half-reactions: one for oxidation (e− is product), and one for reduction (e− is reactant). For example: Cu 2+ + 2 e− Cu Zn 2+ + 2 e− -----------------Cu 2+ + Zn 2+ + Cu (reduction of Cu 2+) (oxidation of Zn) (overall redox reaction) 3

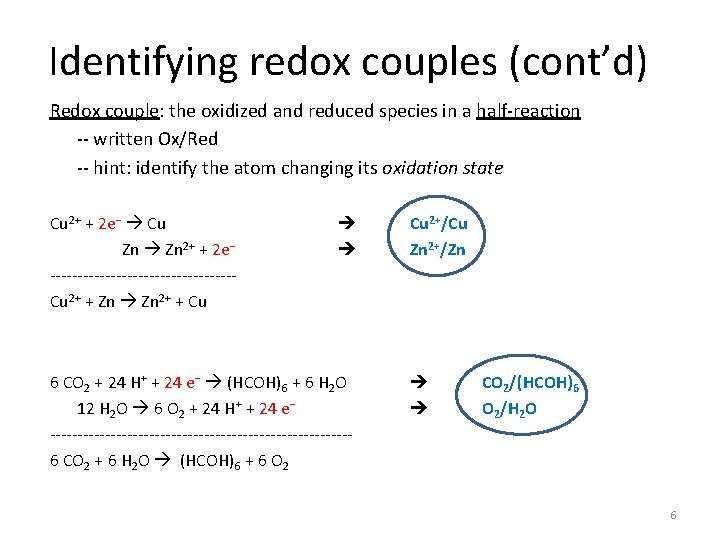
Half-reactions (cont’d) Any redox reaction can be written as a sum of two half-reactions: one for oxidation (e− is product), and one for reduction (e− is reactant). For example: Cu 2+ + 2 e− Cu Zn 2+ + 2 e− -----------------Cu 2+ + Zn 2+ + Cu (reduction of Cu 2+) (oxidation of Zn) (overall redox reaction) 6 CO 2 + 24 H+ + 24 e− (HCOH)6 + 6 H 2 O 12 H 2 O 6 O 2 + 24 H+ + 24 e− ---------------------------6 CO 2 + 6 H 2 O (HCOH)6 + 6 O 2 (reduction of C 4+ in CO 2) (oxidation of O 2− in H 2 O) (overall redox reaction) 4

Identifying redox couples Redox couple: the oxidized and reduced species in a half-reaction -- written Ox/Red -- hint: identify the atom changing its oxidation state Cu 2+ + 2 e− Cu Zn 2+ + 2 e− -----------------Cu 2+ + Zn 2+ + Cu 6 CO 2 + 24 H+ + 24 e− (HCOH)6 + 6 H 2 O 12 H 2 O 6 O 2 + 24 H+ + 24 e− ---------------------------6 CO 2 + 6 H 2 O (HCOH)6 + 6 O 2 Cu 2+/Cu Zn 2+/Zn ? ? /? ? 5

Identifying redox couples (cont’d) Redox couple: the oxidized and reduced species in a half-reaction -- written Ox/Red -- hint: identify the atom changing its oxidation state Cu 2+ + 2 e− Cu Zn 2+ + 2 e− -----------------Cu 2+ + Zn 2+ + Cu 6 CO 2 + 24 H+ + 24 e− (HCOH)6 + 6 H 2 O 12 H 2 O 6 O 2 + 24 H+ + 24 e− ---------------------------6 CO 2 + 6 H 2 O (HCOH)6 + 6 O 2 Cu 2+/Cu Zn 2+/Zn CO 2/(HCOH)6 O 2/H 2 O 6

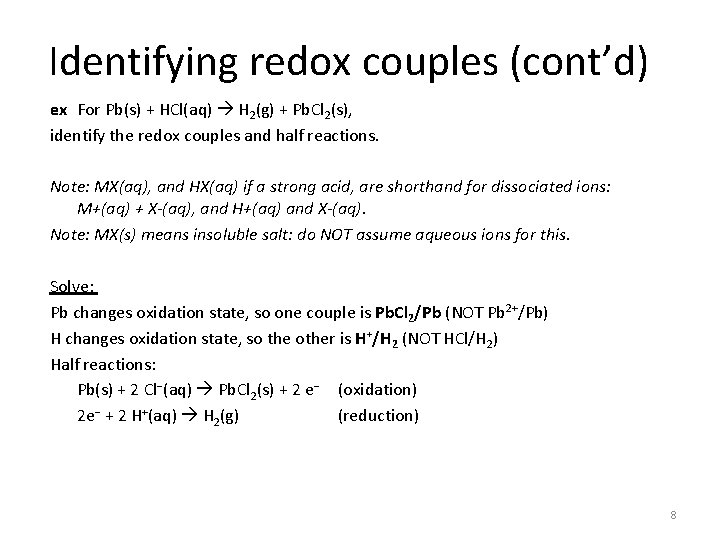
Identifying redox couples (cont’d) ex For Pb(s) + HCl(aq) H 2(g) + Pb. Cl 2(s), identify the redox couples and half reactions. Note: MX(aq), and HX(aq) if a strong acid, are shorthand for dissociated ions: M+(aq) + X-(aq), and H+(aq) and X-(aq). Note: MX(s) means insoluble salt: do NOT assume aqueous ions for this. Solve: 7

Identifying redox couples (cont’d) ex For Pb(s) + HCl(aq) H 2(g) + Pb. Cl 2(s), identify the redox couples and half reactions. Note: MX(aq), and HX(aq) if a strong acid, are shorthand for dissociated ions: M+(aq) + X-(aq), and H+(aq) and X-(aq). Note: MX(s) means insoluble salt: do NOT assume aqueous ions for this. Solve: Pb changes oxidation state, so one couple is Pb. Cl 2/Pb (NOT Pb 2+/Pb) H changes oxidation state, so the other is H+/H 2 (NOT HCl/H 2) Half reactions: Pb(s) + 2 Cl−(aq) Pb. Cl 2(s) + 2 e− (oxidation) 2 e− + 2 H+(aq) H 2(g) (reduction) 8

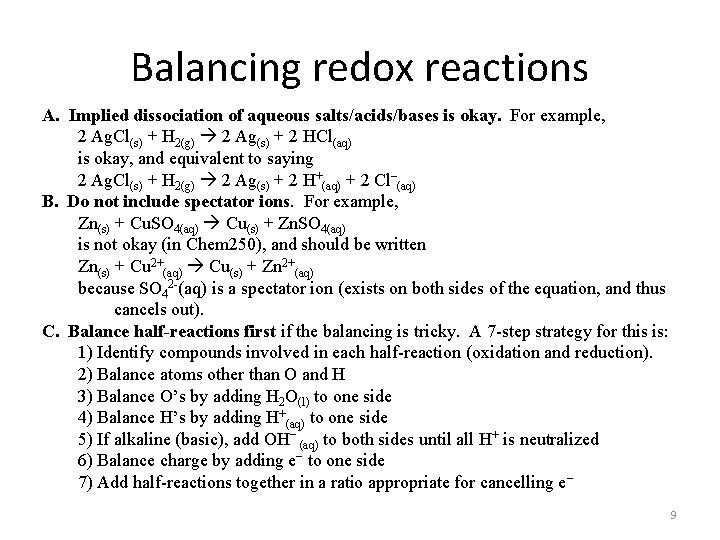
Balancing redox reactions A. Implied dissociation of aqueous salts/acids/bases is okay. For example, 2 Ag. Cl(s) + H 2(g) 2 Ag(s) + 2 HCl(aq) is okay, and equivalent to saying 2 Ag. Cl(s) + H 2(g) 2 Ag(s) + 2 H+(aq) + 2 Cl–(aq) B. Do not include spectator ions. For example, Zn(s) + Cu. SO 4(aq) Cu(s) + Zn. SO 4(aq) is not okay (in Chem 250), and should be written Zn(s) + Cu 2+(aq) Cu(s) + Zn 2+(aq) because SO 42 -(aq) is a spectator ion (exists on both sides of the equation, and thus cancels out). C. Balance half-reactions first if the balancing is tricky. A 7 -step strategy for this is: 1) Identify compounds involved in each half-reaction (oxidation and reduction). 2) Balance atoms other than O and H 3) Balance O’s by adding H 2 O(l) to one side 4) Balance H’s by adding H+(aq) to one side 5) If alkaline (basic), add OH− (aq) to both sides until all H+ is neutralized 6) Balance charge by adding e− to one side 7) Add half-reactions together in a ratio appropriate for cancelling e− 9

Balancing redox reactions (cont’d) ex Balance the following reaction, in alkaline (basic) media: [Mn. O 4]−(aq) + [C 2 O 4]2−(aq) Mn(OH)2(s) + CO 2(g) Solve: Here are the 7 steps again: 1) Identify compounds involved in each half-reaction (oxidation and reduction). 2) Balance atoms other than O and H 3) Balance O’s by adding H 2 O(l) to one side 4) Balance H’s by adding H+(aq) to one side 5) If alkaline (basic), add OH− (aq) to both sides until all H+ is neutralized 6) Balance charge by adding e− to one side 7) Add half-reactions together in a ratio appropriate for cancelling e− 10

Balancing redox reactions (cont’d) ex Balance the following reaction, in alkaline (basic) media: [Mn. O 4]−(aq) + [C 2 O 4]2−(aq) Mn(OH)2(s) + CO 2(g) Solve: Here are the 7 steps again: 1) Identify compounds involved in each half-reaction (oxidation and reduction). 2) Balance atoms other than O and H 3) Balance O’s by adding H 2 O(l) to one side 4) Balance H’s by adding H+(aq) to one side 5) If alkaline (basic), add OH− (aq) to both sides until all H+ is neutralized 6) Balance charge by adding e− to one side 7) Add half-reactions together in a ratio appropriate for cancelling e− Now: Reduction of Mn 7+ to Mn 2+ 1 [Mn. O 4]− Mn(OH)2 2 [Mn. O 4]− Mn(OH)2 3 [Mn. O 4]− Mn(OH)2 + 2 H 2 O 4 6 H+ + [Mn. O 4]− Mn(OH)2 + 2 H 2 O 11

Balancing redox reactions (cont’d) ex Balance the following reaction, in alkaline (basic) media: [Mn. O 4]−(aq) + [C 2 O 4]2−(aq) Mn(OH)2(s) + CO 2(g) Solve: Here are the 7 steps again: 1) Identify compounds involved in each half-reaction (oxidation and reduction). 2) Balance atoms other than O and H 3) Balance O’s by adding H 2 O(l) to one side 4) Balance H’s by adding H+(aq) to one side 5) If alkaline (basic), add OH− (aq) to both sides until all H+ is neutralized 6) Balance charge by adding e− to one side 7) Add half-reactions together in a ratio appropriate for cancelling e− Now: Reduction of Mn 7+ to Mn 2+ 1 [Mn. O 4]− Mn(OH)2 2 [Mn. O 4]− Mn(OH)2 3 [Mn. O 4]− Mn(OH)2 + 2 H 2 O 4 6 H+ + [Mn. O 4]− Mn(OH)2 + 2 H 2 O 5 6 H 2 O + [Mn. O 4]− Mn(OH)2 + 2 H 2 O + 6 OH− 6 5 e − + 4 H 2 O + [Mn. O 4]− Mn(OH)2 + 6 OH − [uh oh, two spectator H 2 O…] [good. Next…. ] 12

Balancing redox reactions (cont’d) ex Balance the following reaction, in alkaline (basic) media: [Mn. O 4]−(aq) + [C 2 O 4]2−(aq) Mn(OH)2(s) + CO 2(g) Solve: Here are the 7 steps again: 1) Identify compounds involved in each half-reaction (oxidation and reduction). 2) Balance atoms other than O and H 3) Balance O’s by adding H 2 O(l) to one side 4) Balance H’s by adding H+(aq) to one side 5) If alkaline (basic), add OH− (aq) to both sides until all H+ is neutralized 6) Balance charge by adding e− to one side 7) Add half-reactions together in a ratio appropriate for cancelling e− Now: Oxidation of C 3+ to C 4+ 1 [C 2 O 4]2− CO 2 2 [C 2 O 4]2− 2 CO 2 3 [C 2 O 4]2− 2 CO 2 4 [C 2 O 4]2− 2 CO 2 5 [C 2 O 4]2− 2 CO 2 6 [C 2 O 4]2− 2 CO 2 + 2 e− [good. Next: overall…. ] 13

Balancing redox reactions (cont’d) ex Balance the following reaction, in alkaline (basic) media: [Mn. O 4]−(aq) + [C 2 O 4]2−(aq) Mn(OH)2(s) + CO 2(g) Solve: Here are the 7 steps again: 1) Identify compounds involved in each half-reaction (oxidation and reduction). 2) Balance atoms other than O and H 3) Balance O’s by adding H 2 O(l) to one side 4) Balance H’s by adding H+(aq) to one side 5) If alkaline (basic), add OH− (aq) to both sides until all H+ is neutralized 6) Balance charge by adding e− to one side 7) Add half-reactions together in a ratio appropriate for cancelling e− Step 7: To add together, multiply reduction reaction (with 5 e−) by 2, and oxidation reaction (with 2 e−) by 5, to cancel e−: 10 e− + 8 H 2 O + 2 [Mn. O 4]− 2 Mn(OH)2 + 12 OH− 5 [C 2 O 4]2− 10 CO 2 + 10 e− ---------------------------------------- 14

Balancing redox reactions (cont’d) ex Balance the following reaction, in alkaline (basic) media: [Mn. O 4]−(aq) + [C 2 O 4]2−(aq) Mn(OH)2(s) + CO 2(g) Solve: Here are the 7 steps again: 1) Identify compounds involved in each half-reaction (oxidation and reduction). 2) Balance atoms other than O and H 3) Balance O’s by adding H 2 O(l) to one side 4) Balance H’s by adding H+(aq) to one side 5) If alkaline (basic), add OH− (aq) to both sides until all H+ is neutralized 6) Balance charge by adding e− to one side 7) Add half-reactions together in a ratio appropriate for cancelling e− Step 7: To add together, multiply reduction reaction (with 5 e−) by 2, and oxidation reaction (with 2 e−) by 5, to cancel e−: 10 e− + 8 H 2 O + 2 [Mn. O 4]− 2 Mn(OH)2 + 12 OH− 5 [C 2 O 4]2− 10 CO 2 + 10 e− ---------------------------------------- 2 [Mn. O 4]−(aq) + 5 [C 2 O 4]2−(aq) + 8 H 2 O(l) 2 Mn(OH)2(s) + 10 CO 2(g) + 12 OH−(aq) 15

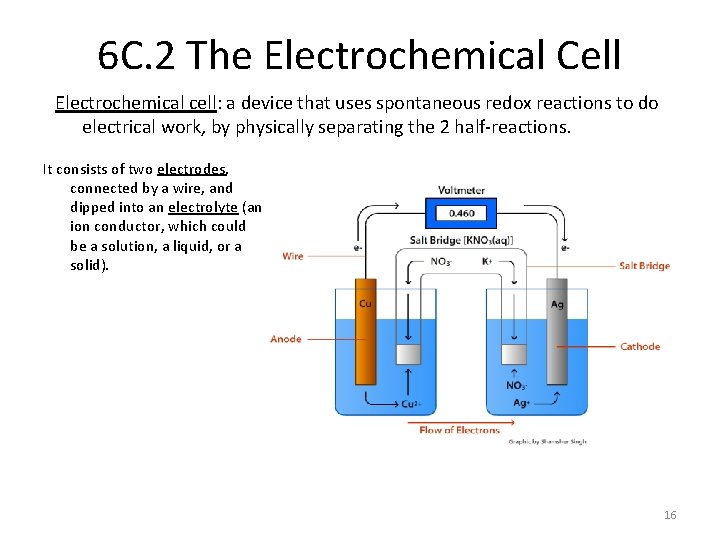
6 C. 2 The Electrochemical Cell Electrochemical cell: a device that uses spontaneous redox reactions to do electrical work, by physically separating the 2 half-reactions. It consists of two electrodes, connected by a wire, and dipped into an electrolyte (an ion conductor, which could be a solution, a liquid, or a solid). 16

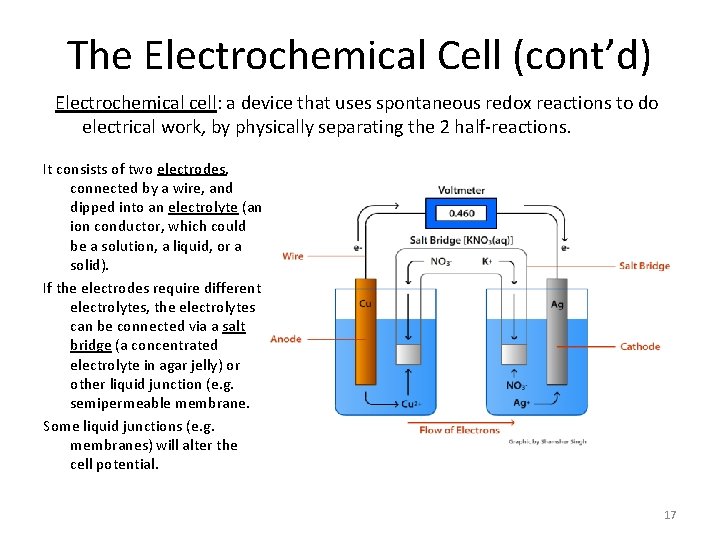
The Electrochemical Cell (cont’d) Electrochemical cell: a device that uses spontaneous redox reactions to do electrical work, by physically separating the 2 half-reactions. It consists of two electrodes, connected by a wire, and dipped into an electrolyte (an ion conductor, which could be a solution, a liquid, or a solid). If the electrodes require different electrolytes, the electrolytes can be connected via a salt bridge (a concentrated electrolyte in agar jelly) or other liquid junction (e. g. semipermeable membrane. Some liquid junctions (e. g. membranes) will alter the cell potential. 17

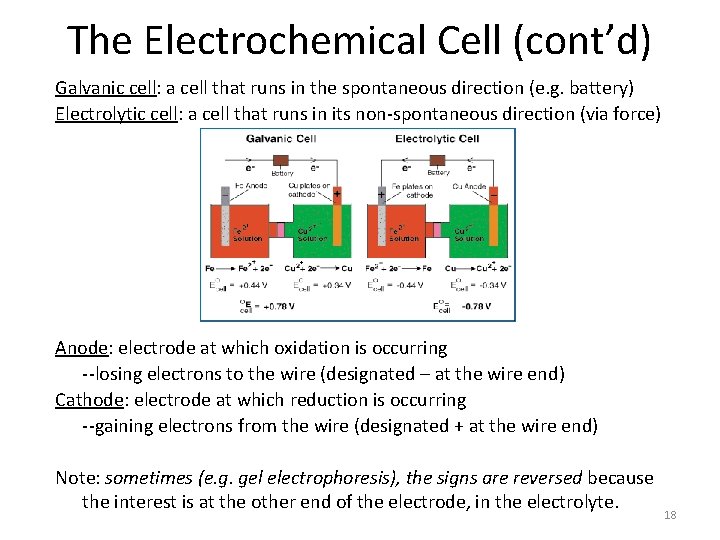
The Electrochemical Cell (cont’d) Galvanic cell: a cell that runs in the spontaneous direction (e. g. battery) Electrolytic cell: a cell that runs in its non-spontaneous direction (via force) Anode: electrode at which oxidation is occurring --losing electrons to the wire (designated – at the wire end) Cathode: electrode at which reduction is occurring --gaining electrons from the wire (designated + at the wire end) Note: sometimes (e. g. gel electrophoresis), the signs are reversed because the interest is at the other end of the electrode, in the electrolyte. 18

Corrosion: loss of metal due to oxidation: M(s) Mn+(aq) + n e−. --Anodes corrode (intentionally or not). --Fighting corrosion is a major application of electrochemistry. Example 1: Bridge Rusting Test. The iron in steel bridges will rust, but only when in contact with O 2 and H 2 O. The test used in Saskatchewan for active rusting of the iron in steel bridges replaces the natural oxidizing agent O 2 with Cu 2+ (aqueous copper sulphate). The overall “cell reaction” in this test is Fe(s) + Cu 2+(aq) Fe 2+(aq) + Cu(s) Iron is the anode, copper is the cathode, and concrete is the electrolyte. A substantial voltage reading (E < − 0. 35 V) indicates that O 2/H 2 O are present in sufficient amounts that active rusting is likely occurring. Readings are taken all over the bridge to determine how much of the bridge is actively rusting. 19

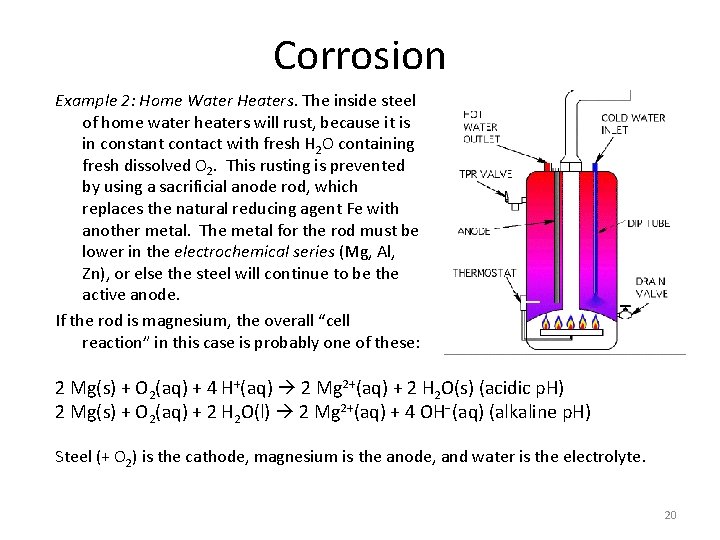
Corrosion Example 2: Home Water Heaters. The inside steel of home water heaters will rust, because it is in constant contact with fresh H 2 O containing fresh dissolved O 2. This rusting is prevented by using a sacrificial anode rod, which replaces the natural reducing agent Fe with another metal. The metal for the rod must be lower in the electrochemical series (Mg, Al, Zn), or else the steel will continue to be the active anode. If the rod is magnesium, the overall “cell reaction” in this case is probably one of these: 2 Mg(s) + O 2(aq) + 4 H+(aq) 2 Mg 2+(aq) + 2 H 2 O(s) (acidic p. H) 2 Mg(s) + O 2(aq) + 2 H 2 O(l) 2 Mg 2+(aq) + 4 OH−(aq) (alkaline p. H) Steel (+ O 2) is the cathode, magnesium is the anode, and water is the electrolyte. 20

6 C. 3 The Cell Potential (Voltage) • 21

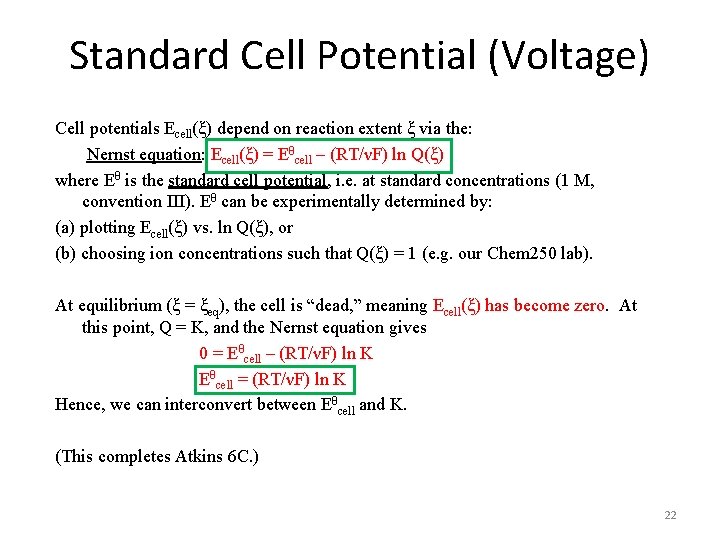
Standard Cell Potential (Voltage) Cell potentials Ecell(ξ) depend on reaction extent ξ via the: Nernst equation: Ecell(ξ) = Eθcell – (RT/νF) ln Q(ξ) where Eθ is the standard cell potential, i. e. at standard concentrations (1 M, convention III). Eθ can be experimentally determined by: (a) plotting Ecell(ξ) vs. ln Q(ξ), or (b) choosing ion concentrations such that Q(ξ) = 1 (e. g. our Chem 250 lab). At equilibrium (ξ = ξeq), the cell is “dead, ” meaning Ecell(ξ) has become zero. At this point, Q = K, and the Nernst equation gives 0 = Eθcell – (RT/νF) ln K Eθcell = (RT/νF) ln K Hence, we can interconvert between Eθcell and K. (This completes Atkins 6 C. ) 22