Redox Year 12 Chemistry What is Redox REDOX






- Slides: 31

Redox Year 12 Chemistry

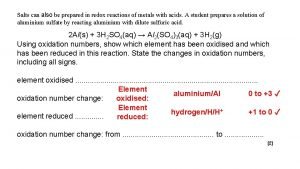
What is Redox? • REDOX stands for REDuction/Oxidation 2 Mg(s) + O 2(g) 2 Mg. O(s) Fe 2 O 3(s) + 3 CO(g) 2 Fe(s) + 3 CO 2(g) Zn(s) + Cu 2+(aq) Zn 2+(aq) + Cu(s) Redox reactions involve the transfer of electrons • Zn metal has been oxidised as it has lost electrons, and the Cu 2+ has been reduced as it has gained electrons. • Zn 2+ + 2 e and Cu 2++ 2 e Cu • Oxidation refers to a loss of electrons • Reduction refers to a gain of electrons

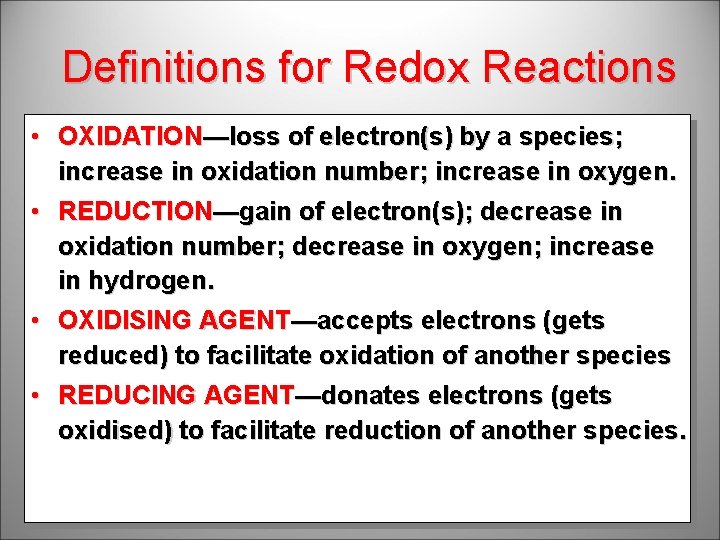
Definitions for Redox Reactions • OXIDATION—loss of electron(s) by a species; increase in oxidation number; increase in oxygen. • REDUCTION—gain of electron(s); decrease in oxidation number; decrease in oxygen; increase in hydrogen. • OXIDISING AGENT—accepts electrons (gets reduced) to facilitate oxidation of another species • REDUCING AGENT—donates electrons (gets oxidised) to facilitate reduction of another species.

• Oxidation and reduction always occur together Zn(s) + Cu 2+(aq) Zn 2+(aq) + Cu(s) Zn 2+ + 2 e and Cu 2++ 2 e Cu • Reduction (gaining electrons) can’t happen without an oxidation to provide the electrons. L ose E lectrons Oxidation Is L oss G ain E lectrons R eduction Is Gain

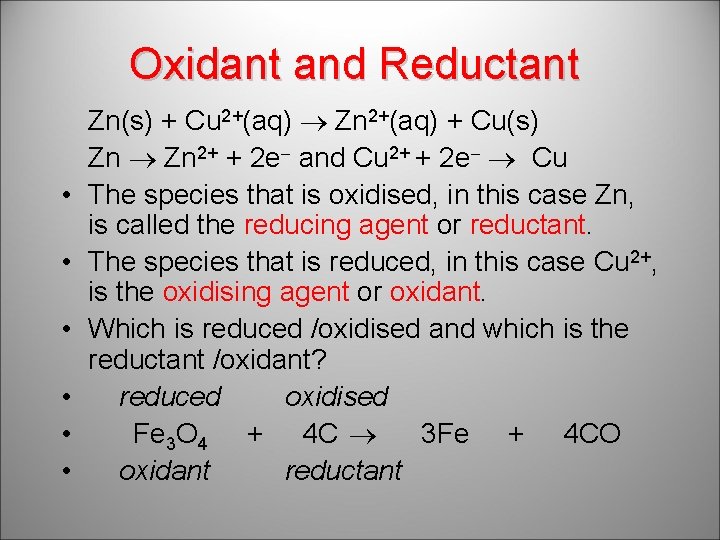
Oxidant and Reductant • • • Zn(s) + Cu 2+(aq) Zn 2+(aq) + Cu(s) Zn 2+ + 2 e and Cu 2+ + 2 e Cu The species that is oxidised, in this case Zn, is called the reducing agent or reductant. The species that is reduced, in this case Cu 2+, is the oxidising agent or oxidant. Which is reduced /oxidised and which is the reductant /oxidant? reduced oxidised Fe 3 O 4 + 4 C 3 Fe + 4 CO oxidant reductant

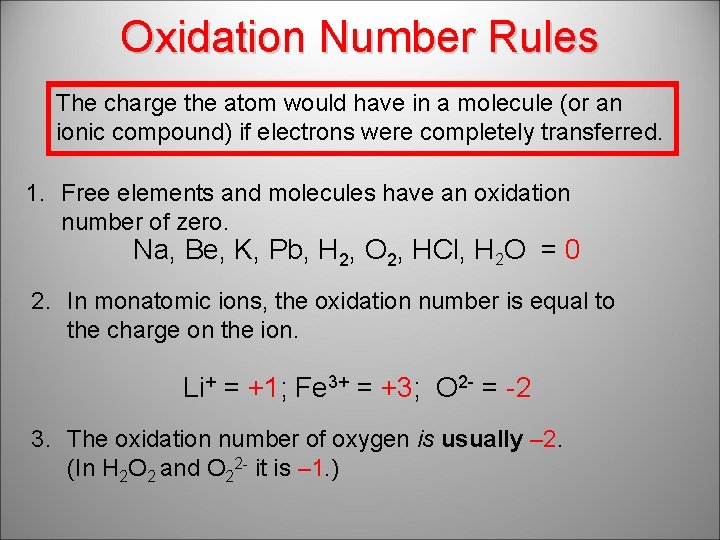
Oxidation Number Rules The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred. 1. Free elements and molecules have an oxidation number of zero. Na, Be, K, Pb, H 2, O 2, HCl, H 2 O = 0 2. In monatomic ions, the oxidation number is equal to the charge on the ion. Li+ = +1; Fe 3+ = +3; O 2 - = -2 3. The oxidation number of oxygen is usually – 2. (In H 2 O 2 and O 22 - it is – 1. )

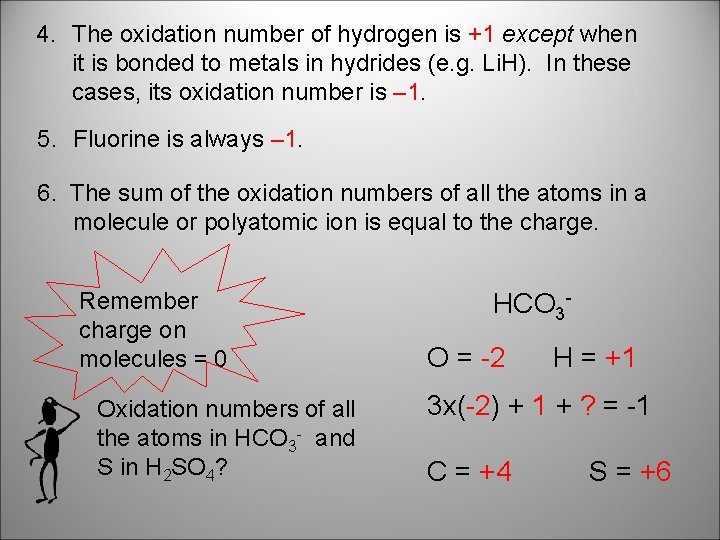
4. The oxidation number of hydrogen is +1 except when it is bonded to metals in hydrides (e. g. Li. H). In these cases, its oxidation number is – 1. 5. Fluorine is always – 1. 6. The sum of the oxidation numbers of all the atoms in a molecule or polyatomic ion is equal to the charge. Remember charge on molecules = 0 Oxidation numbers of all the atoms in HCO 3 - and S in H 2 SO 4? HCO 3 O = -2 H = +1 3 x(-2) + 1 + ? = -1 C = +4 S = +6

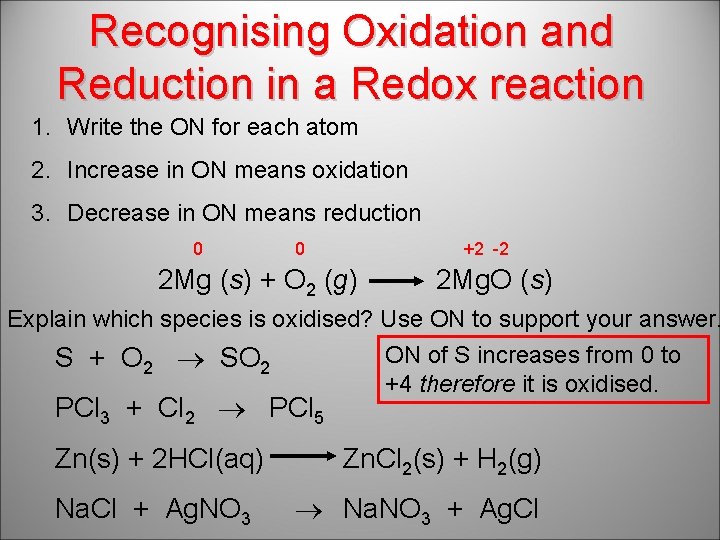
Recognising Oxidation and Reduction in a Redox reaction 1. Write the ON for each atom 2. Increase in ON means oxidation 3. Decrease in ON means reduction 0 0 +2 -2 2 Mg (s) + O 2 (g) 2 Mg. O (s) Explain which species is oxidised? Use ON to support your answer. ON of S increases from 0 to S + O 2 SO 2 +4 therefore it is oxidised. PCl 3 + Cl 2 PCl 5 Zn(s) + 2 HCl(aq) Na. Cl + Ag. NO 3 Zn. Cl 2(s) + H 2(g) Na. NO 3 + Ag. Cl

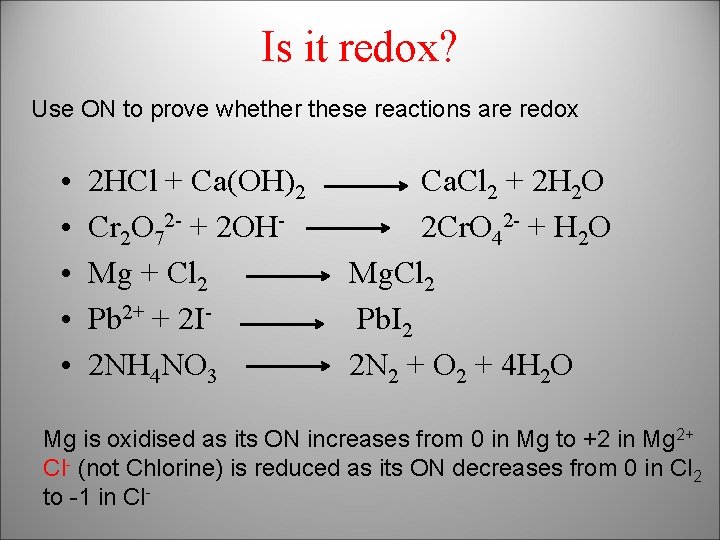
Is it redox? Use ON to prove whether these reactions are redox • • • 2 HCl + Ca(OH)2 Cr 2 O 72 - + 2 OHMg + Cl 2 Pb 2+ + 2 I 2 NH 4 NO 3 Ca. Cl 2 + 2 H 2 O 2 Cr. O 42 - + H 2 O Mg. Cl 2 Pb. I 2 2 N 2 + O 2 + 4 H 2 O Mg is oxidised as its ON increases from 0 in Mg to +2 in Mg 2+ Cl- (not Chlorine) is reduced as its ON decreases from 0 in Cl 2 to -1 in Cl-

Test Yourself Q- Define oxidation and reduction and represent each as a chemical equation. A- oxidation = loss of e– … X X+ + e– reduction = gain of e– … X + e– X– Q- Why are 2 Na + Cl 2 2 Na. Cl & 2 H 2 + O 2 2 H 2 O considered redox reactions? A- Both involve the transfer of electrons (Na, Cl 2 , H 2 and O 2 have ON=0. After reaction ON are Na+ = 1, Cl- = -1, H+ = +1 and O 2 - = -2 Q- Is it possible to oxidise a material without reducing something else? A- No. A lost e– is taken up by something else.

Test Yourself Q- Define oxidising and reducing agent. A- An oxidising agent causes oxidation by being reduced itself and a reducing agent causes reduction by being oxidised itself. Q- Explain using equations why Ca + Cl 2 Ca. Cl 2 is a redox reaction. A- Ca. Cl 2 is an ionic compound made of positive calcium ion and negative chlorine ions Ca 2+ + 2 e–, Cl 2 + 2 e– 2 Cl–. Thus Ca is losing electrons (oxidation) and Cl is gaining electrons (reduction).

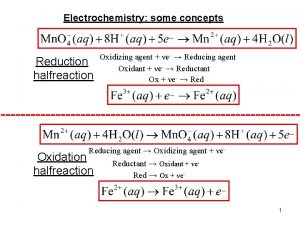
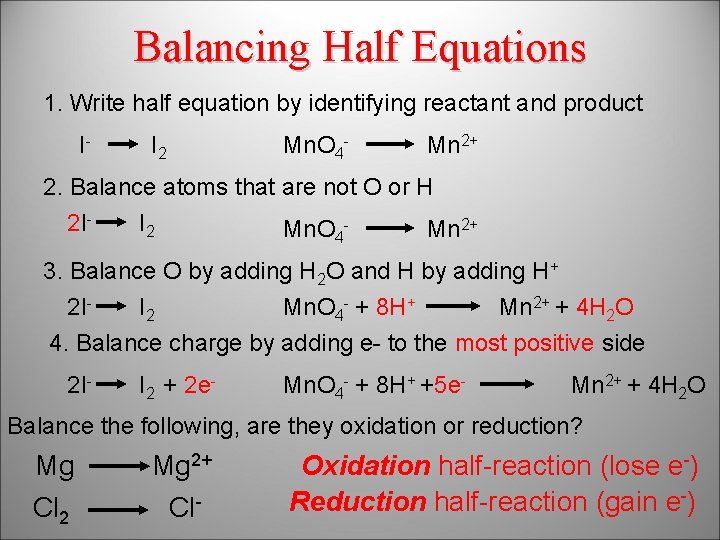
Balancing Half Equations 1. Write half equation by identifying reactant and product I- I 2 Mn. O 4 - Mn 2+ 2. Balance atoms that are not O or H 2 II 2 Mn. O 4 Mn 2+ 3. Balance O by adding H 2 O and H by adding H+ 2 II 2 Mn. O 4 - + 8 H+ Mn 2+ + 4 H 2 O 4. Balance charge by adding e- to the most positive side 2 I- I 2 + 2 e- Mn. O 4 - + 8 H+ +5 e- Mn 2+ + 4 H 2 O Balance the following, are they oxidation or reduction? Mg Mg 2+ + 2 e- Oxidation half-reaction (lose e-) -) Reduction half-reaction (gain e Cl 2 Cl Cl. Cl 2 2+ 2 e

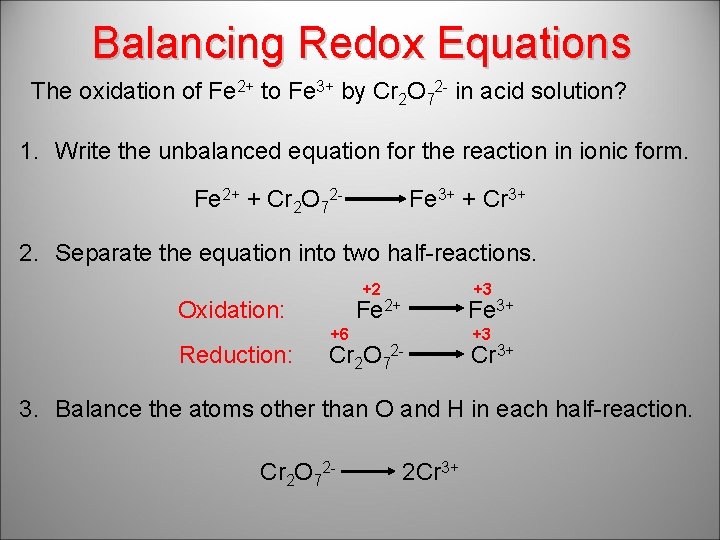
Balancing Redox Equations The oxidation of Fe 2+ to Fe 3+ by Cr 2 O 72 - in acid solution? 1. Write the unbalanced equation for the reaction in ionic form. Fe 2+ + Cr 2 O 72 - Fe 3+ + Cr 3+ 2. Separate the equation into two half-reactions. +2 Oxidation: Reduction: +3 Fe 2+ +6 Cr 2 O 7 Fe 3+ 2 - +3 Cr 3+ 3. Balance the atoms other than O and H in each half-reaction. Cr 2 O 72 - 2 Cr 3+

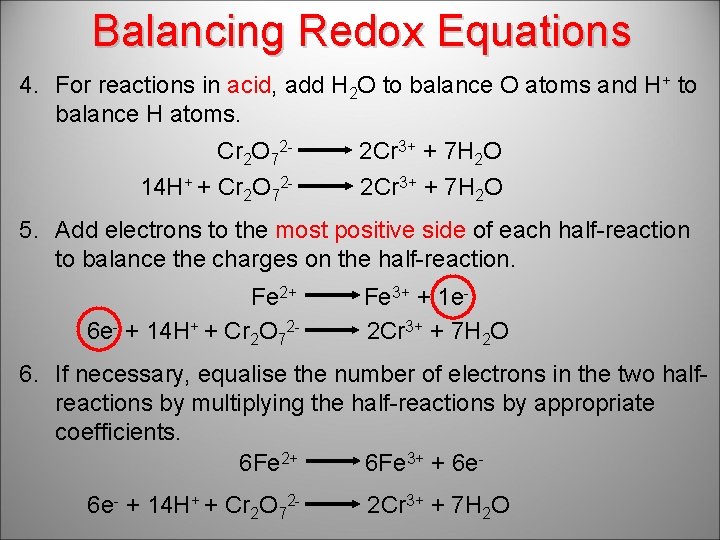
Balancing Redox Equations 4. For reactions in acid, add H 2 O to balance O atoms and H+ to balance H atoms. Cr 2 O 7214 H+ + Cr 2 O 72 - 2 Cr 3+ + 7 H 2 O 5. Add electrons to the most positive side of each half-reaction to balance the charges on the half-reaction. Fe 2+ 6 e- + 14 H+ + Cr 2 O 72 - Fe 3+ + 1 e 2 Cr 3+ + 7 H 2 O 6. If necessary, equalise the number of electrons in the two halfreactions by multiplying the half-reactions by appropriate coefficients. 6 Fe 2+ 6 Fe 3+ + 6 e 6 e- + 14 H+ + Cr 2 O 72 - 2 Cr 3+ + 7 H 2 O

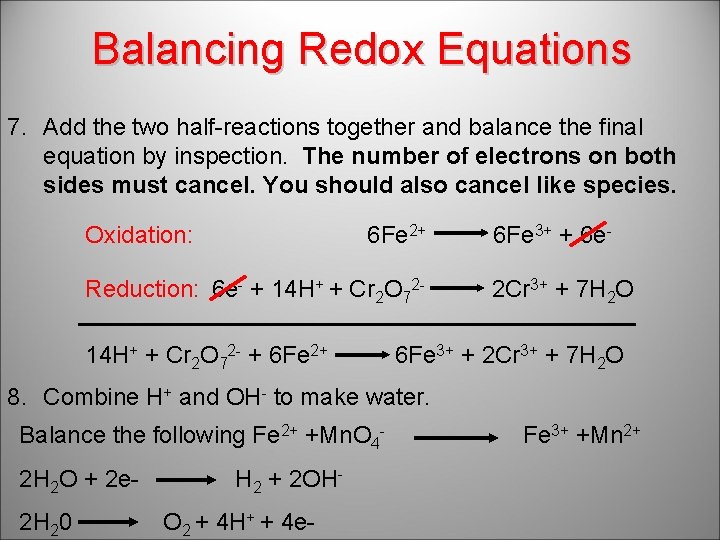
Balancing Redox Equations 7. Add the two half-reactions together and balance the final equation by inspection. The number of electrons on both sides must cancel. You should also cancel like species. Oxidation: 6 Fe 2+ Reduction: 6 e- + 14 H+ + Cr 2 O 72 - + 6 Fe 2+ 6 Fe 3+ + 6 e 2 Cr 3+ + 7 H 2 O 6 Fe 3+ + 2 Cr 3+ + 7 H 2 O 8. Combine H+ and OH- to make water. Balance the following Fe 2+ +Mn. O 42 H 2 O + 2 e 2 H 20 H 2 + 2 OHO 2 + 4 H+ + 4 e- Fe 3+ +Mn 2+

Halogens as Oxidants • Fluorine is so powerful an oxidant that it oxidises water to oxygen. 2 F 2 + 2 H 2 O 4 HF + O 2 A halogen higher in the Group can oxidise the ions of one lower down. • Chlorine reacts with bromide and iodide Cl 2 + 2 Br 2 Cl- + Br 2 Cl 2 + 2 I 2 Cl- + I 2 • Bromine reacts with iodide Br 2 + 2 I 2 Br- + I 2 • Iodine does not react with either of the other halide ions.

Test Yourself • Write a balanced equation for Chlorine reacting with Magnesium and use oxidation numbers to explain which element is oxidised. 0 0 Cl 2 + Mg +2 -1 Mg 2+ + 2 Cl- • Mg changes its oxidation number from 0 to +2, an increase in oxidation number means it is oxidised. Chlorine decreases its oxidation number from 0 to -1 so it is reduced.

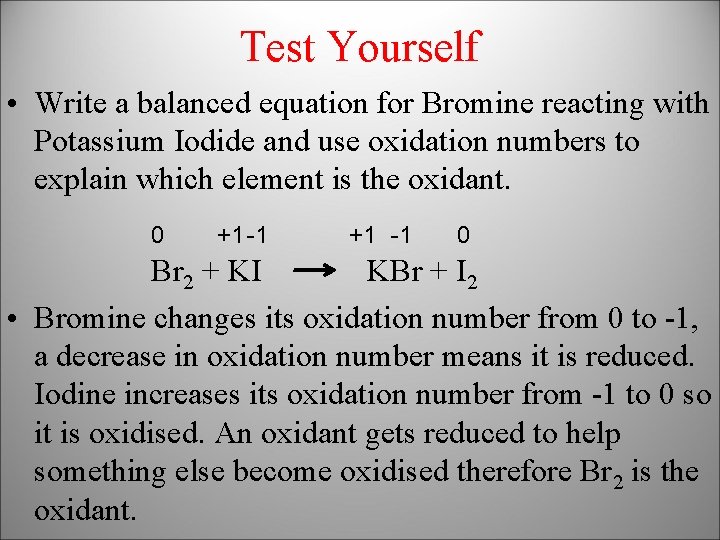
Test Yourself • Write a balanced equation for Bromine reacting with Potassium Iodide and use oxidation numbers to explain which element is the oxidant. 0 +1 -1 0 Br 2 + KI KBr + I 2 • Bromine changes its oxidation number from 0 to -1, a decrease in oxidation number means it is reduced. Iodine increases its oxidation number from -1 to 0 so it is oxidised. An oxidant gets reduced to help something else become oxidised therefore Br 2 is the oxidant.

Halogens can Oxidise Water • Halogens are not very soluble in water • They do react with water Cl 2(s) + H 2 O(l) HCl(aq) + HOCl(aq) • Hypochlorous acid (HOCl) and hypochlorite (OCl-) are the main components of free active chlorine used in disinfectants and swimming pools.

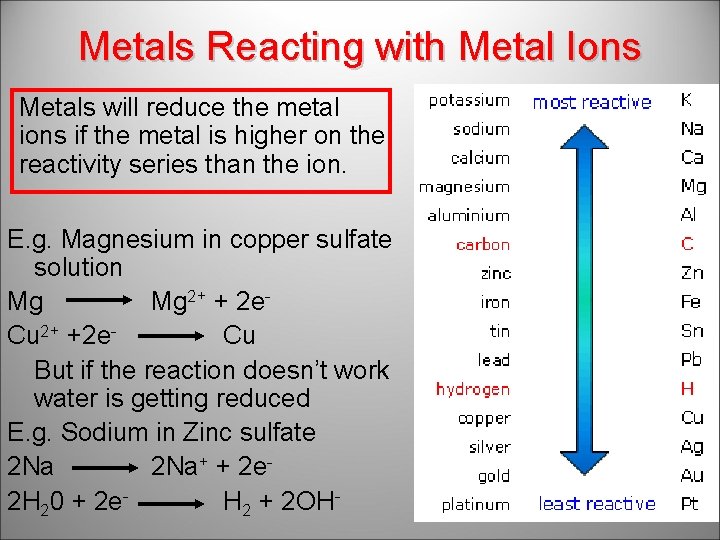
Metals Reacting with Metal Ions Metals will reduce the metal ions if the metal is higher on the reactivity series than the ion. E. g. Magnesium in copper sulfate solution Mg Mg 2+ + 2 e. Cu 2+ +2 e. Cu But if the reaction doesn’t work water is getting reduced E. g. Sodium in Zinc sulfate 2 Na+ + 2 e 2 H 20 + 2 e. H 2 + 2 OH-

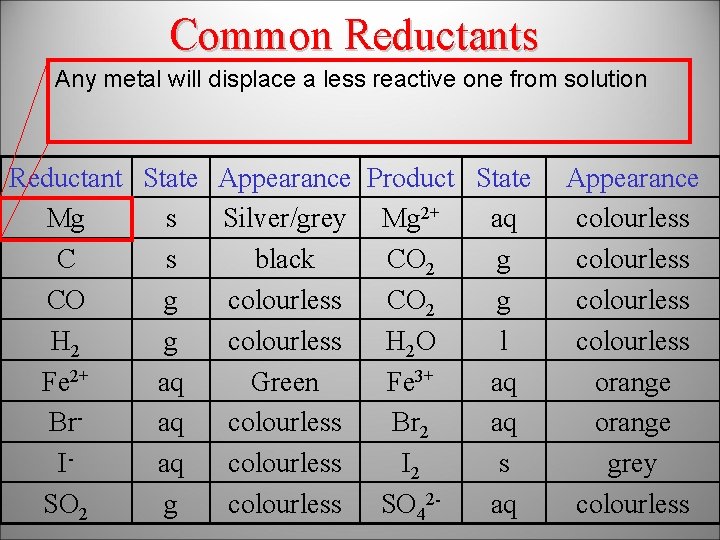
Common Reductants Any metal will displace a less reactive one from solution K>Na>Li>Ca>Mg>Al>Zn>Fe>Sn>Pb>Cu>Ag Reductant State Appearance Product State Mg s Silver/grey Mg 2+ aq C s black CO 2 g CO g colourless CO 2 g H 2 g colourless H 2 O l Fe 2+ aq Green Fe 3+ aq Braq colourless Br 2 aq Iaq colourless I 2 s SO 2 g colourless SO 42 aq Appearance colourless orange grey colourless

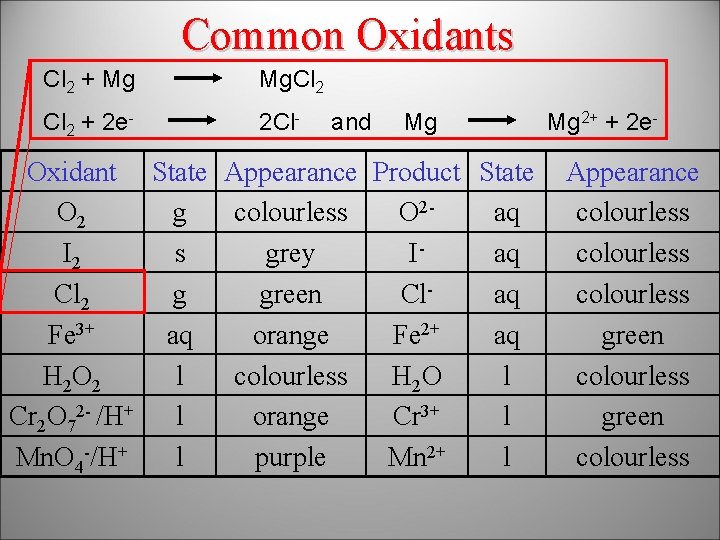
Common Oxidants Cl 2 + Mg Mg. Cl 2 + 2 e- 2 Cl- and Mg Oxidant State Appearance Product State O 2 g colourless O 2 aq I 2 s grey Iaq Cl 2 g green Claq Fe 3+ aq orange Fe 2+ aq H 2 O 2 l colourless H 2 O l Cr 2 O 72 - /H+ l orange Cr 3+ l Mn. O 4 -/H+ l purple Mn 2+ l Mg 2+ + 2 e- Appearance colourless green colourless

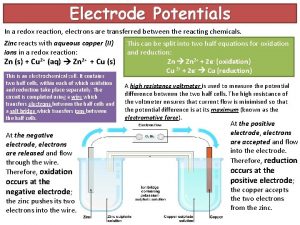
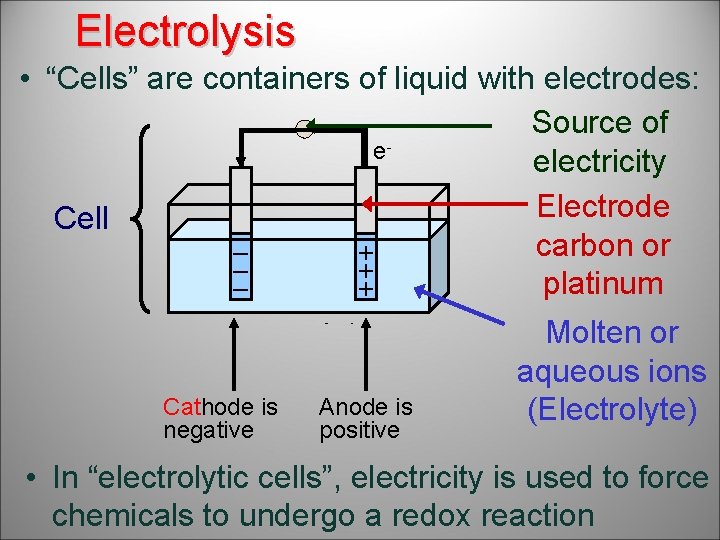
Electrolysis – used to separate ions • “Cells” are containers of liquid with electrodes: Source of eelectricity Electrode Cell carbon or – + platinum – + Cations Anions Reduced Oxidised Cathode is negative Anode is positive Molten or aqueous ions (Electrolyte) • In “electrolytic cells”, electricity is used to force chemicals to undergo a redox reaction

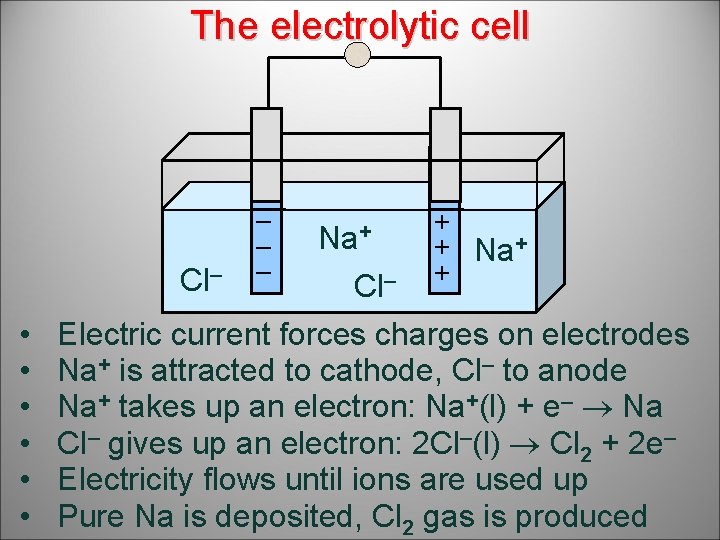
The electrolytic cell Cl– • • • – – – Na+ Cl– + + Na+ + Electric current forces charges on electrodes Na+ is attracted to cathode, Cl– to anode Na+ takes up an electron: Na+(l) + e– Na Cl– gives up an electron: 2 Cl–(l) Cl 2 + 2 e– Electricity flows until ions are used up Pure Na is deposited, Cl 2 gas is produced

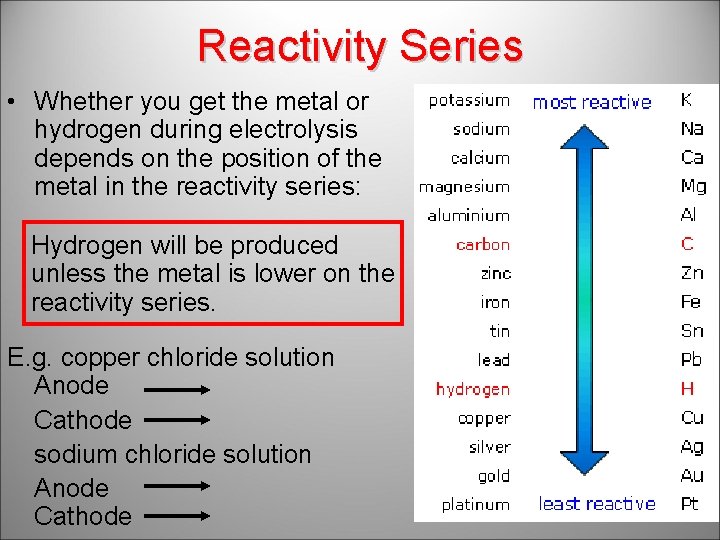
Reactivity Series • Whether you get the metal or hydrogen during electrolysis depends on the position of the metal in the reactivity series: Hydrogen will be produced unless the metal is lower on the reactivity series. E. g. copper chloride solution Anode chlorine Cathode copper sodium chloride solution Anode chlorine Cathode hydrogen

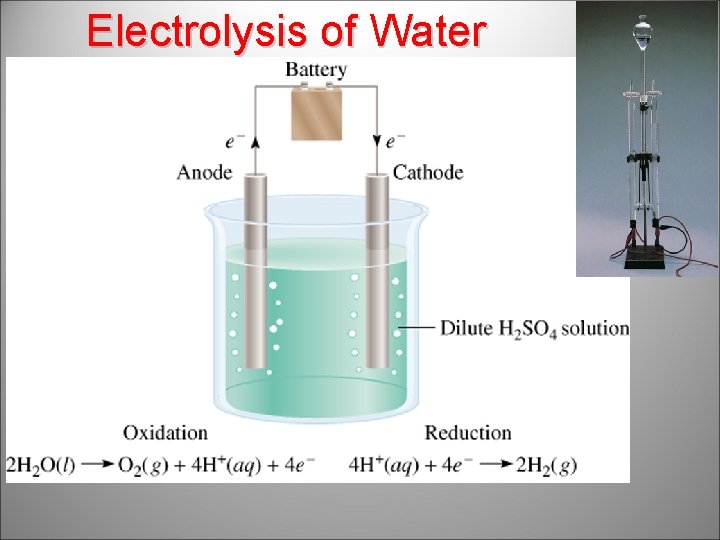
Electrolysis of Water

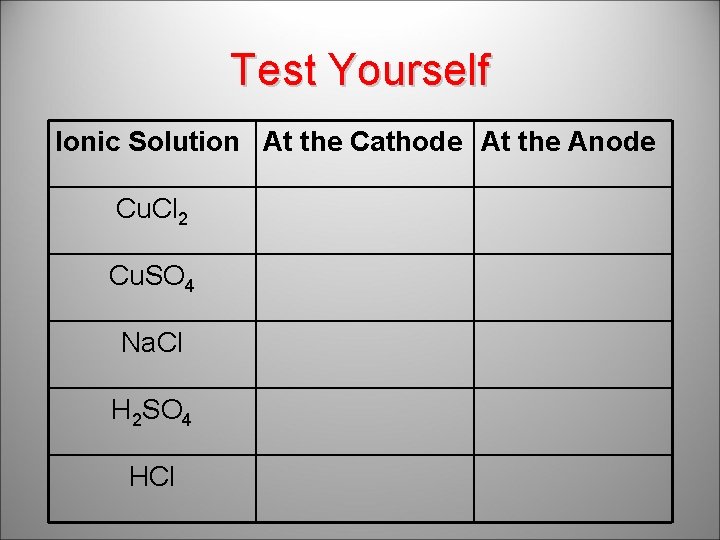
Test Yourself Ionic Solution At the Cathode At the Anode Cu. Cl 2 Cu. SO 4 Cu O 2 Na. Cl H 2 Cl 2 H 2 SO 4 H 2 O 2 HCl H 2 Cl 2

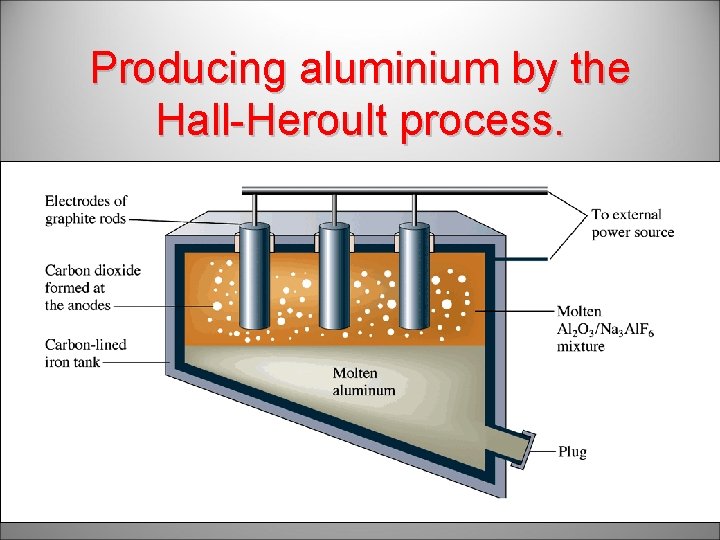
Producing aluminium by the Hall-Heroult process.

The Hall – Heroult Process for Aluminium • Electrolysis of molten Al 2 O 3 mixed with cryolite – lowers melting point • Cell still operates at high temperature – 1000 o. C • Aluminium was a precious metal in 1886 (US$4/lb)

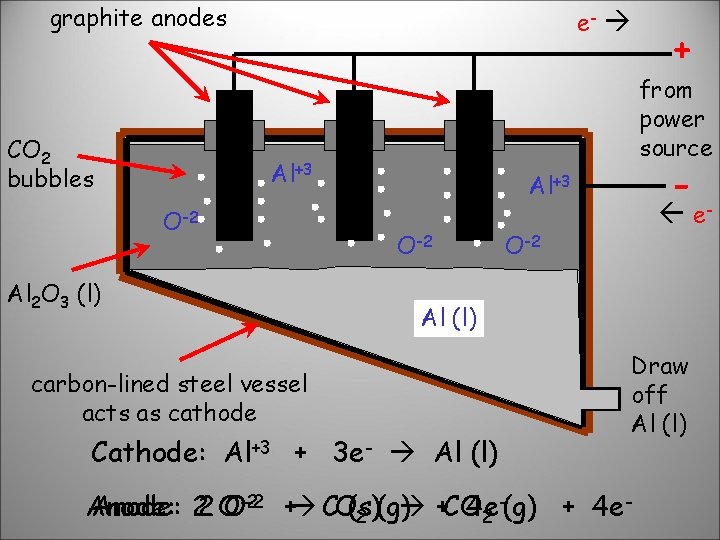
graphite anodes CO 2 bubbles e- from power source Al+3 O-2 Al 2 O 3 (l) + - Al+3 O-2 e- O-2 Al (l) carbon-lined steel vessel acts as cathode Cathode: Al+3 + 3 e- Al (l) Draw off Al (l) -(g) + 4 e. Anode: 22 O O-2 -2 + CO(s) (g) + CO 4 e 2 2

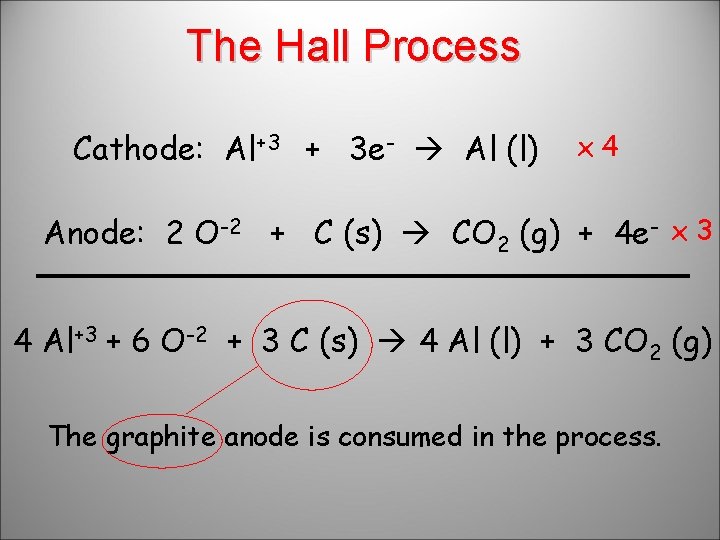
The Hall Process Cathode: Al+3 + 3 e- Al (l) x 4 Anode: 2 O-2 + C (s) CO 2 (g) + 4 e- x 3 4 Al+3 + 6 O-2 + 3 C (s) 4 Al (l) + 3 CO 2 (g) The graphite anode is consumed in the process.
 Sad year 6 leavers poem
Sad year 6 leavers poem Organic vs inorganic chemistry
Organic vs inorganic chemistry Ib chemistry functional groups
Ib chemistry functional groups Redox reaction examples
Redox reaction examples Single replacement redox reaction
Single replacement redox reaction Redox volumetric analysis
Redox volumetric analysis Rules for balancing redox reactions
Rules for balancing redox reactions Redox os
Redox os Redox reaction leo ger
Redox reaction leo ger Reacciones redox 2 bachillerato
Reacciones redox 2 bachillerato Redox titration curve
Redox titration curve Standard electrode potential
Standard electrode potential Reducing agent strength table
Reducing agent strength table Hvad er oxidationstal
Hvad er oxidationstal Redox tutorial
Redox tutorial Define redox titration
Define redox titration Reacciones redox
Reacciones redox Akumulatory przepływowe redox
Akumulatory przepływowe redox Redox reaction in alkaline medium
Redox reaction in alkaline medium Surco mayor adn
Surco mayor adn Balancing complex redox reactions
Balancing complex redox reactions Electrolytic cell khan academy
Electrolytic cell khan academy Using light to make food
Using light to make food Red cat redox
Red cat redox Redox real life example
Redox real life example Oxidation state
Oxidation state Redox stoichiometry
Redox stoichiometry Ipo4 oxidation number
Ipo4 oxidation number Redox potential table
Redox potential table Redox reactions definition
Redox reactions definition Hall heroult process
Hall heroult process Bateria redox
Bateria redox