Catalyst What is a mass spectrometer and how




















- Slides: 31

Catalyst What is a mass spectrometer and how does it work? What kind of ion is produced to create a mass spectrum? 3. What is a mass spectrum? 1. 2. End

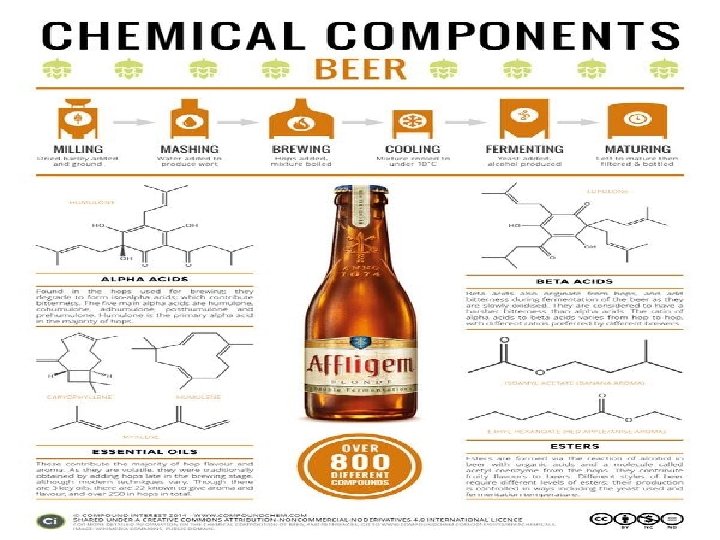
Naming is Hard! Let’s work on it �Haven’t you always wanted to know what’s actually going into your body? �Naming Practice �Stations practice �Naming big molecules �Naming little molecules �Naming organic molecules �Naming inorganic molecules �Name it all!


What Substances are We Dealing With and How Much?



Lesson 2. 7 – Guaca. Mole, and Molecular and Empirical Formulas

Today’s Learning Targets � LT 2. 11 – I can explain the concept of the mole and why it is an adequate means by which to express the amount of given substance. � LT 2. 12 – I can determine the empirical and molecular formula for any compound given its constituent parts. I can also calculate percent composition for each element in that compound.

Remember the Mole?

The Mole = Just a Number! �Recall that Avogadro proposed that 1 mole = 6. 022 x 1023 particles. �If you have 1 mole this means you have 6. 022 x 1023 of this substance. �This works because particles are really small!

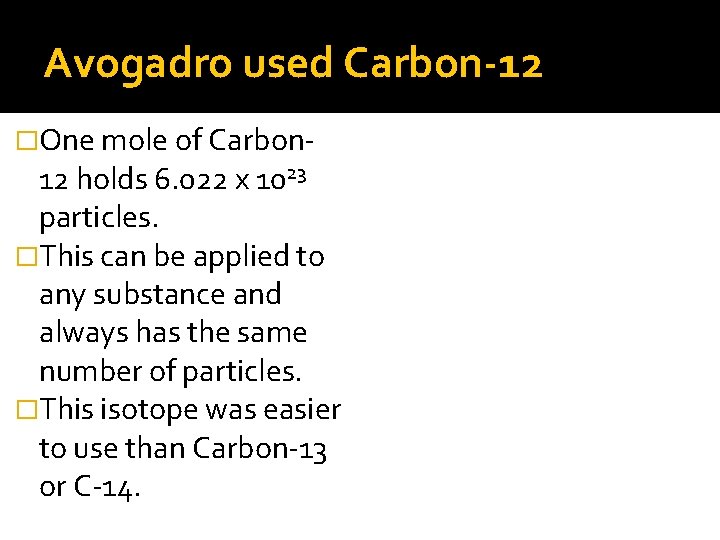
Avogadro used Carbon-12 �One mole of Carbon- 12 holds 6. 022 x 1023 particles. �This can be applied to any substance and always has the same number of particles. �This isotope was easier to use than Carbon-13 or C-14.

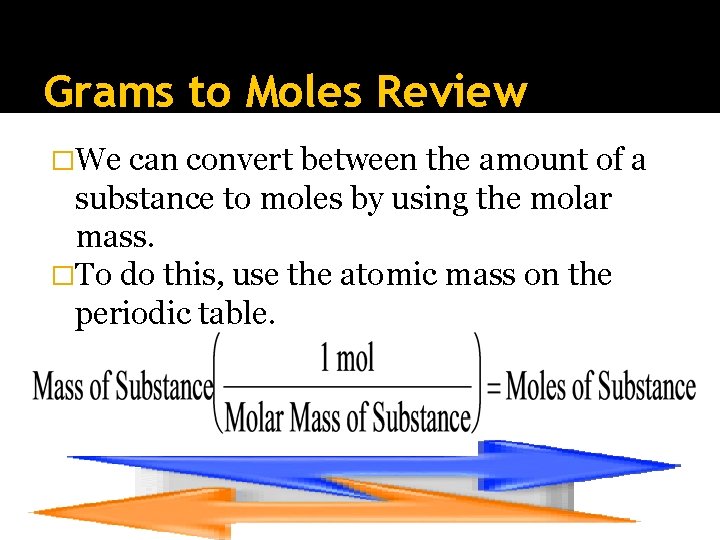
Grams to Moles Review �We can convert between the amount of a substance to moles by using the molar mass. �To do this, use the atomic mass on the periodic table.

Table Talk �You have 5. 380 g of C 6 H 12 O 6, how many moles do you have?

Percent Composition by Mass

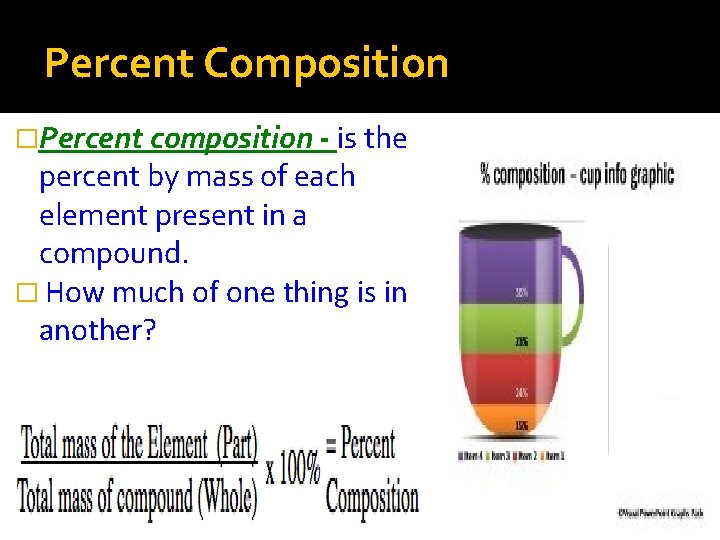
Percent Composition �Percent composition - is the percent by mass of each element present in a compound. � How much of one thing is in another?

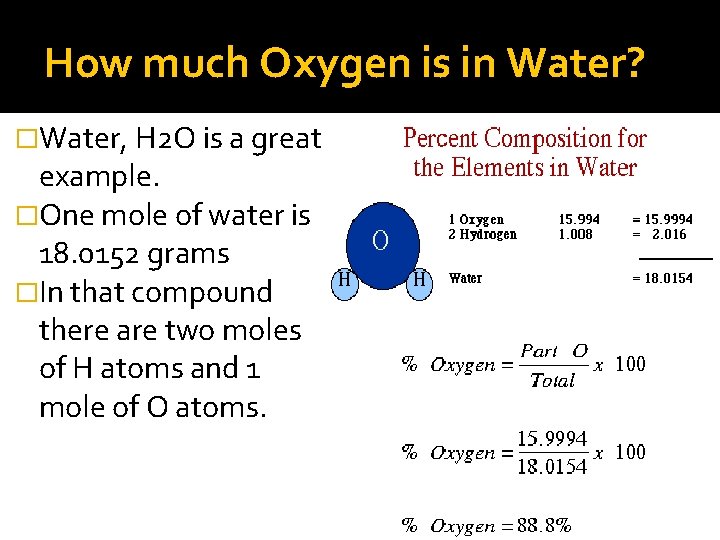
How much Oxygen is in Water? �Water, H 2 O is a great example. �One mole of water is 18. 0152 grams �In that compound there are two moles of H atoms and 1 mole of O atoms.

Class Example �Calculate the percent by weight of sodium (Na) and chlorine (Cl) in sodium chloride (Na. Cl) molecule.

Molecular and Empirical Formulas

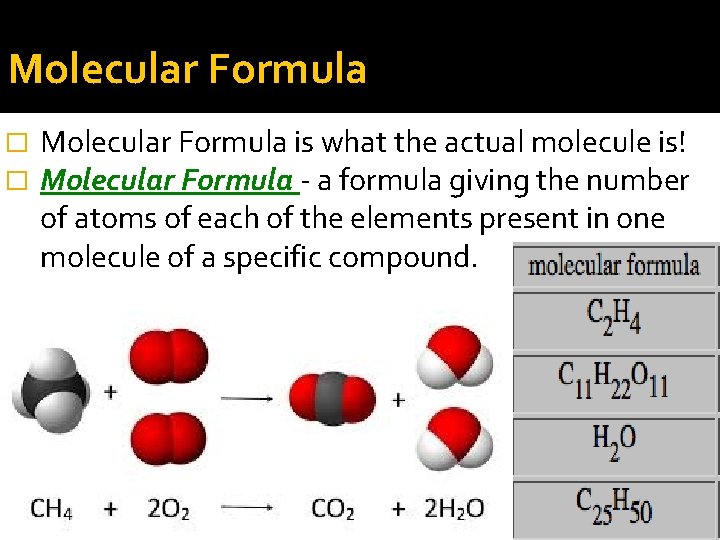
Molecular Formula � � Molecular Formula is what the actual molecule is! Molecular Formula - a formula giving the number of atoms of each of the elements present in one molecule of a specific compound.

Empirical Formula Empirical means observed, or determined from experimentation! �Empirical formula is the smallest whole number ratio of moles of each element in a compound. �Ca. Cl 2 --> there is 1 mole of calcium for every 2 moles of chlorine �

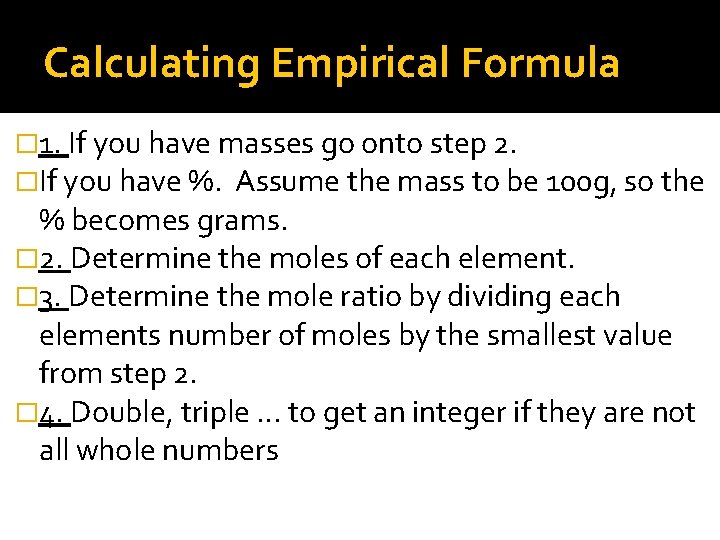
Calculating Empirical Formula � 1. If you have masses go onto step 2. �If you have %. Assume the mass to be 100 g, so the % becomes grams. � 2. Determine the moles of each element. � 3. Determine the mole ratio by dividing each elements number of moles by the smallest value from step 2. � 4. Double, triple … to get an integer if they are not all whole numbers

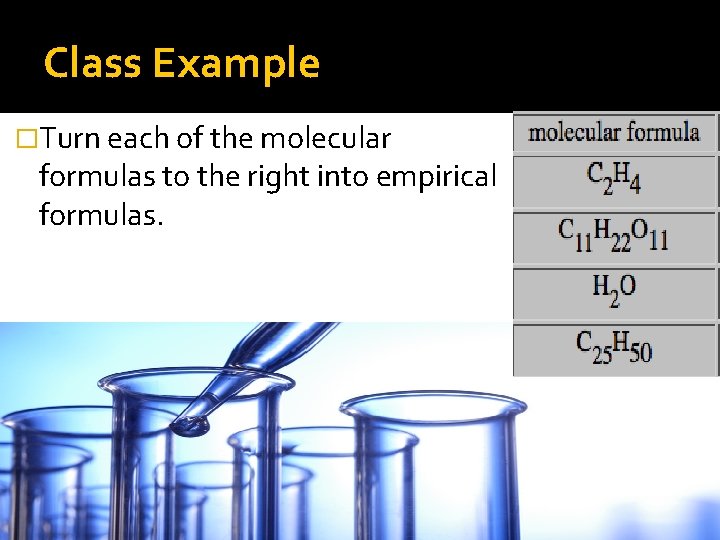
Class Example �Turn each of the molecular formulas to the right into empirical formulas.

Table Talk �The atomic weight of element X is 100. g/mol. 50. 0 grams of X combines with 32. 0 g of oxygen. What is the simplest formula for the resulting compound? The atomic weight of oxygen = 16. 0 g/mol.

Stop and Jot �Which of the following substances has an empirical formula that is different from that of the others? (a) Erythrose: C 4 H 8 O 4 (b) Propionic acid: C 3 H 6 O 2 (c) Acetic acid C 2 H 4 O 2 (d) Glucose C 6 H 12 O 6

KHAN ACADEMY �KHAN YOU DO IT? �http: //www. khanacademy. org/test- prep/mcat/physicalprocesses/stoichiometry/v/molecular-andempirical-formulas �Watch video and answer Q’s

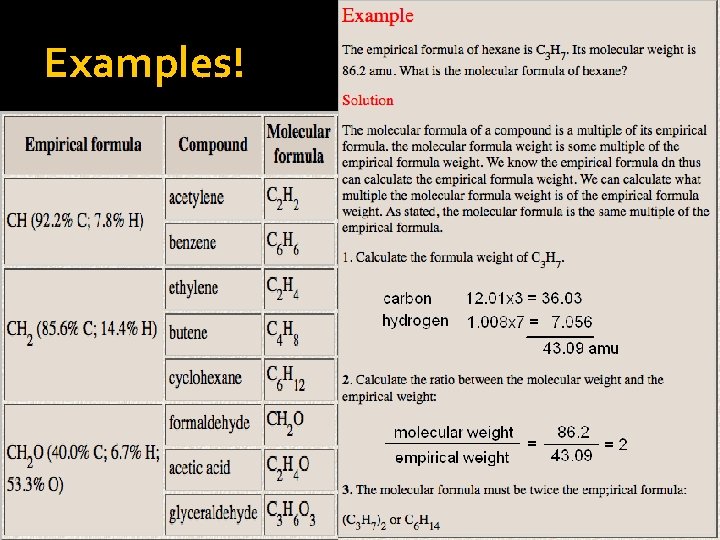
Examples!

Naming Simple Organic Compounds

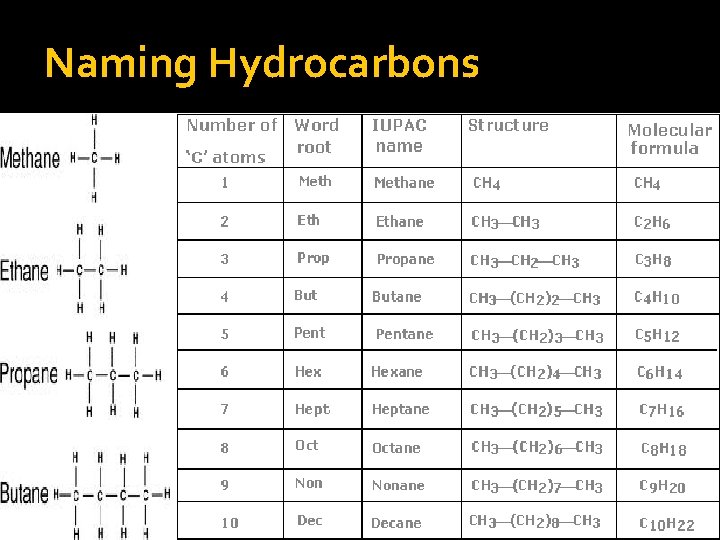
Naming Hydrocarbons

Unit 2 Stations Review �Complete the stations review independently or with your table.


Closing Time �Quantitative on Ch. 2 on Monday �Answers to practices