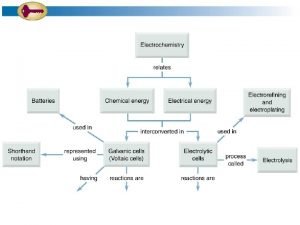
18 24 The following picture of a galvanic





- Slides: 10



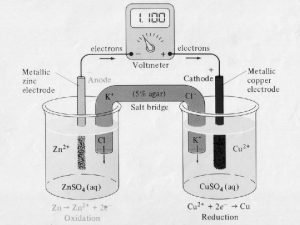
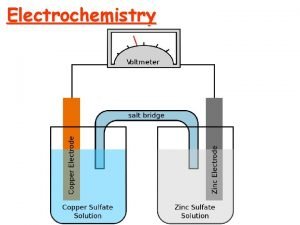
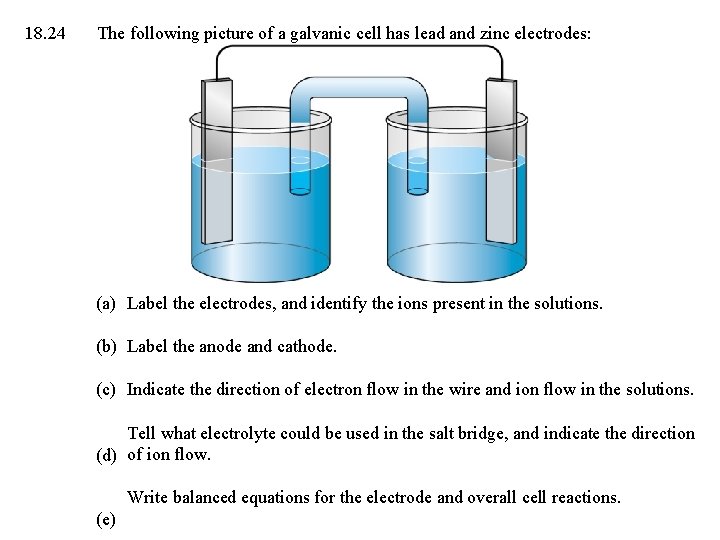
18. 24 The following picture of a galvanic cell has lead and zinc electrodes: (a) Label the electrodes, and identify the ions present in the solutions. (b) Label the anode and cathode. (c) Indicate the direction of electron flow in the wire and ion flow in the solutions. Tell what electrolyte could be used in the salt bridge, and indicate the direction (d) of ion flow. Write balanced equations for the electrode and overall cell reactions. (e)

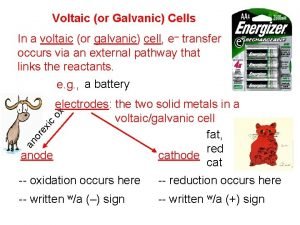
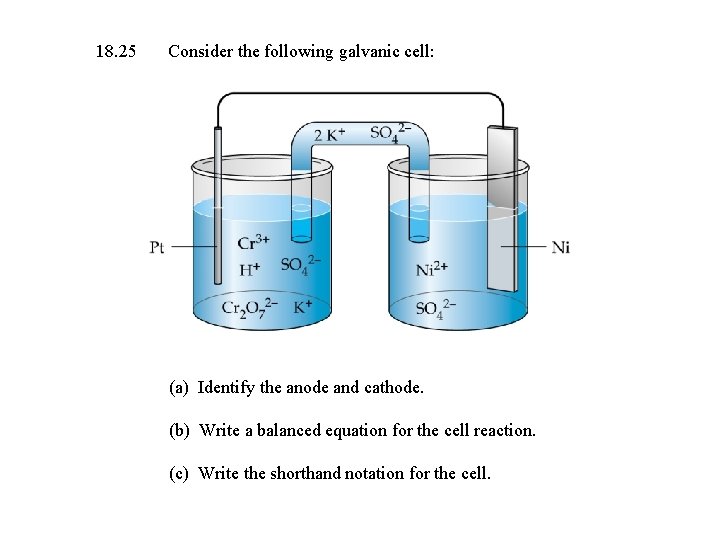
18. 25 Consider the following galvanic cell: (a) Identify the anode and cathode. (b) Write a balanced equation for the cell reaction. (c) Write the shorthand notation for the cell.

18. 26 Consider the following galvanic cells: (1) Cu(s) | Cu 2+ (1 M) || Fe 3+ (1 M), Fe 2+ (1 M) | Pt(s) (2) Cu(s) | Cu 2+ (1 M) || Fe 3+ (1 M), Fe 2+ (5 M) | Pt(s) (3) Cu(s) | Cu 2+ (0. 1 M) || Fe 3+ (0. 1 M), Fe 2+ (0. 1 M) | Pt(s) (a) Write a balanced equation for each cell reaction. (b) Sketch each cell. Label the anode and cathode, and indicate the direction of electron and ion flow. (c) Which of the three cells has the largest cell potential? Which has the smallest cell potential? Explain.

18. 27 Sketch a cell with inert electrodes suitable for electrolysis of aqueous Cu. Br 2 (a) Label the anode and cathode. (b) Indicate the direction of electron and ion flow. (c) Write balanced equations for the anode, cathode, and overall cell reactions.

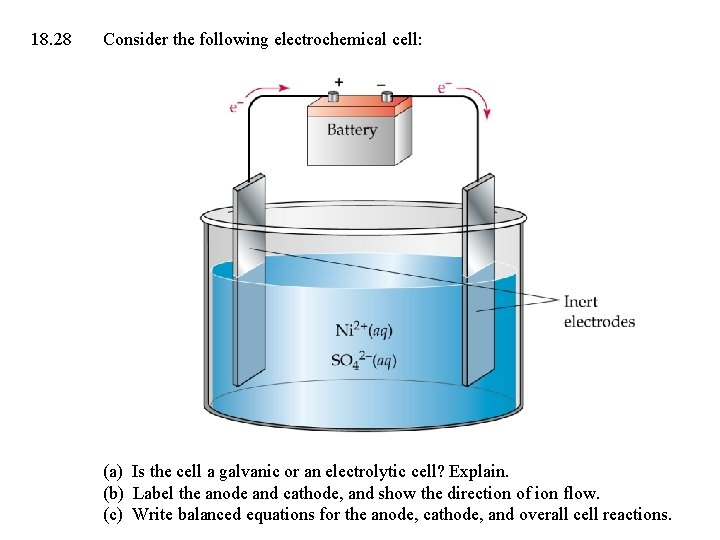
18. 28 Consider the following electrochemical cell: (a) Is the cell a galvanic or an electrolytic cell? Explain. (b) Label the anode and cathode, and show the direction of ion flow. (c) Write balanced equations for the anode, cathode, and overall cell reactions.

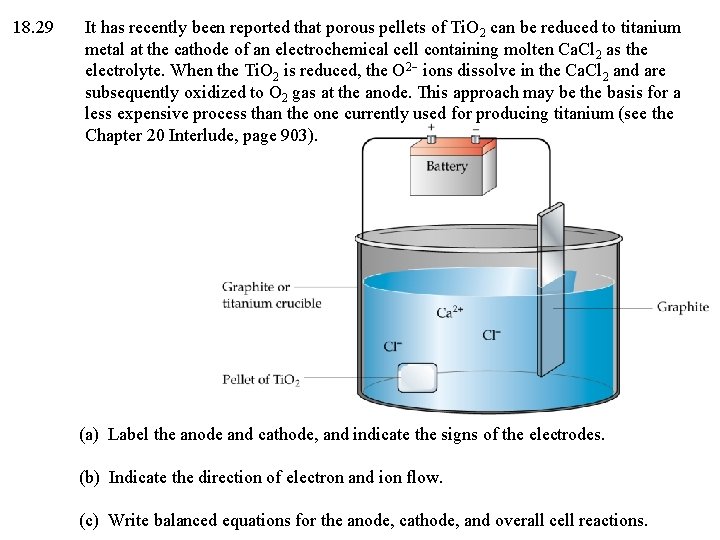
18. 29 It has recently been reported that porous pellets of Ti. O 2 can be reduced to titanium metal at the cathode of an electrochemical cell containing molten Ca. Cl 2 as the electrolyte. When the Ti. O 2 is reduced, the O 2 ions dissolve in the Ca. Cl 2 and are subsequently oxidized to O 2 gas at the anode. This approach may be the basis for a less expensive process than the one currently used for producing titanium (see the Chapter 20 Interlude, page 903). (a) Label the anode and cathode, and indicate the signs of the electrodes. (b) Indicate the direction of electron and ion flow. (c) Write balanced equations for the anode, cathode, and overall cell reactions.

18. 30 Consider a Daniell cell with 1. 0 M ion concentrations: Does the cell voltage increase, decrease, or remain the same when each of the following changes is made? Explain. (a) 5. 0 M Cu. SO 4 is added to the cathode compartment. (b) 5. 0 M H 2 SO 4 is added to the cathode compartment. (c) 5. 0 M Zn(NO 3)2 is added to the anode compartment. (d) 1. 0 M Zn(NO 3)2 is added to the anode compartment.

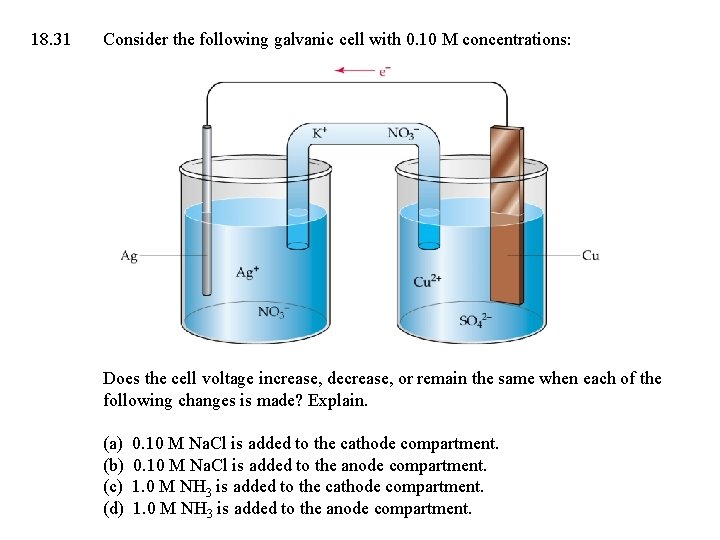
18. 31 Consider the following galvanic cell with 0. 10 M concentrations: Does the cell voltage increase, decrease, or remain the same when each of the following changes is made? Explain. (a) (b) (c) (d) 0. 10 M Na. Cl is added to the cathode compartment. 0. 10 M Na. Cl is added to the anode compartment. 1. 0 M NH 3 is added to the cathode compartment. 1. 0 M NH 3 is added to the anode compartment.