ELECTROCHEMISTRY 1 2 Electron Transfer Reactions Electron transfer











- Slides: 54

ELECTROCHEMISTRY 1

2 Electron Transfer Reactions • Electron transfer reactions are oxidationreduction or redox reactions. • Results in the generation of an electric current (electricity) or be caused by imposing an electric current. • Therefore, this field of chemistry is often called ELECTROCHEMISTRY.

Terminology for Redox Reactions 3 • OXIDATION—loss of electron(s) by a species; increase in oxidation number; increase in oxygen. • REDUCTION—gain of electron(s); decrease in © oxidation number; decrease in oxygen; increase in hydrogen. • OXIDIZING AGENT—electron acceptor; species is reduced. • REDUCING AGENT—electron donor; species is oxidized.

Oxidation and reduction happen at the same time There is no net gain or loss of electrons. e You can’t just create them or destroy them!

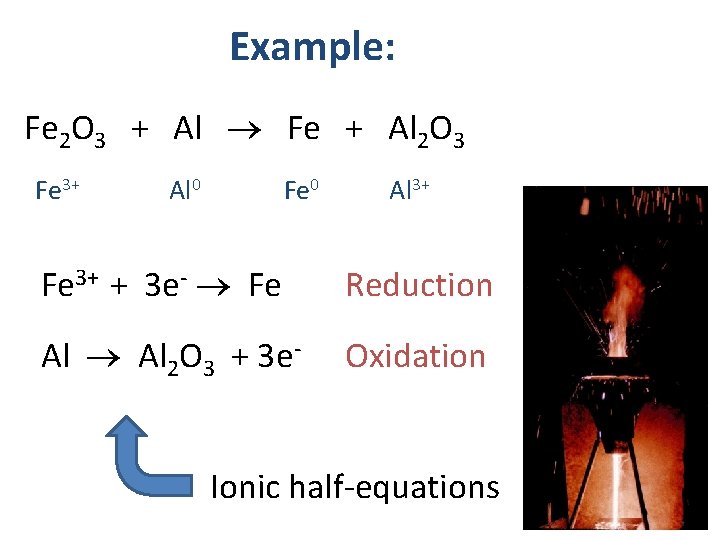
Example: Fe 2 O 3 + Al Fe + Al 2 O 3 Fe 3+ Al 0 Fe 0 Al 3+ Fe 3+ + 3 e- Fe Reduction Al 2 O 3 + 3 e- Oxidation Ionic half-equations

Remember: OIL - Oxidation Is Loss of electrons RIG - Reduction Is Gain of electrons And often. . . (as a quick and simple way to tell): Oxidation is gain in oxygen or loss of hydrogen Reduction is loss of oxygen or gain of hydrogen

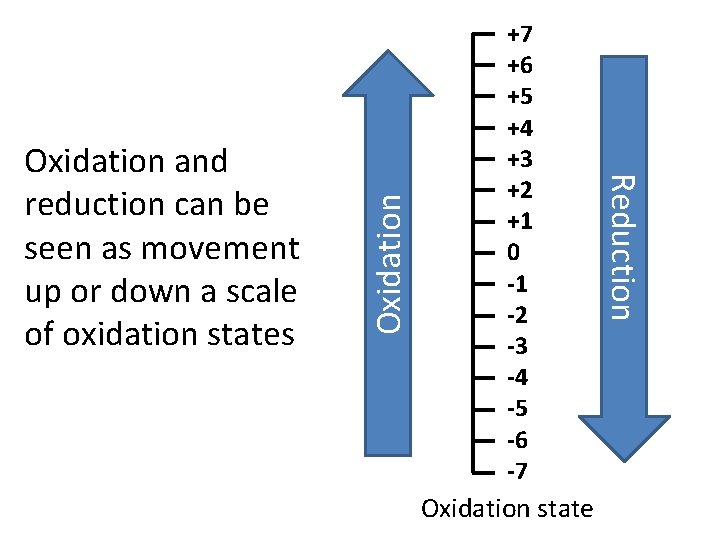
Oxidation state Oxidation Reduction Oxidation and reduction can be seen as movement up or down a scale of oxidation states +7 +6 +5 +4 +3 +2 +1 0 -1 -2 -3 -4 -5 -6 -7

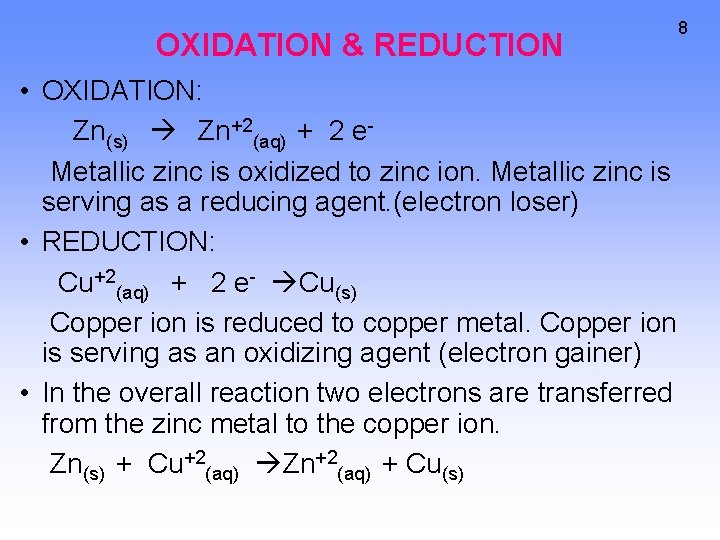
OXIDATION & REDUCTION • OXIDATION: Zn(s) Zn+2(aq) + 2 e. Metallic zinc is oxidized to zinc ion. Metallic zinc is serving as a reducing agent. (electron loser) • REDUCTION: Cu+2(aq) + 2 e- Cu(s) Copper ion is reduced to copper metal. Copper ion is serving as an oxidizing agent (electron gainer) • In the overall reaction two electrons are transferred from the zinc metal to the copper ion. Zn(s) + Cu+2(aq) Zn+2(aq) + Cu(s) 8

You can’t have one… without the other! • Reduction (gaining electrons) can’t happen without an oxidation to provide the electrons. • You can’t have 2 oxidations or 2 reductions in the same equation. Reduction has to occur at the cost of oxidation 9

OXIDATION NUMBERS 10 • Before discussing the mechanics of oxidation numbers it is important to realize that: • (1) oxidation numbers have no physical significance. They are merely a way to tell which substances gain or lose electrons and how many. • (2) oxidation numbers are assigned to atoms never molecules. Molecules contain atoms with oxidation numbers but they themselves cannot be assigned oxidation numbers. • (3) there a several rules used to assign oxidation numbers. They must be observed carefully.

OXIDATION NUMBERS RULES • (1) Elements have oxidation numbers of zero. For example Cu has an oxidation number of zero. In Cl 2 each atom of chlorine has an oxidation number of zero. • (2) The oxidation number of a monatomic ion (consisting of one atom) is the charge on the ion. In Cu+2 the oxidation number is +2. In Cl- the oxidation number is – 1. • (3) The oxidation number of combined oxygen is always – 2 except in peroxides such as hydrogen peroxide, H 2 O 2. In CO 2 each oxygen atom has an oxidation number of – 2. In SO 3 each oxygen is – 2. 11

FINDING UNKNOWN OXIDATION NUMBERS 12 • Problem: What is the oxidation number of Mn in Mn. O 4 -, the permanganate ion? • Solution: Mn is unknown Each O is – 2 All of the oxidation numbers must add up to – 1 Mn + 4(-2) = -1, Mn = +7 in permanganate • Problem: What is the oxidation number of Cr in K 2 Cr 2 O 7, potassium dichromate? • Solution: Cr is unknown Each K from Column I is +1 Each O is – 2 All the oxidation numbers add up to 0 (no charge is shown for K 2 Cr 2 O 7) 2(+1) + 2 Cr + 7(-2) = 0, 2 Cr = 12, Cr = 12 / 2 = +6 Cr = +6 in potassium dichromate

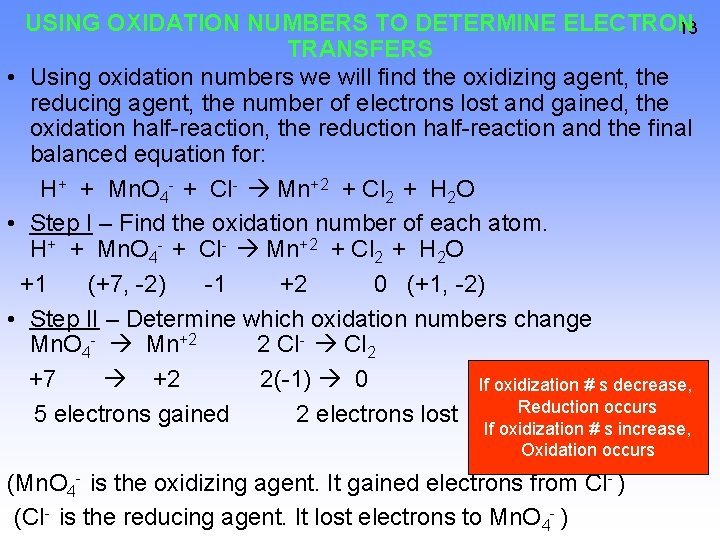
USING OXIDATION NUMBERS TO DETERMINE ELECTRON 13 TRANSFERS • Using oxidation numbers we will find the oxidizing agent, the reducing agent, the number of electrons lost and gained, the oxidation half-reaction, the reduction half-reaction and the final balanced equation for: H+ + Mn. O 4 - + Cl- Mn+2 + Cl 2 + H 2 O • Step I – Find the oxidation number of each atom. H+ + Mn. O 4 - + Cl- Mn+2 + Cl 2 + H 2 O +1 (+7, -2) -1 +2 0 (+1, -2) • Step II – Determine which oxidation numbers change Mn. O 4 - Mn+2 2 Cl- Cl 2 +7 +2 2(-1) 0 If oxidization # s decrease, Reduction occurs 5 electrons gained 2 electrons lost If oxidization # s increase, Oxidation occurs (Mn. O 4 - is the oxidizing agent. It gained electrons from Cl- ) (Cl- is the reducing agent. It lost electrons to Mn. O 4 - )

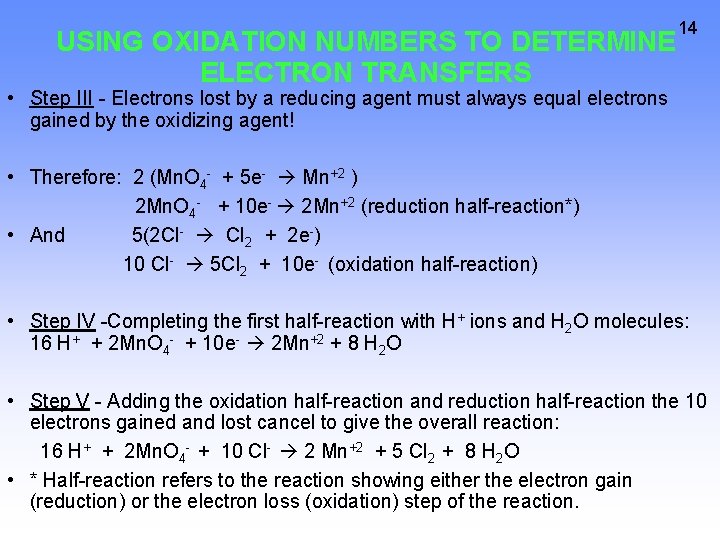
USING OXIDATION NUMBERS TO DETERMINE ELECTRON TRANSFERS 14 • Step III - Electrons lost by a reducing agent must always equal electrons gained by the oxidizing agent! • Therefore: 2 (Mn. O 4 - + 5 e- Mn+2 ) 2 Mn. O 4 - + 10 e- 2 Mn+2 (reduction half-reaction*) • And 5(2 Cl- Cl 2 + 2 e-) 10 Cl- 5 Cl 2 + 10 e- (oxidation half-reaction) • Step IV -Completing the first half-reaction with H+ ions and H 2 O molecules: 16 H+ + 2 Mn. O 4 - + 10 e- 2 Mn+2 + 8 H 2 O • Step V - Adding the oxidation half-reaction and reduction half-reaction the 10 electrons gained and lost cancel to give the overall reaction: 16 H+ + 2 Mn. O 4 - + 10 Cl- 2 Mn+2 + 5 Cl 2 + 8 H 2 O • * Half-reaction refers to the reaction showing either the electron gain (reduction) or the electron loss (oxidation) step of the reaction.

15 OXIDATION-REDUCTION REACTIONS Direct Redox Reaction Oxidizing and reducing agents in direct contact. Cu(s) + 2 Ag+(aq) ---> Cu 2+(aq) + 2 Ag(s)

16 OXIDATION-REDUCTION REACTIONS Indirect Redox Reaction A battery functions by transferring electrons through an external wire from the reducing agent to the oxidizing agent.

Why Study Electrochemistry? • Batteries • Corrosion • Industrial production of chemicals such as Cl 2, Na. OH, F 2 and Al • Biological redox reactions The heme group 17

18 Balancing Equations for Redox Reactions Some redox reactions have equations that must be balanced by special techniques. Mn. O 4 - + 5 Fe 2+ + 8 H+ ---> Mn 2+ + 5 Fe 3+ + 4 H 2 O Mn = +7 Fe = +2 Mn = +2 Fe = +3

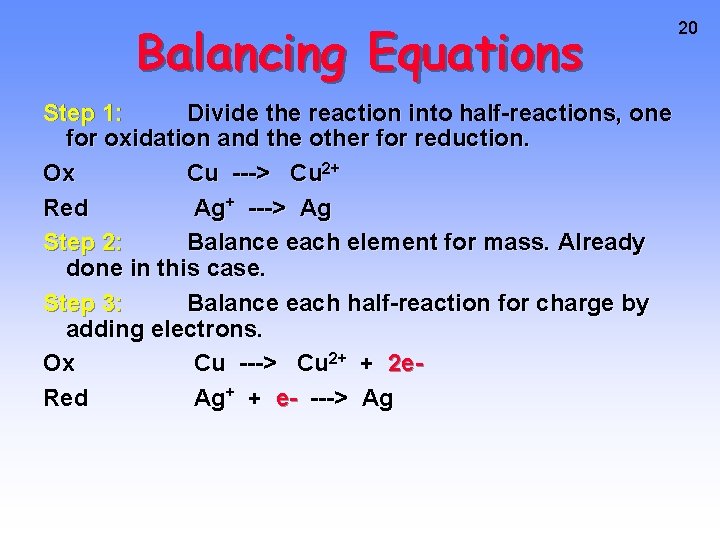
19 Balancing Equations Consider the reduction of Ag+ ions with copper metal. Cu + Ag+ --give--> Cu 2+ + Ag

Balancing Equations Step 1: Divide the reaction into half-reactions, one for oxidation and the other for reduction. Ox Cu ---> Cu 2+ Red Ag+ ---> Ag Step 2: Balance each element for mass. Already done in this case. Step 3: Balance each half-reaction for charge by adding electrons. Ox Cu ---> Cu 2+ + 2 e. Red Ag+ + e- ---> Ag 20

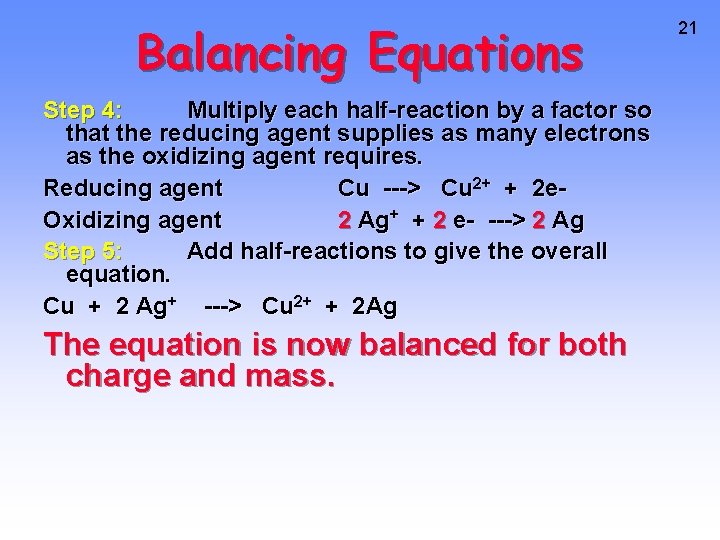
Balancing Equations Step 4: Multiply each half-reaction by a factor so that the reducing agent supplies as many electrons as the oxidizing agent requires. Reducing agent Cu ---> Cu 2+ + 2 e. Oxidizing agent 2 Ag+ + 2 e- ---> 2 Ag Step 5: Add half-reactions to give the overall equation. Cu + 2 Ag+ ---> Cu 2+ + 2 Ag The equation is now balanced for both charge and mass. 21

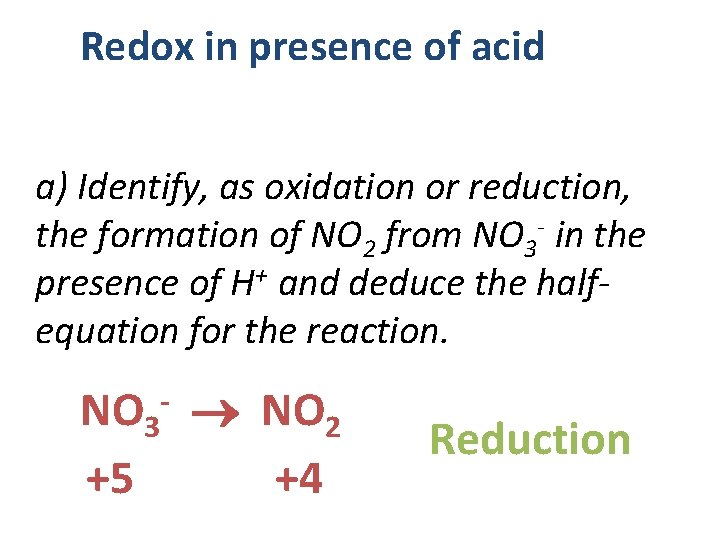
Redox in presence of acid a) Identify, as oxidation or reduction, the formation of NO 2 from NO 3 - in the presence of H+ and deduce the halfequation for the reaction. NO 3 NO 2 +5 +4 - Reduction

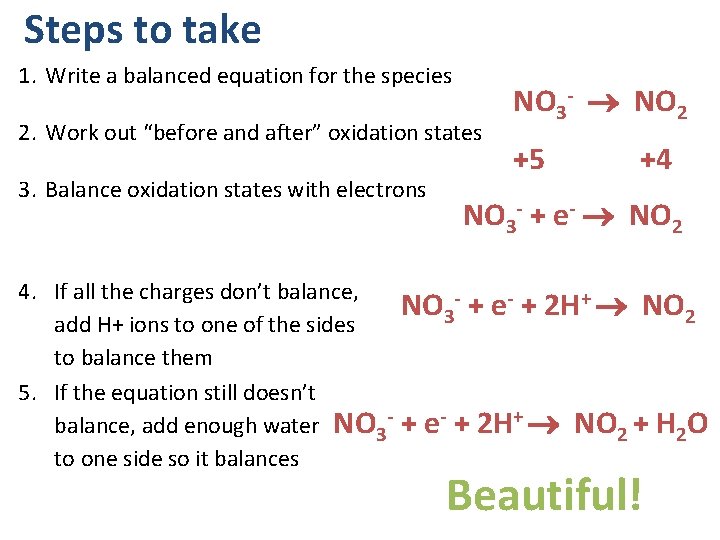
Steps to take 1. Write a balanced equation for the species 2. Work out “before and after” oxidation states 3. Balance oxidation states with electrons NO 3 - NO 2 +5 +4 NO 3 - + e- NO 2 4. If all the charges don’t balance, NO 3 - + e- + 2 H+ NO 2 add H+ ions to one of the sides to balance them 5. If the equation still doesn’t balance, add enough water NO 3 - + e- + 2 H+ NO 2 + H 2 O to one side so it balances Beautiful!

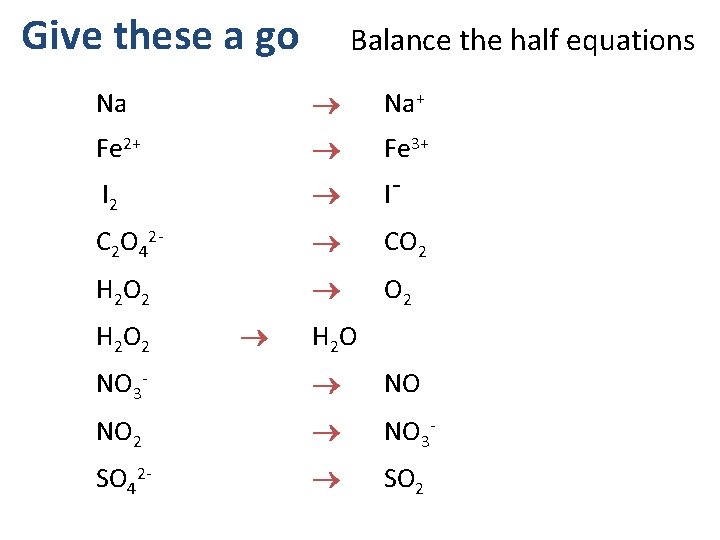
Give these a go Balance the half equations Na Na+ Fe 2+ Fe 3+ I 2 I¯ C 2 O 42 - CO 2 H 2 O NO 3 - NO NO 2 NO 3 - SO 42 - SO 2

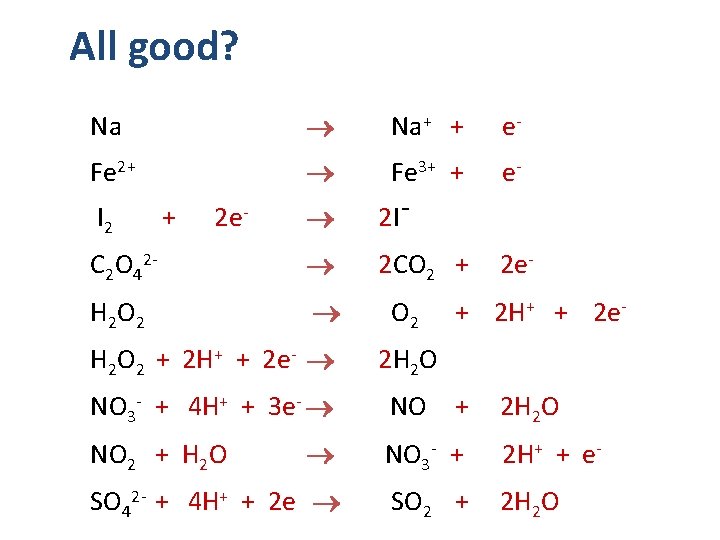
All good? Na Na+ + e- Fe 2+ Fe 3+ + e- 2 I¯ C 2 O 42 - 2 CO 2 + H 2 O 2 I 2 + 2 e- H 2 O 2 + 2 H+ + 2 e- O 2 2 e- + 2 H+ + 2 e- 2 H 2 O NO 3 - + 4 H+ + 3 e- NO + 2 H 2 O NO 3 - + 2 H+ + e- SO 42 - + 4 H+ + 2 e SO 2 + 2 H 2 O NO 2 + H 2 O

Combining half equations Just a mashing together of two half-equations! . . . followed by some satisfying cancelling-out.

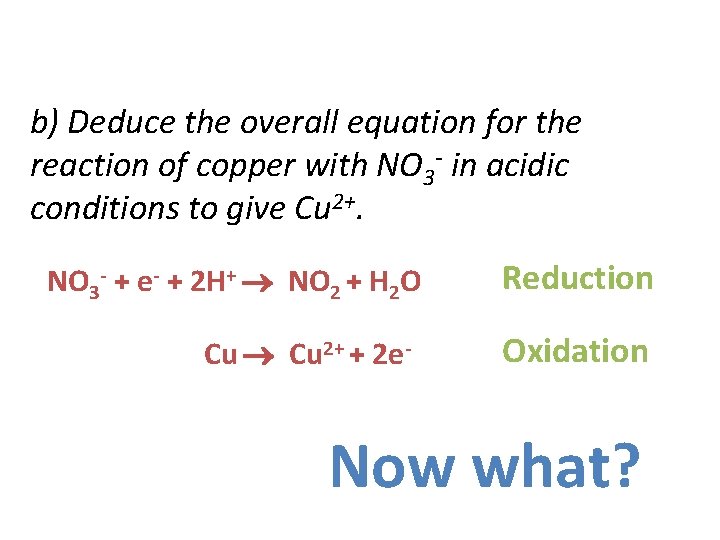
b) Deduce the overall equation for the reaction of copper with NO 3 - in acidic conditions to give Cu 2+. NO 3 - + e- + 2 H+ NO 2 + H 2 O Cu 2+ + 2 e- Reduction Oxidation Now what?

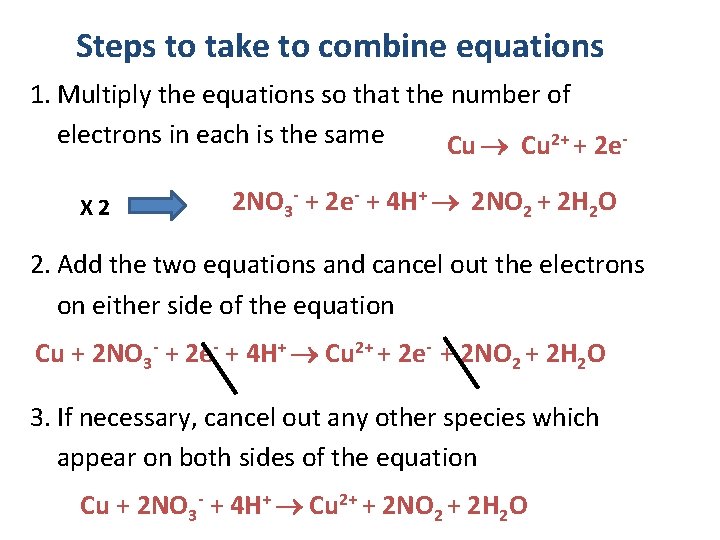
Steps to take to combine equations 1. Multiply the equations so that the number of electrons in each is the same Cu 2+ + 2 e. X 2 2 NO 3 - + 2 e- + 4 H+ 2 NO 2 + 2 H 2 O 2. Add the two equations and cancel out the electrons on either side of the equation Cu + 2 NO 3 - + 2 e- + 4 H+ Cu 2+ + 2 e- + 2 NO 2 + 2 H 2 O 3. If necessary, cancel out any other species which appear on both sides of the equation Cu + 2 NO 3 - + 4 H+ Cu 2+ + 2 NO 2 + 2 H 2 O

Give these a go 1. Fe 2+ ions are oxidised to Fe 3+ ions by Cl. O 3 - ions in acidic conditions. The Cl. O 3 - ions are reduced to Clions. Write the overall reaction. Fe 2+ Fe 3+ + e- + Cl. O 3 - + 6 e- + 6 H+ Cl- + 3 H 2 O 6 Fe 2+ + Cl. O 3 - + 6 H+ Fe 3+ + Cl- + 3 H 2 O 2. Write an overall reaction for Mn. O 4 - reducing H 2 O 2 to O 2 and creating Mn 2+. 2 Mn. O 4¯ + 5 H 2 O 2 + 6 H+ 2 Mn 2+ + 5 O 2 + 8 H 2 O

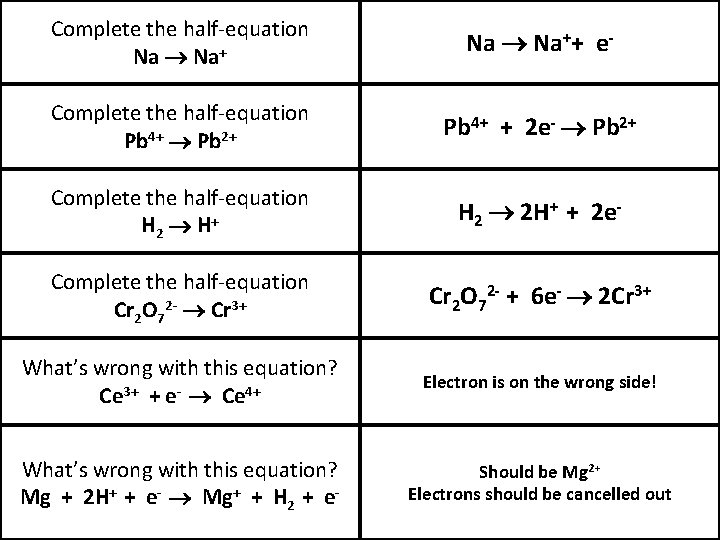
Complete the half-equation Na Na++ e- Complete the half-equation Pb 4+ Pb 2+ Pb 4+ + 2 e- Pb 2+ Complete the half-equation H 2 H+ H 2 2 H+ + 2 e- Complete the half-equation Cr 2 O 72 - Cr 3+ Cr 2 O 72 - + 6 e- 2 Cr 3+ What’s wrong with this equation? Ce 3+ + e- Ce 4+ Electron is on the wrong side! What’s wrong with this equation? Mg + 2 H+ + e- Mg+ + H 2 + e- Should be Mg 2+ Electrons should be cancelled out

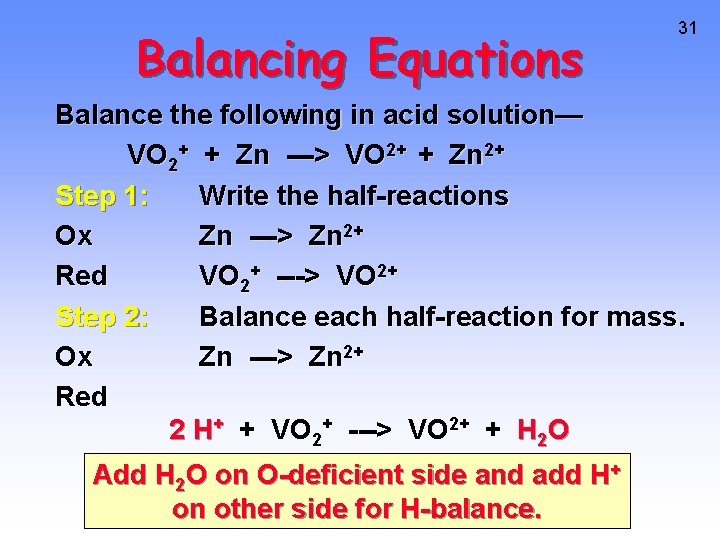
Balancing Equations 31 Balance the following in acid solution— VO 2+ + Zn ---> VO 2+ + Zn 2+ Step 1: Write the half-reactions Ox Zn ---> Zn 2+ Red VO 2+ ---> VO 2+ Step 2: Balance each half-reaction for mass. Ox Zn ---> Zn 2+ Red 2 H+ + VO 2+ ---> VO 2+ + H 2 O Add H 2 O on O-deficient side and add H+ on other side for H-balance.

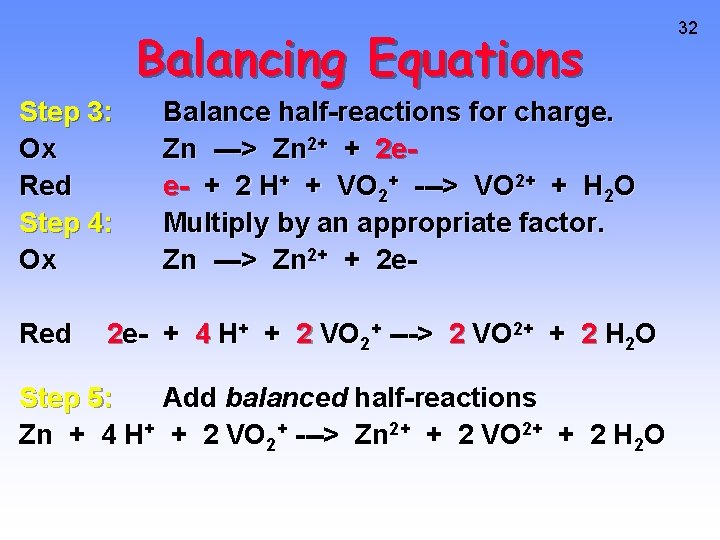
Balancing Equations Step 3: Ox Red Step 4: Ox Red Balance half-reactions for charge. Zn ---> Zn 2+ + 2 ee- + 2 H+ + VO 2+ ---> VO 2+ + H 2 O Multiply by an appropriate factor. Zn ---> Zn 2+ + 2 e- + 4 H+ + 2 VO 2+ ---> 2 VO 2+ + 2 H 2 O Step 5: Add balanced half-reactions Zn + 4 H+ + 2 VO 2+ ---> Zn 2+ + 2 VO 2+ + 2 H 2 O 32

33 Tips on Balancing Equations • Never add O 2, O atoms, or O 2 - to balance oxygen. • Never add H 2 or H atoms to balance hydrogen. • Be sure to write the correct charges on all the ions. • Check your work at the end to make sure mass and charge are balanced. • PRACTICE!

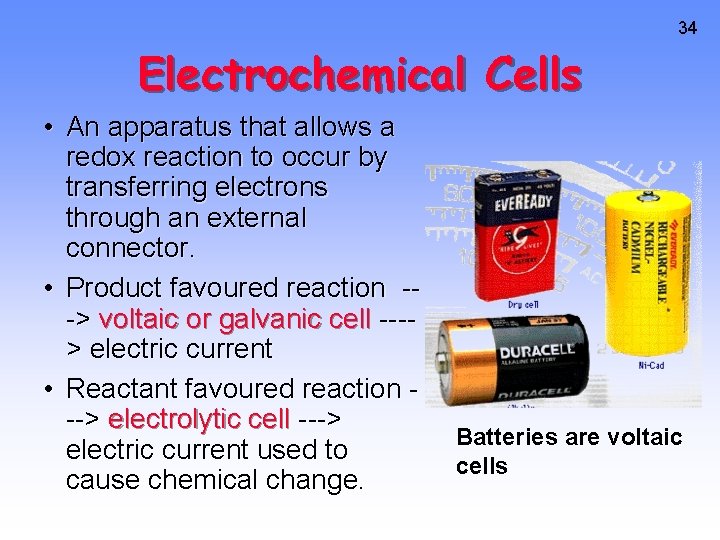
34 Electrochemical Cells • An apparatus that allows a redox reaction to occur by transferring electrons through an external connector. • Product favoured reaction --> voltaic or galvanic cell ---> electric current • Reactant favoured reaction --> electrolytic cell ---> electric current used to cause chemical change. Batteries are voltaic cells

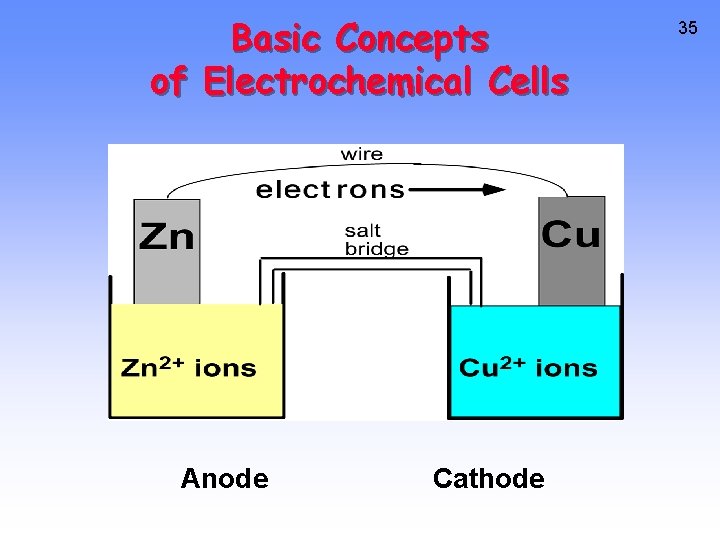
Basic Concepts of Electrochemical Cells Anode Cathode 35

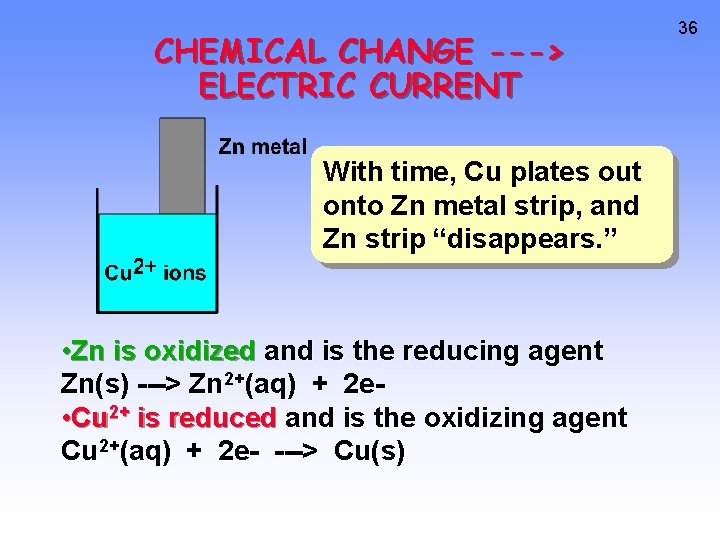
CHEMICAL CHANGE ---> ELECTRIC CURRENT With time, Cu plates out onto Zn metal strip, and Zn strip “disappears. ” • Zn is oxidized and is the reducing agent Zn(s) ---> Zn 2+(aq) + 2 e • Cu 2+ is reduced and is the oxidizing agent Cu 2+(aq) + 2 e- ---> Cu(s) 36

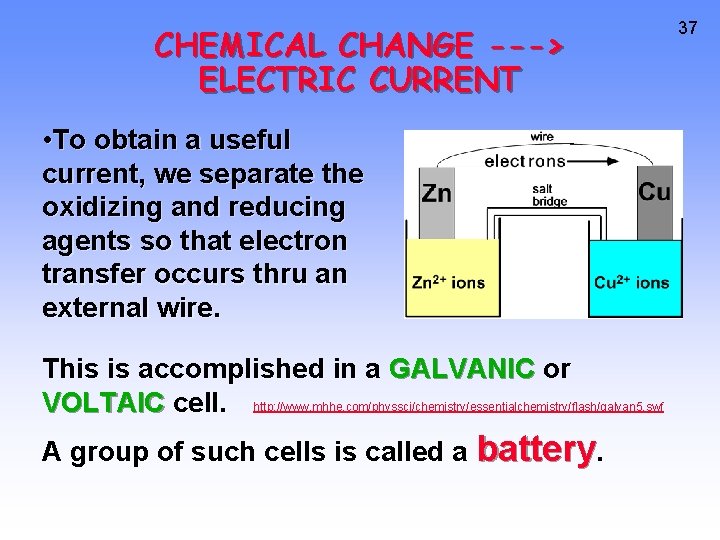
CHEMICAL CHANGE ---> ELECTRIC CURRENT • To obtain a useful current, we separate the oxidizing and reducing agents so that electron transfer occurs thru an external wire. This is accomplished in a GALVANIC or VOLTAIC cell. http: //www. mhhe. com/physsci/chemistry/essentialchemistry/flash/galvan 5. swf A group of such cells is called a battery. 37

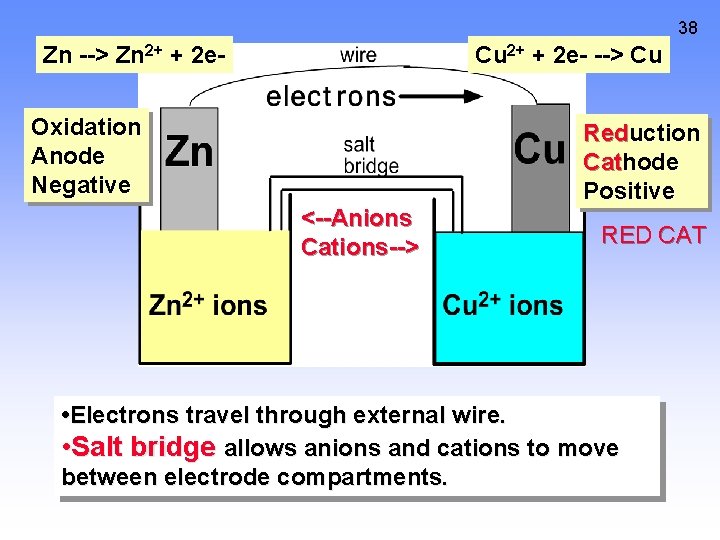
38 Zn --> Zn 2+ + 2 e- Cu 2+ + 2 e- --> Cu Oxidation Anode Negative <--Anions Cations--> Reduction Cathode Positive RED CAT • Electrons travel through external wire. • Salt bridge allows anions and cations to move between electrode compartments.

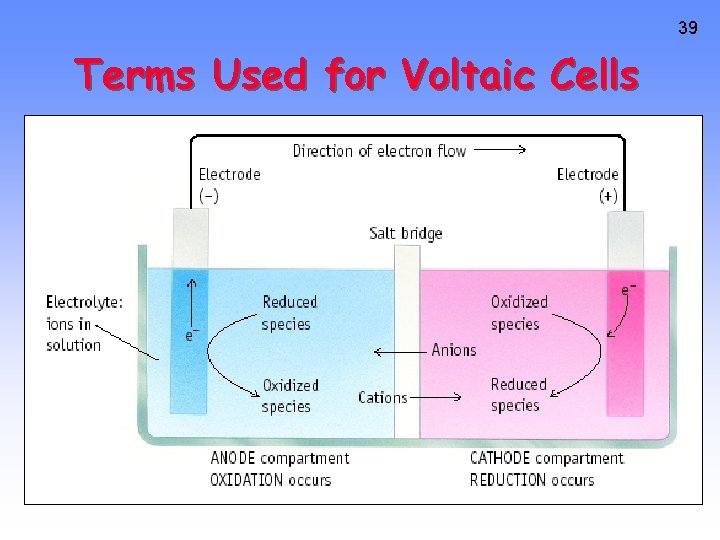
39 Terms Used for Voltaic Cells

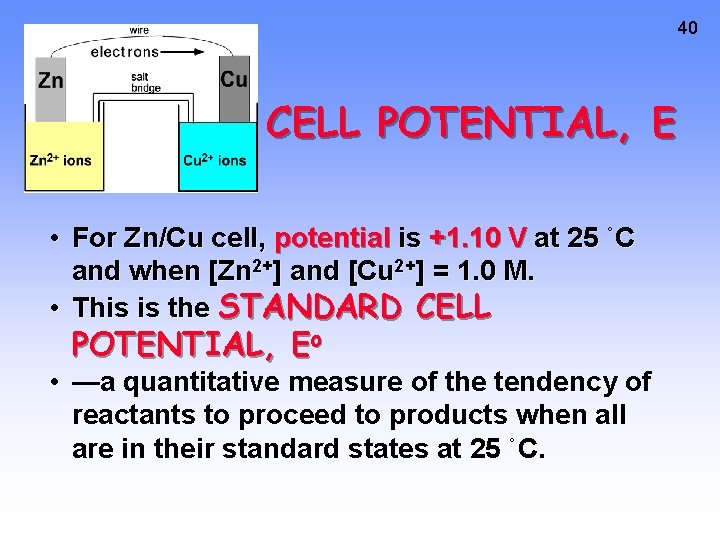
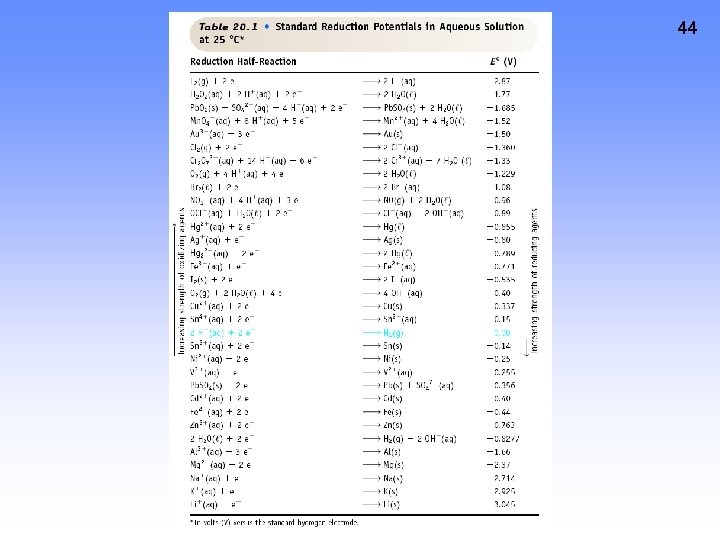
40 CELL POTENTIAL, E • For Zn/Cu cell, potential is +1. 10 V at 25 ˚C and when [Zn 2+] and [Cu 2+] = 1. 0 M. • This is the STANDARD CELL POTENTIAL, Eo • —a quantitative measure of the tendency of reactants to proceed to products when all are in their standard states at 25 ˚C.

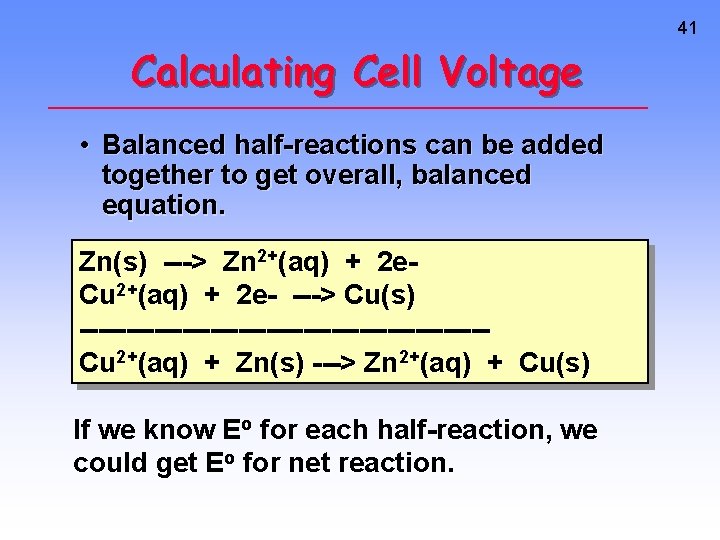
41 Calculating Cell Voltage • Balanced half-reactions can be added together to get overall, balanced equation. Zn(s) ---> Zn 2+(aq) + 2 e. Cu 2+(aq) + 2 e- ---> Cu(s) ----------------------Cu 2+(aq) + Zn(s) ---> Zn 2+(aq) + Cu(s) If we know Eo for each half-reaction, we could get Eo for net reaction.

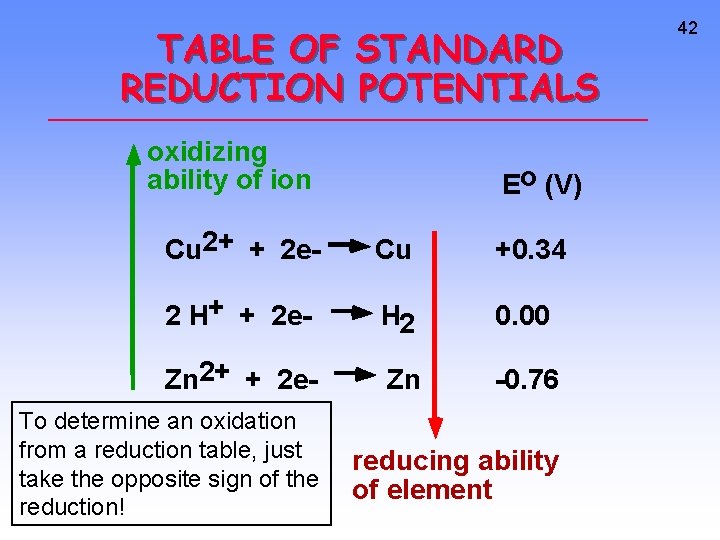
TABLE OF STANDARD REDUCTION POTENTIALS oxidizing ability of ion Eo (V) Cu 2+ + 2 e- Cu +0. 34 2 H+ + 2 e- H 2 0. 00 Zn 2+ + 2 e- Zn -0. 76 To determine an oxidation from a reduction table, just take the opposite sign of the reduction! reducing ability of element 42

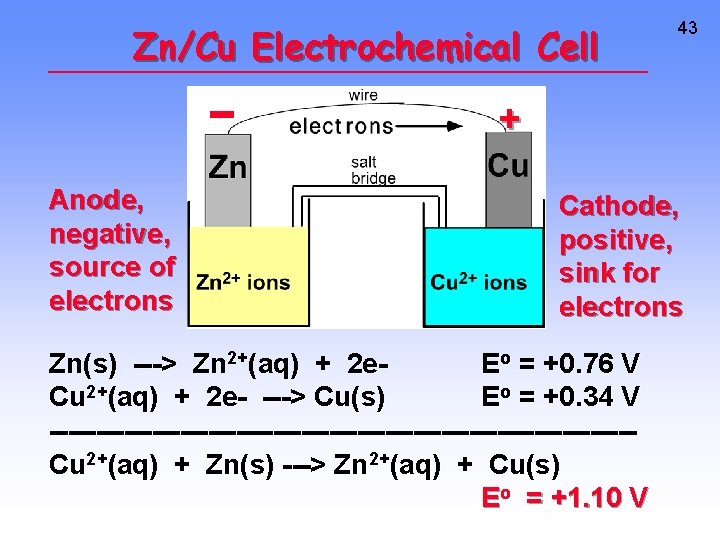
Zn/Cu Electrochemical Cell 43 + Anode, negative, source of electrons Cathode, positive, sink for electrons Zn(s) ---> Zn 2+(aq) + 2 e. Eo = +0. 76 V Cu 2+(aq) + 2 e- ---> Cu(s) Eo = +0. 34 V -------------------------------Cu 2+(aq) + Zn(s) ---> Zn 2+(aq) + Cu(s) Eo = +1. 10 V

44

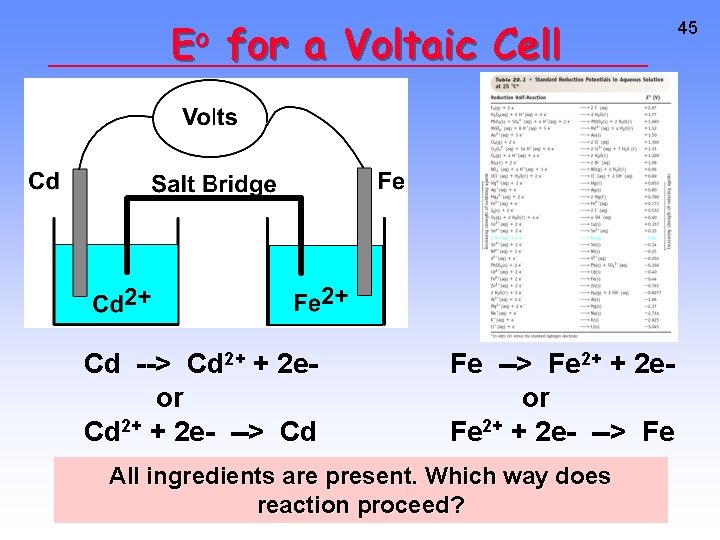
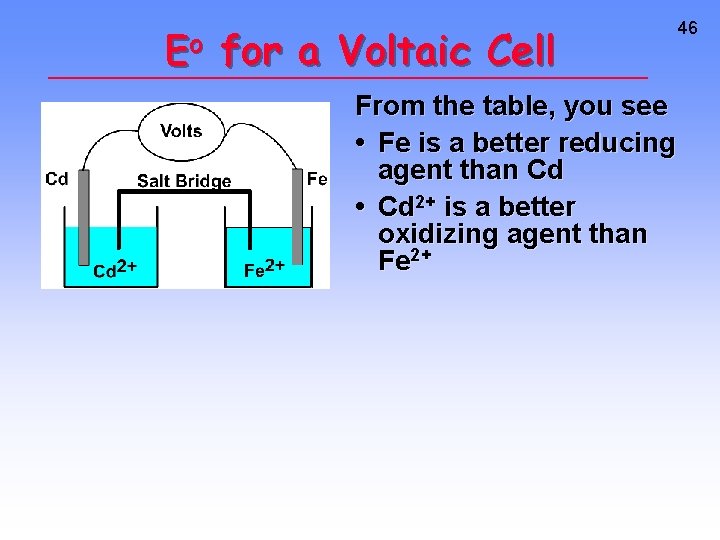
Eo for a Voltaic Cell Cd --> Cd 2+ + 2 eor Cd 2+ + 2 e- --> Cd Fe --> Fe 2+ + 2 eor Fe 2+ + 2 e- --> Fe All ingredients are present. Which way does reaction proceed? 45

Eo for a Voltaic Cell From the table, you see • Fe is a better reducing agent than Cd • Cd 2+ is a better oxidizing agent than Fe 2+ 46

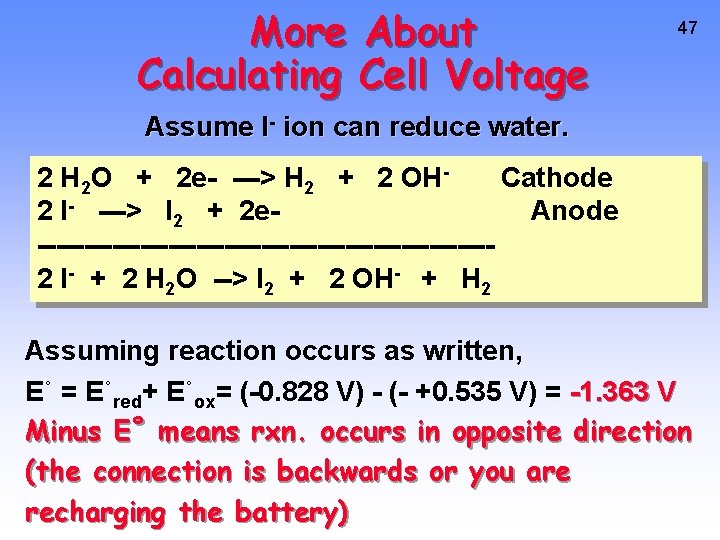
More About Calculating Cell Voltage 47 Assume I- ion can reduce water. 2 H 2 O + 2 e- ---> H 2 + 2 OHCathode 2 I- ---> I 2 + 2 e. Anode ------------------------2 I- + 2 H 2 O --> I 2 + 2 OH- + H 2 Assuming reaction occurs as written, E˚ = E˚red+ E˚ox= (-0. 828 V) - (- +0. 535 V) = -1. 363 V Minus E˚ means rxn. occurs in opposite direction (the connection is backwards or you are recharging the battery)

Charging a Battery When you charge a battery, you are forcing the electrons backwards (from the + to the -). To do this, you will need a higher voltage backwards than forwards. This is why the ammeter in your car often goes slightly higher while your battery is charging, and then returns to normal. In your car, the battery charger is called an alternator. If you have a dead battery, it could be the battery needs to be replaced OR the alternator is not charging the battery properly. 48

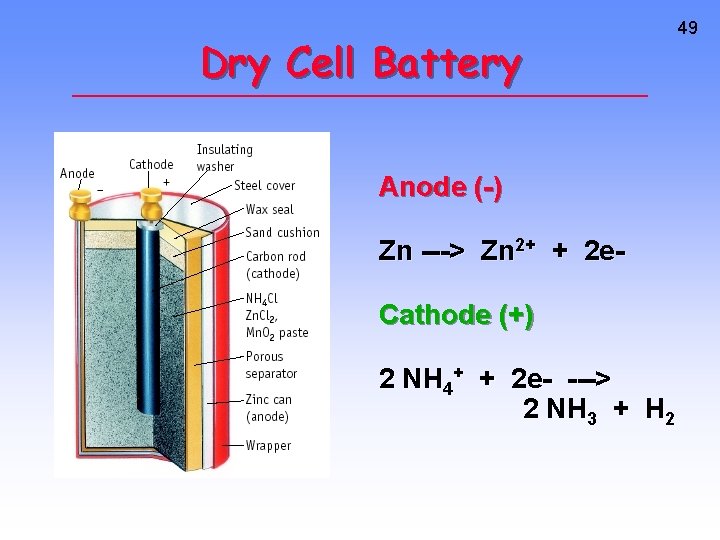
Dry Cell Battery Anode (-) Zn ---> Zn 2+ + 2 e. Cathode (+) 2 NH 4+ + 2 e- ---> 2 NH 3 + H 2 49

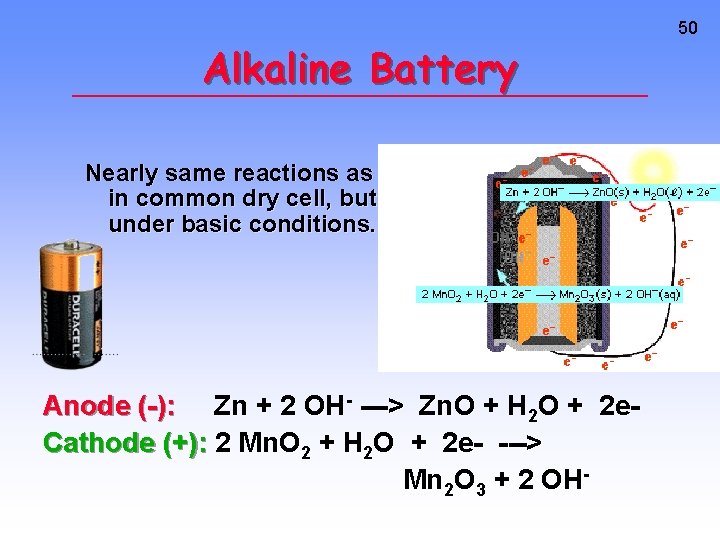
Alkaline Battery Nearly same reactions as in common dry cell, but under basic conditions. Anode (-): Zn + 2 OH- ---> Zn. O + H 2 O + 2 e. Cathode (+): 2 Mn. O 2 + H 2 O + 2 e- ---> Mn 2 O 3 + 2 OH- 50

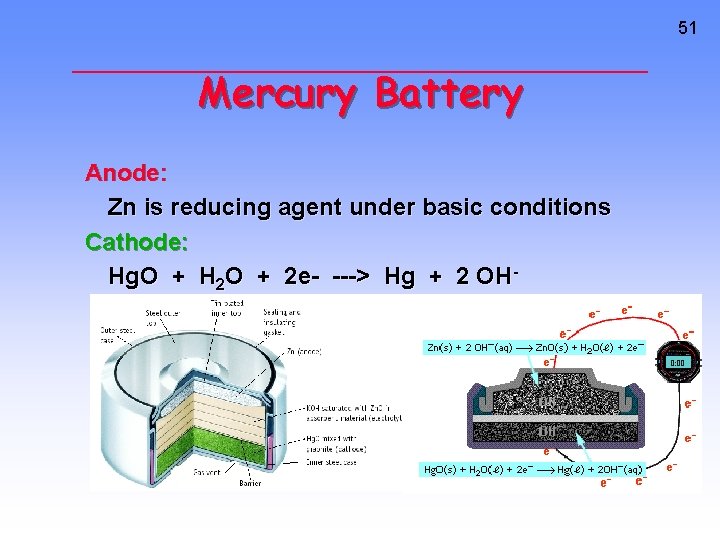
51 Mercury Battery Anode: Zn is reducing agent under basic conditions Cathode: Hg. O + H 2 O + 2 e- ---> Hg + 2 OH-

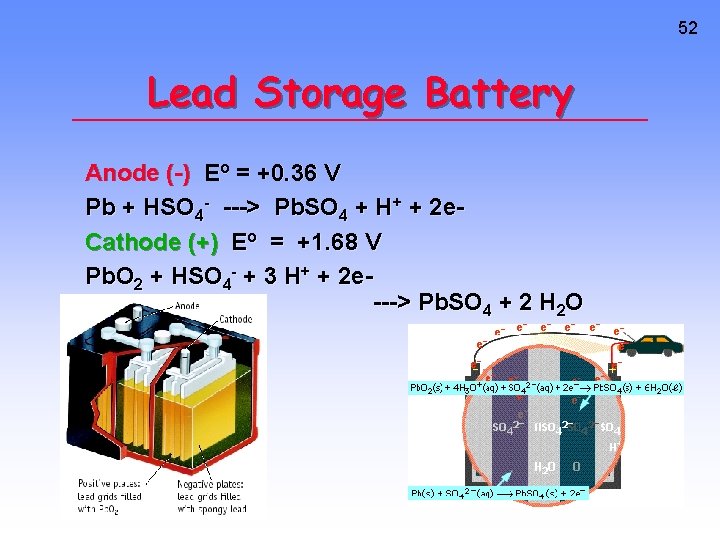
52 Lead Storage Battery Anode (-) Eo = +0. 36 V Pb + HSO 4 - ---> Pb. SO 4 + H+ + 2 e. Cathode (+) Eo = +1. 68 V Pb. O 2 + HSO 4 - + 3 H+ + 2 e---> Pb. SO 4 + 2 H 2 O

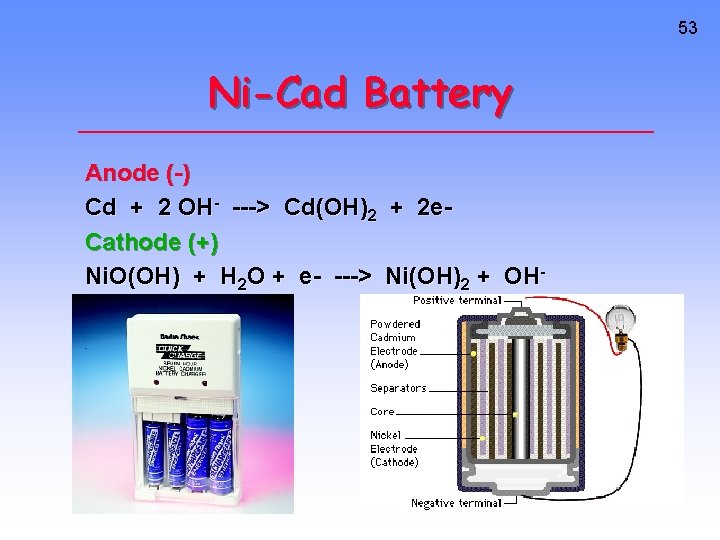
53 Ni-Cad Battery Anode (-) Cd + 2 OH- ---> Cd(OH)2 + 2 e. Cathode (+) Ni. O(OH) + H 2 O + e- ---> Ni(OH)2 + OH-

H 2 as a Fuel Cars can use electricity generated by H 2/O 2 fuel cells. H 2 carried in tanks or generated from hydrocarbons 54