Electrochemistry homework Chapter 18 Electrochemistry AP Practice test










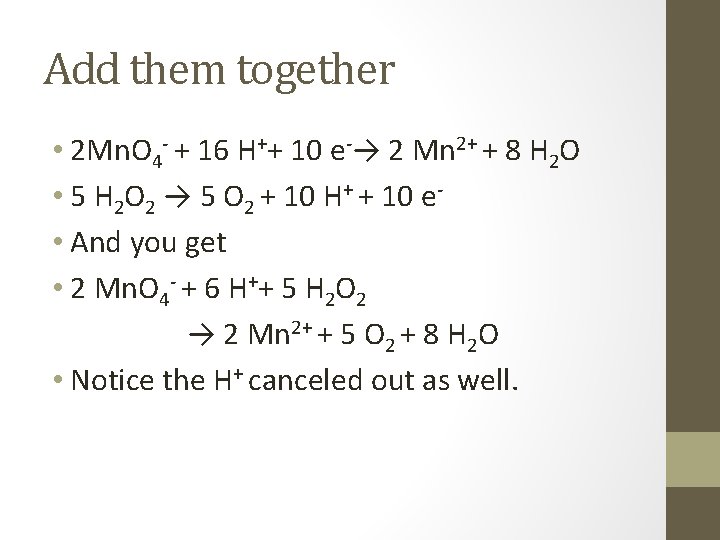





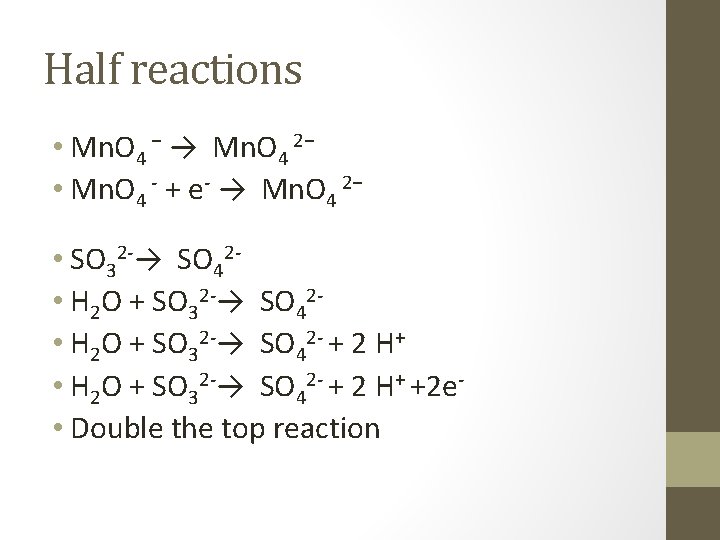

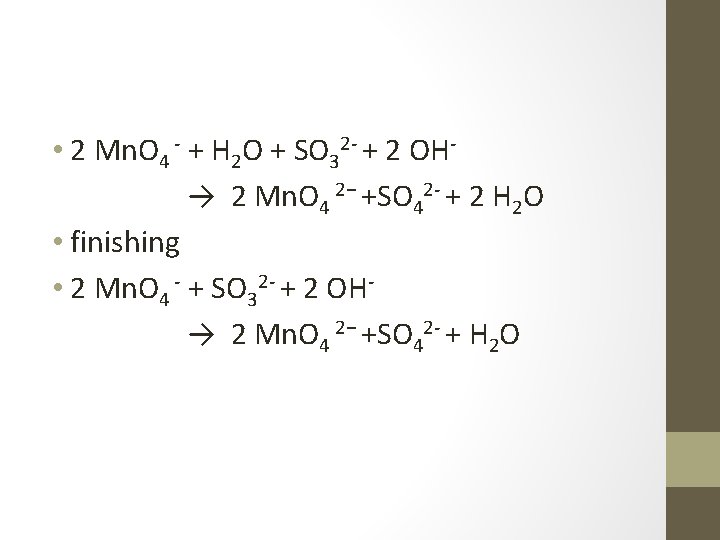







- Slides: 42

Electrochemistry

homework • Chapter 18 • Electrochemistry • AP Practice test

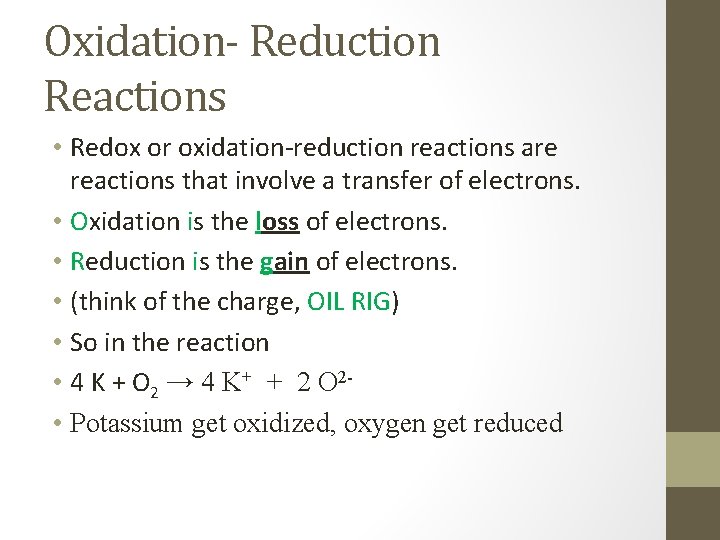
Oxidation- Reduction Reactions • Redox or oxidation-reduction reactions are reactions that involve a transfer of electrons. • Oxidation is the loss of electrons. • Reduction is the gain of electrons. • (think of the charge, OIL RIG) • So in the reaction • 4 K + O 2 → 4 K+ + 2 O 2 • Potassium get oxidized, oxygen get reduced

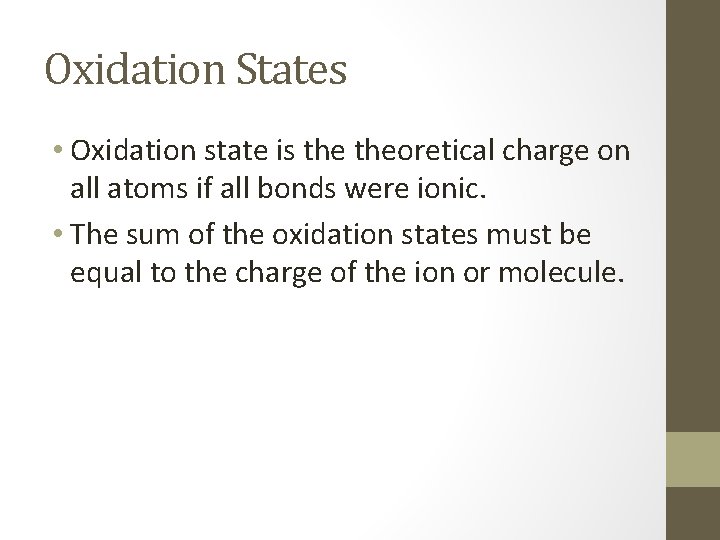
Oxidation States • Oxidation state is theoretical charge on all atoms if all bonds were ionic. • The sum of the oxidation states must be equal to the charge of the ion or molecule.

Using oxidation states • In the reaction… • 2 Na +2 H 2 O → 2 Na. OH + H 2 • 0 +1 -2 +1 0 • Note the changes • Sodium went from 0 to 1 • 2 of the hydrogen atoms went from +1 to 0 (the other two were unchanged)

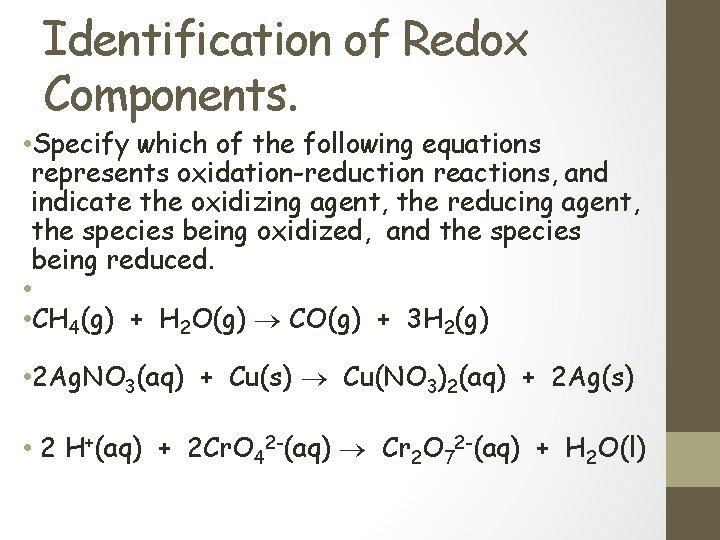
Identification of Redox Components. • Specify which of the following equations represents oxidation-reduction reactions, and indicate the oxidizing agent, the reducing agent, the species being oxidized, and the species being reduced. • • CH 4(g) + H 2 O(g) CO(g) + 3 H 2(g) • 2 Ag. NO 3(aq) + Cu(s) Cu(NO 3)2(aq) + 2 Ag(s) • 2 H+(aq) + 2 Cr. O 42 -(aq) Cr 2 O 72 -(aq) + H 2 O(l)

• CH 4(g) + H 2 O(g) CO(g) + 3 H 2(g) • -4 +1 +1 -2 +4 -2 0 • C is oxidized, and H is reduced • C is reducing agent, H is oxidizing agent • 2 Ag. NO 3(aq) + Cu(s) Cu(NO 3)2(aq) + 2 Ag(s) • +1 +5 -2 0 +2 +5 -2 0 • Ag is reduced, Cu is oxidized • Ag is oxidizing agent, Cu is reducing agent • Net Ionic • 2 Ag+(aq) + Cu(s) Cu 2+ (aq) + 2 Ag(s) • +1 0 +2 0 • Ag is reduced, Cu is oxidized • 2 H+(aq) + 2 Cr. O 42 -(aq) Cr 2 O 72 -(aq) + H 2 O(l) • +1 +6 -2 +1 -2 • This is not a redox reaction

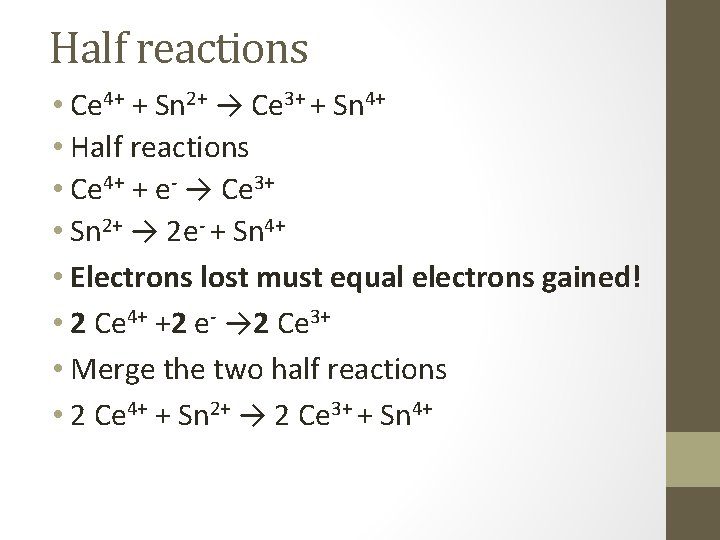
Half reactions • Ce 4+ + Sn 2+ → Ce 3+ + Sn 4+ • Half reactions • Ce 4+ + e- → Ce 3+ • Sn 2+ → 2 e- + Sn 4+ • Electrons lost must equal electrons gained! • 2 Ce 4+ +2 e- → 2 Ce 3+ • Merge the two half reactions • 2 Ce 4+ + Sn 2+ → 2 Ce 3+ + Sn 4+

Redox reactions in acidic solutions • It will be noted in the problem • Balance all elements except hydrogen and oxygen. • Balance oxygen by adding H 2 O (which is always prevalent in an acidic solution) • Balance hydrogen by adding H+ • Then balance the charge adding electrons and proceed normally.

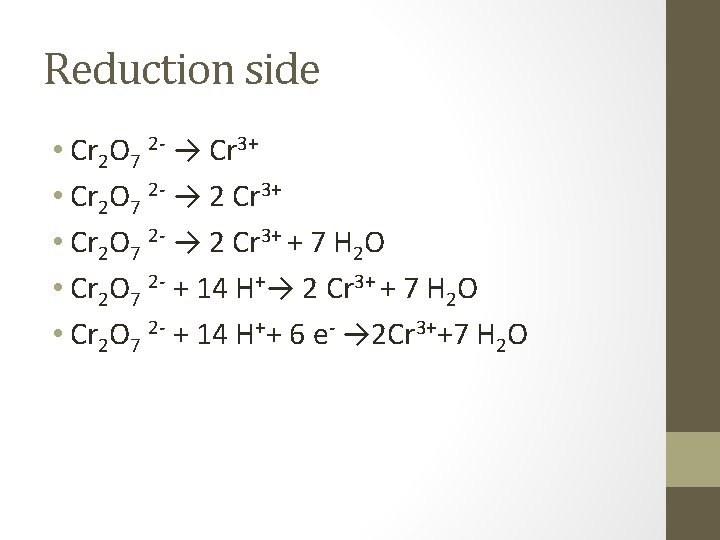
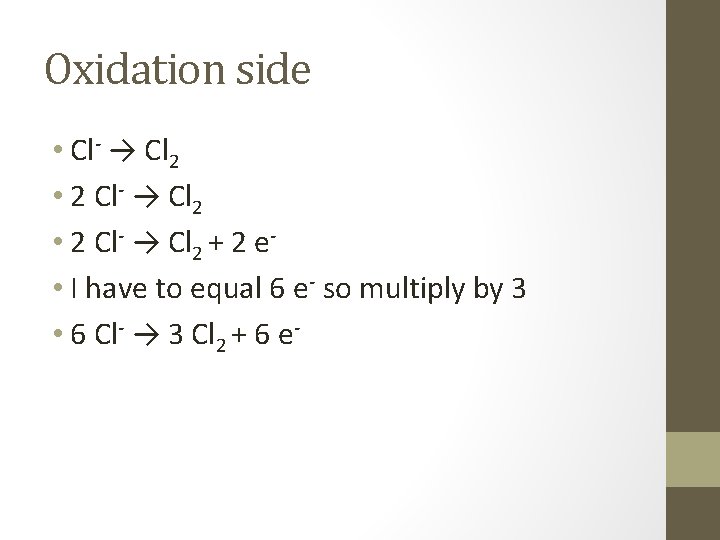
Example • In an acidic solution • Cr 2 O 7 2 - + Cl- → Cr 3+ + Cl 2 • Half reactions • Cr 2 O 7 2 - → Cr 3+ • Cl- → Cl 2

Reduction side • Cr 2 O 7 2 - → Cr 3+ • Cr 2 O 7 2 - → 2 Cr 3+ + 7 H 2 O • Cr 2 O 7 2 - + 14 H+→ 2 Cr 3+ + 7 H 2 O • Cr 2 O 7 2 - + 14 H++ 6 e- → 2 Cr 3++7 H 2 O

Oxidation side • Cl- → Cl 2 • 2 Cl- → Cl 2 + 2 e • I have to equal 6 e- so multiply by 3 • 6 Cl- → 3 Cl 2 + 6 e-

Combine my half reactions • Cr 2 O 7 2 - + 14 H++ 6 e- → 2 Cr 3+ + 7 H 2 O • 6 Cl- → 3 Cl 2 + 6 e • And you get • Cr 2 O 7 2 -+14 H++6 Cl-→ 2 Cr 3++3 Cl 2+7 H 2 O • The electrons cancel out.

Example • In an acidic solution • Mn. O 4 - + H 2 O 2 → Mn 2+ + O 2 • Half reactions • Mn. O 4 - → Mn 2+ • H 2 O 2 → O 2

Top Equation • Mn. O 4 - → Mn 2+ + 4 H 2 O • Mn. O 4 - + 8 H+→ Mn 2+ + 4 H 2 O • Mn. O 4 - + 8 H++ 5 e-→ Mn 2+ + 4 H 2 O

Bottom Equation • H 2 O 2 → O 2 + 2 H + + 2 e • I need to equal 5 e- so… • That won’t work… • 2 Mn. O 4 - + 16 H++ 10 e-→ 2 Mn 2+ + 8 H 2 O • 5 H 2 O 2 → 5 O 2 + 10 H+ + 10 e-
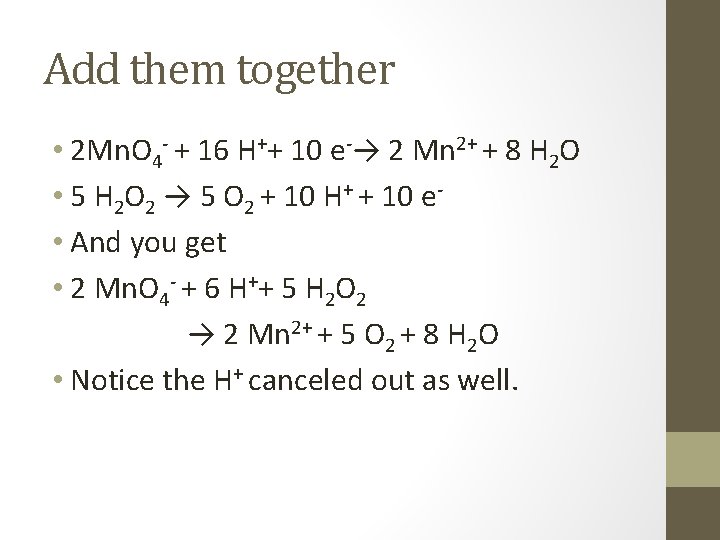
Add them together • 2 Mn. O 4 - + 16 H++ 10 e-→ 2 Mn 2+ + 8 H 2 O • 5 H 2 O 2 → 5 O 2 + 10 H+ + 10 e • And you get • 2 Mn. O 4 - + 6 H++ 5 H 2 O 2 → 2 Mn 2+ + 5 O 2 + 8 H 2 O • Notice the H+ canceled out as well.

Balancing Redox Equations in a basic solution • Look for the words basic or alkaline • Follow all rules for an acidic solution. • After you have completed the acidic reaction add OH- to each side to neutralize any H+. • Combine OH- and H+ to make H 2 O. • Cancel out any extra waters from both sides of the equation.

Example • We will use the same equation as before • In a basic solution • Mn. O 4 - + H 2 O 2 → Mn 2+ + O 2 • 2 Mn. O 4 - + 6 H++ 5 H 2 O 2 → 2 Mn 2+ + 5 O 2 + 8 H 2 O

Basic solution • Since this is a basic solution we can’t have excess H+. • We will add OH- to each side to neutralize all H+ • 2 Mn. O 4 - + 6 H++ 5 H 2 O 2 + 6 OH→ 2 Mn 2+ +5 O 2 +8 H 2 O + 6 OH • We added 6 OH- because there were 6 H+

Cont. • H+ + OH- → H 2 O • Combine the hydroxide and hydrogen on the reactant side to make water • 2 Mn. O 4 - + 6 H 2 O + 5 H 2 O 2 → 2 Mn 2++ 5 O 2+ 8 H 2 O + 6 OH • Cancel out waters on both sides 2 Mn. O 4 - + 5 H 2 O 2 → 2 Mn 2+ + 5 O 2 +2 H 2 O +6 OH-

Another example • In a basic solution • Mn. O 4 − + SO 32 -→Mn. O 4 2− + SO 42 • Half reactions • Mn. O 4 − → Mn. O 4 2− • SO 32 -→ SO 42 -
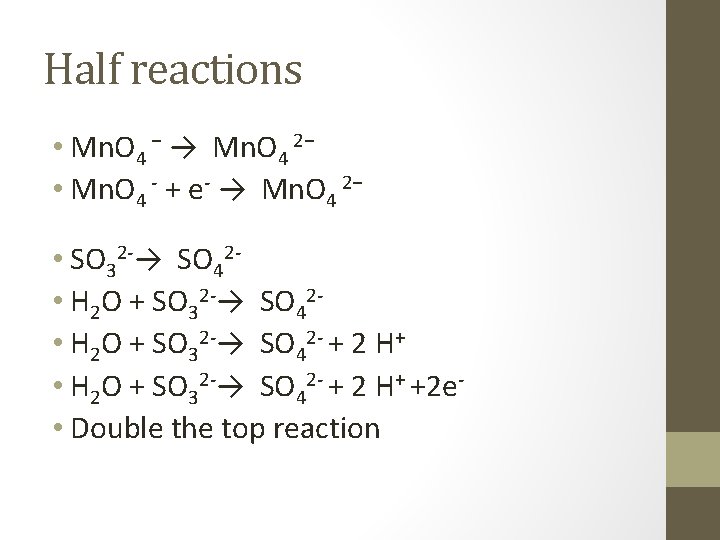
Half reactions • Mn. O 4 − → Mn. O 4 2− • Mn. O 4 - + e- → Mn. O 4 2− • SO 32 -→ SO 42 • H 2 O + SO 32 -→ SO 42 - + 2 H+ +2 e • Double the top reaction

• 2 Mn. O 4 - + 2 e- → 2 Mn. O 4 2− • H 2 O + SO 32 -→ SO 42 - + 2 H+ +2 e • Combine them • 2 Mn. O 4 - + H 2 O + SO 32→ 2 Mn. O 4 2− +SO 42 - + 2 H+ • Add OH 2 Mn. O 4 - + H 2 O + SO 32 - + 2 OH→ 2 Mn. O 4 2−+SO 42 - +2 H++2 OH-
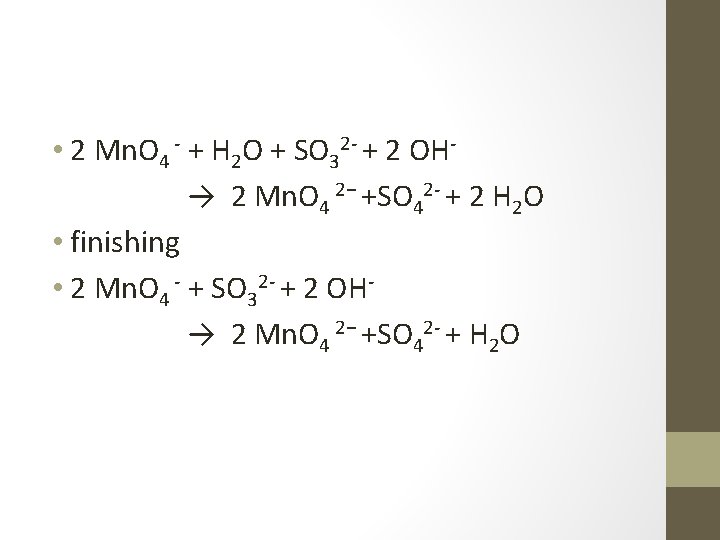
• 2 Mn. O 4 - + H 2 O + SO 32 - + 2 OH→ 2 Mn. O 4 2− +SO 42 - + 2 H 2 O • finishing • 2 Mn. O 4 - + SO 32 - + 2 OH→ 2 Mn. O 4 2− +SO 42 - + H 2 O

Major Redox Points to Remember. • Any redox reaction can be treated as the sum of the reduction and oxidation halfreactions. • Mass (atoms) and charge are conserved in each half-reaction. • Electrons lost in one half-reaction are gained in the other. • Even though the half-reactions are treated separately, electron loss and electron gain occur simultaneously.

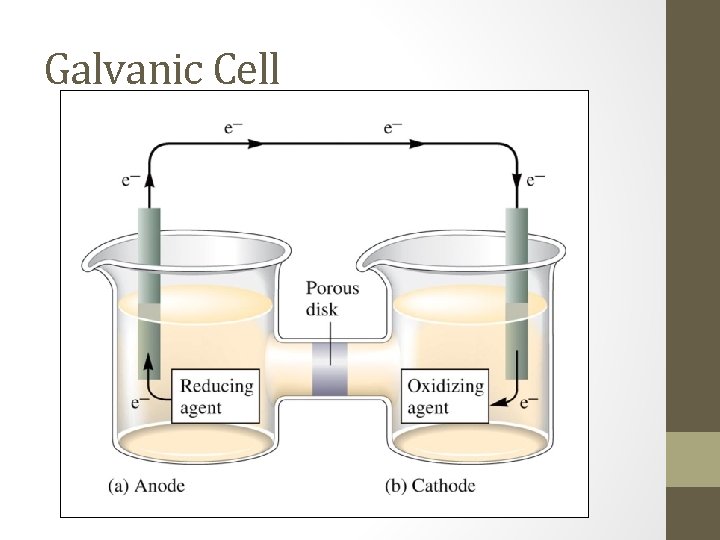
Galvanic Cell

Galvanic cell • A galvanic cell is a device in which chemical energy is changed to electrical energy. • A galvanic cell uses a thermodynanamically favored or spontaneous redox reaction to produce an electric current that can be used to do work. The system does work on the surroundings. • A redox reaction involves the transfer of electrons from the reducing agent to the oxidizing agent.

Oxidation v reduction • Oxidation. • * Involves a loss of electrons. • * Increase in oxidation number. • * “To get more positive. ” • * Occurs at the anode of a galvanic cell. • An Ox • Reduction. • * Involves a gain of electrons. • * Decrease in oxidation number. • * “To get more negative. ” • * Occurs at the cathode of a galvanic cell. • Red Cat

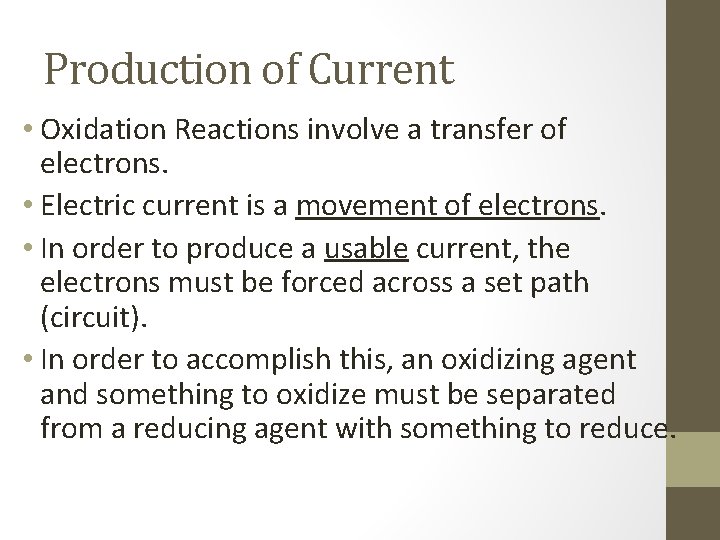
Production of Current • Oxidation Reactions involve a transfer of electrons. • Electric current is a movement of electrons. • In order to produce a usable current, the electrons must be forced across a set path (circuit). • In order to accomplish this, an oxidizing agent and something to oxidize must be separated from a reducing agent with something to reduce.

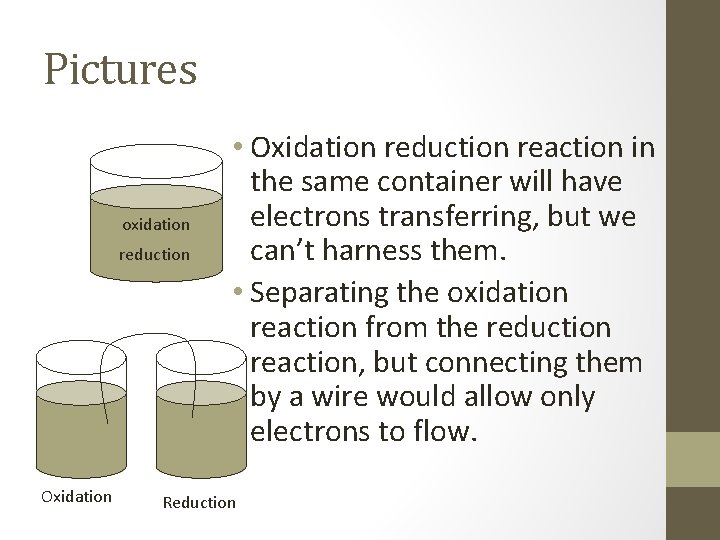
Pictures oxidation reduction Oxidation • Oxidation reduction reaction in the same container will have electrons transferring, but we can’t harness them. • Separating the oxidation reaction from the reduction reaction, but connecting them by a wire would allow only electrons to flow. Reduction

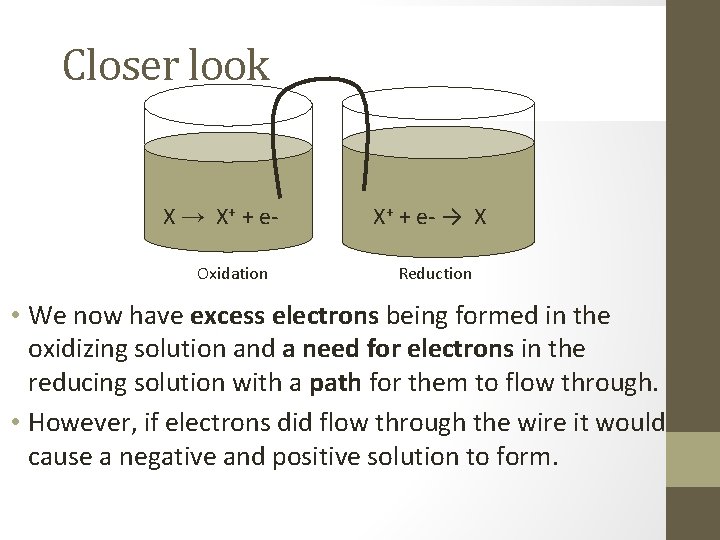
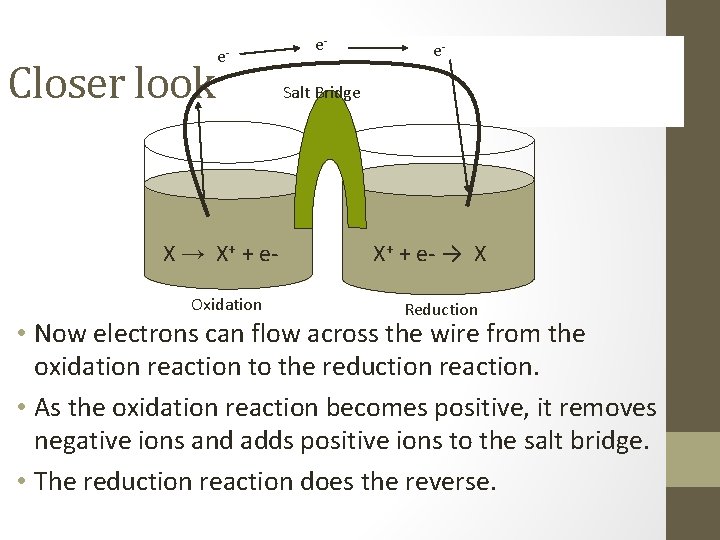
Closer look X → X+ + e. Oxidation X+ + e- → X Reduction • We now have excess electrons being formed in the oxidizing solution and a need for electrons in the reducing solution with a path for them to flow through. • However, if electrons did flow through the wire it would cause a negative and positive solution to form.

That’s not possible • Or at least it would require a lot of energy. • A negative solution would theoretically be formed by adding electrons, and a positive one by removing electrons. • The negative solution would then repel the electrons and stop them from flowing in, and a positive solution would attract the electrons pulling them back where they came from. • Making it so the charged solutions wouldn’t form. • In order for this to work, I would need a way for ions to flow back and forth but keeping the solutions mostly separated.

The Salt Bridge or the Porous Disk. • These devices allow ion flow to occur (circuit completion) without mixing the solutions. They are typically made of sodium sulfate or potassium nitrate

Closer look e- X → X+ + e. Oxidation e- e- Salt Bridge X+ + e- → X Reduction • Now electrons can flow across the wire from the oxidation reaction to the reduction reaction. • As the oxidation reaction becomes positive, it removes negative ions and adds positive ions to the salt bridge. • The reduction reaction does the reverse.

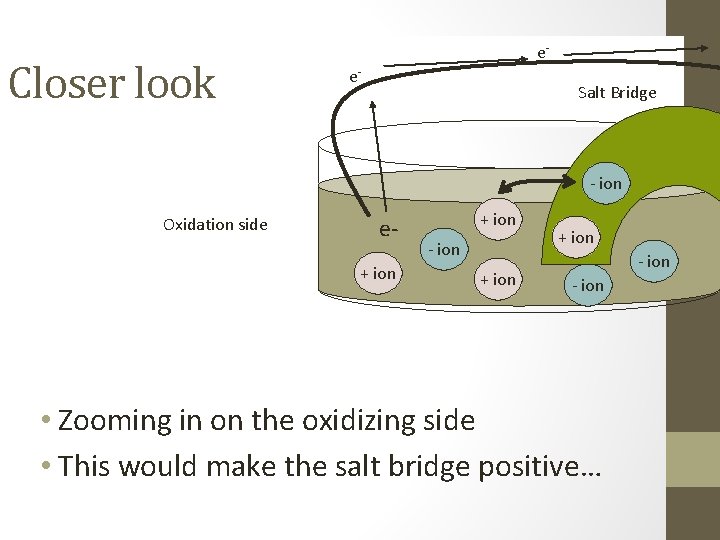
Closer look ee- Salt Bridge - ion Oxidation side e+ ion - ion • Zooming in on the oxidizing side • This would make the salt bridge positive…

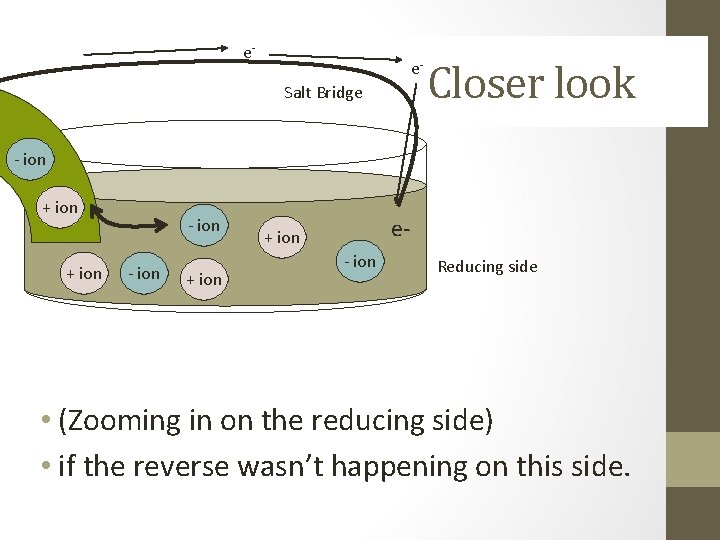
e- e. Salt Bridge Closer look - ion + ion e- + ion - ion Reducing side • (Zooming in on the reducing side) • if the reverse wasn’t happening on this side.

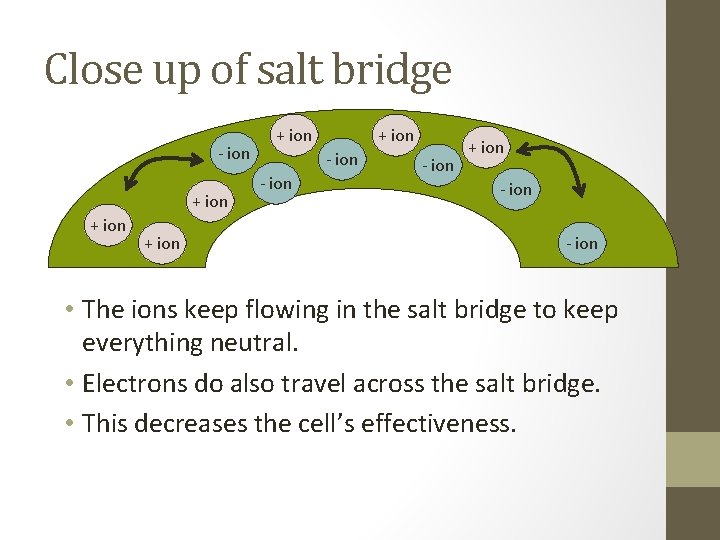
Close up of salt bridge - ion + ion + ion - ion + ion - ion • The ions keep flowing in the salt bridge to keep everything neutral. • Electrons do also travel across the salt bridge. • This decreases the cell’s effectiveness.

Electrochemical cell • This is the basic unit of a battery. • It is also called a galvanic cell, batteries have two or more galvanic cells linked together. • Electrochemical cells always have two terminals. • The terminal where oxidation occurs is called the anode. • The terminal where reduction occurs is called the cathode.

Galvanic Cell

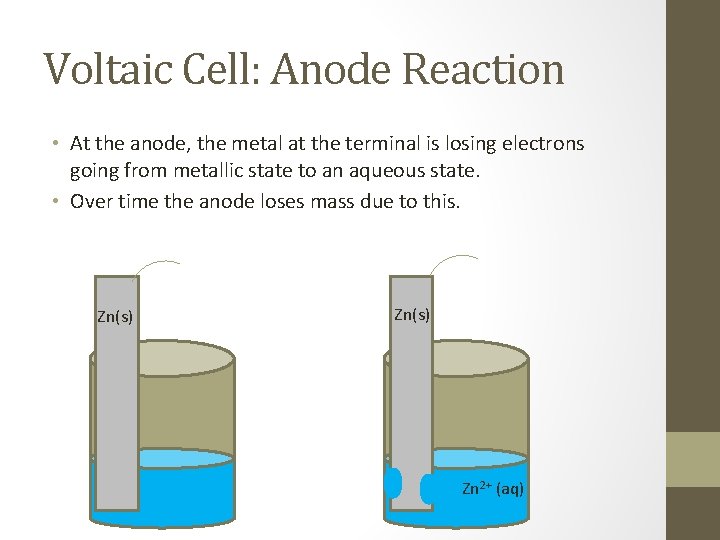
Voltaic Cell: Anode Reaction • At the anode, the metal at the terminal is losing electrons going from metallic state to an aqueous state. • Over time the anode loses mass due to this. Zn(s) Zn 2+ (aq)

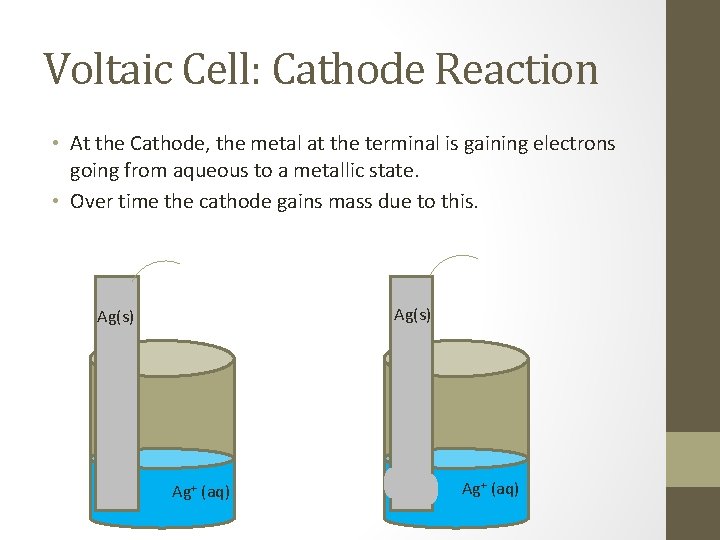
Voltaic Cell: Cathode Reaction • At the Cathode, the metal at the terminal is gaining electrons going from aqueous to a metallic state. • Over time the cathode gains mass due to this. Ag(s) Ag+ (aq)