Chapter 21 Standardized Test Prep Preview Multiple Choice

































- Slides: 37

Chapter 21 Standardized Test Prep Preview • Multiple Choice • Short Response • Extended Response © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Multiple Choice 1. What is another word for “quantum of light”? A. B. C. D. blackbody radiation energy level frequency photon © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Multiple Choice 1. What is another word for “quantum of light”? A. B. C. D. blackbody radiation energy level frequency photon © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Multiple Choice, continued 2. According to classical physics, when a light illuminates a photosensitive surface, what should determine how long it takes before electrons are ejected from the surface? F. G. H. J. frequency intensity photon energy wavelength © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Multiple Choice, continued 2. According to classical physics, when a light illuminates a photosensitive surface, what should determine how long it takes before electrons are ejected from the surface? F. G. H. J. frequency intensity photon energy wavelength © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Multiple Choice, continued 3. According to Einstein’s photon theory of light, what does the intensity of light shining on a metal determine? A. the number of photons hitting the metal in a given time interval B. the energy of photons hitting the metal C. whether or not photoelectrons will be emitted D. KEmax of emitted photoelectrons © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Multiple Choice, continued 3. According to Einstein’s photon theory of light, what does the intensity of light shining on a metal determine? A. the number of photons hitting the metal in a given time interval B. the energy of photons hitting the metal C. whether or not photoelectrons will be emitted D. KEmax of emitted photoelectrons © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Multiple Choice, continued 4. An X-ray photon is scattered by a stationary electron. How does the frequency of this scattered photon compare to its frequency before being scattered? F. G. H. J. The new frequency is higher. The new frequency is lower. The frequency stays the same. The scattered photon has no frequency. © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Multiple Choice, continued 4. An X-ray photon is scattered by a stationary electron. How does the frequency of this scattered photon compare to its frequency before being scattered? F. G. H. J. The new frequency is higher. The new frequency is lower. The frequency stays the same. The scattered photon has no frequency. © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Multiple Choice, continued 5. Which of the following summarizes Thomson’s model of the atom? A. Atoms are hard, uniform, indestructible spheres. B. Electrons are embedded in a sphere of positive charge. C. Electrons orbit the nucleus in the same way that planets orbit the sun. D. Electrons exist only at discrete energy levels. © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Multiple Choice, continued 5. Which of the following summarizes Thomson’s model of the atom? A. Atoms are hard, uniform, indestructible spheres. B. Electrons are embedded in a sphere of positive charge. C. Electrons orbit the nucleus in the same way that planets orbit the sun. D. Electrons exist only at discrete energy levels. © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Multiple Choice, continued 6. What happens when an electron moves from a higher energy level to a lower energy level in an atom? F. Energy is absorbed from a source outside the atom. G. The energy contained in the electromagnetic field inside the atom increases. H. Energy is released across a continuous range of values. J. A photon is emitted with energy equal to the difference in energy between the two levels. © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Multiple Choice, continued 6. What happens when an electron moves from a higher energy level to a lower energy level in an atom? F. Energy is absorbed from a source outside the atom. G. The energy contained in the electromagnetic field inside the atom increases. H. Energy is released across a continuous range of values. J. A photon is emitted with energy equal to the difference in energy between the two levels. © Houghton Mifflin Harcourt Publishing Company

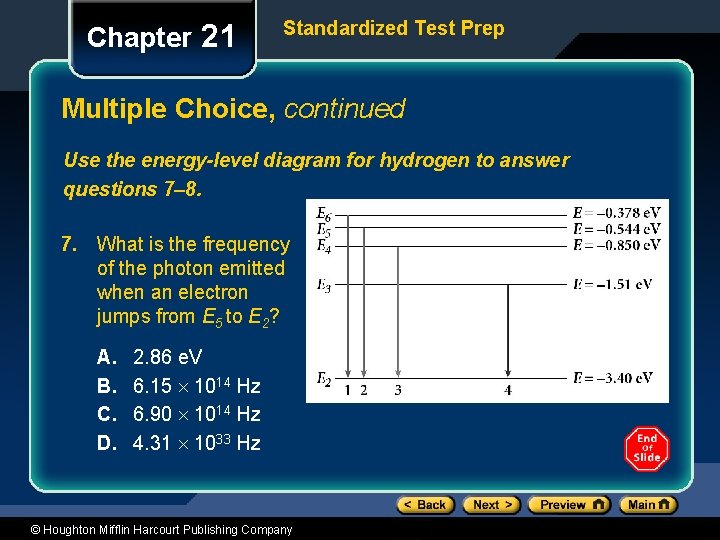
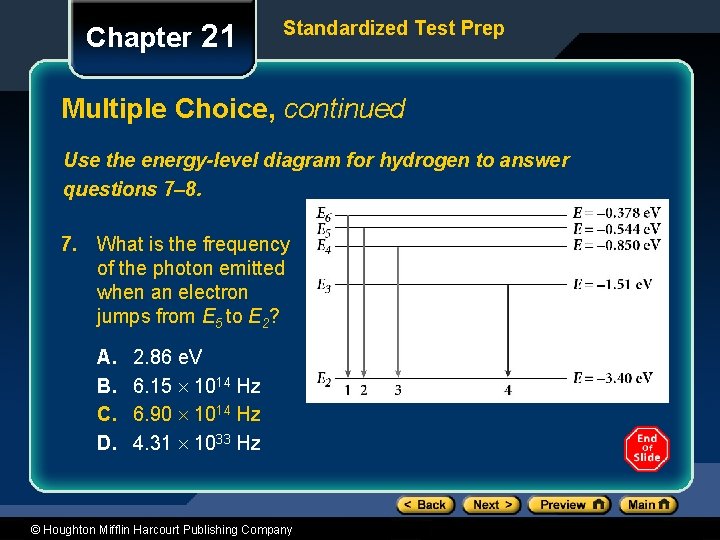
Chapter 21 Standardized Test Prep Multiple Choice, continued Use the energy-level diagram for hydrogen to answer questions 7– 8. 7. What is the frequency of the photon emitted when an electron jumps from E 5 to E 2? A. B. C. D. 2. 86 e. V 6. 15 1014 Hz 6. 90 1014 Hz 4. 31 1033 Hz © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Multiple Choice, continued Use the energy-level diagram for hydrogen to answer questions 7– 8. 7. What is the frequency of the photon emitted when an electron jumps from E 5 to E 2? A. B. C. D. 2. 86 e. V 6. 15 1014 Hz 6. 90 1014 Hz 4. 31 1033 Hz © Houghton Mifflin Harcourt Publishing Company

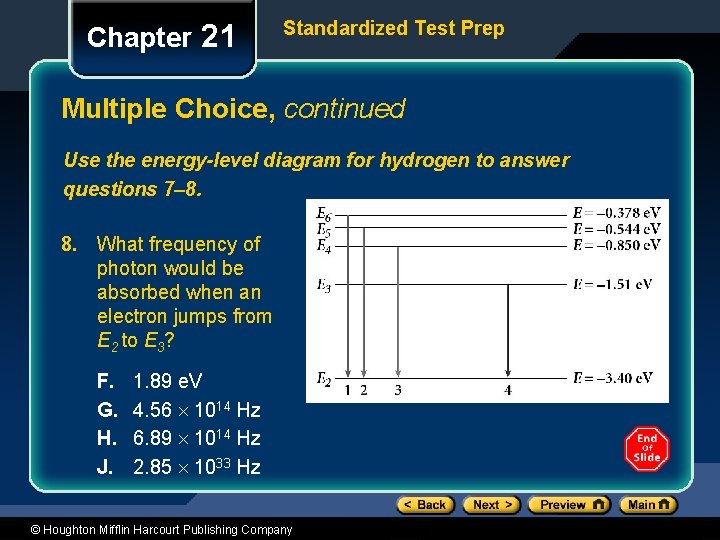
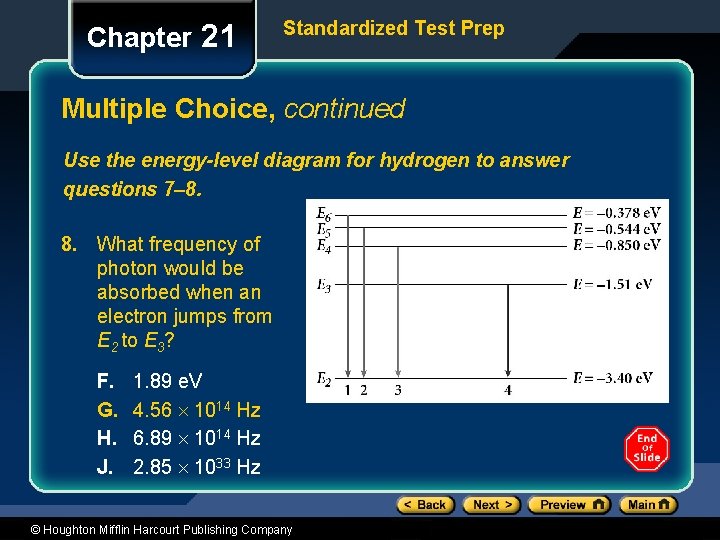
Chapter 21 Standardized Test Prep Multiple Choice, continued Use the energy-level diagram for hydrogen to answer questions 7– 8. 8. What frequency of photon would be absorbed when an electron jumps from E 2 to E 3? F. G. H. J. 1. 89 e. V 4. 56 1014 Hz 6. 89 1014 Hz 2. 85 1033 Hz © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Multiple Choice, continued Use the energy-level diagram for hydrogen to answer questions 7– 8. 8. What frequency of photon would be absorbed when an electron jumps from E 2 to E 3? F. G. H. J. 1. 89 e. V 4. 56 1014 Hz 6. 89 1014 Hz 2. 85 1033 Hz © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Multiple Choice, continued 9. What type of spectrum is created by applying a high potential difference to a pure atomic gas? A. B. C. D. an emission spectrum an absorption spectrum a continuous spectrum a visible spectrum © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Multiple Choice, continued 9. What type of spectrum is created by applying a high potential difference to a pure atomic gas? A. B. C. D. an emission spectrum an absorption spectrum a continuous spectrum a visible spectrum © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Multiple Choice, continued 10. What type of spectrum is used to identify elements in the atmospheres of stars? F. G. H. J. an emission spectrum an absorption spectrum a continuous spectrum a visible spectrum © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Multiple Choice, continued 10. What type of spectrum is used to identify elements in the atmospheres of stars? F. G. H. J. an emission spectrum an absorption spectrum a continuous spectrum a visible spectrum © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Multiple Choice, continued 11. What is the speed of a proton (m = 1. 67 10– 27 kg) with a de Broglie wavelength of 4. 00 10– 14 m? A. B. C. D. 1. 59 10– 30 m/s 1. 01 10– 7 m/s 9. 93 106 m/s 1. 01 107 m/s © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Multiple Choice, continued 11. What is the speed of a proton (m = 1. 67 10– 27 kg) with a de Broglie wavelength of 4. 00 10– 14 m? A. B. C. D. 1. 59 10– 30 m/s 1. 01 10– 7 m/s 9. 93 106 m/s 1. 01 107 m/s © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Multiple Choice, continued 12. What does Heisenberg’s uncertainty principle state? F. It is impossible to simultaneously measure a particle’s position and momentum with infinite accuracy. G. It is impossible to measure both a particle’s position and its momentum. H. The more accurately we know a particle’s position, the more accurately we know the particle’s momentum. J. All measurements are uncertain. © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Multiple Choice, continued 12. What does Heisenberg’s uncertainty principle state? F. It is impossible to simultaneously measure a particle’s position and momentum with infinite accuracy. G. It is impossible to measure both a particle’s position and its momentum. H. The more accurately we know a particle’s position, the more accurately we know the particle’s momentum. J. All measurements are uncertain. © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Short Response 13. What is the energy of a photon of light with frequency f = 2. 80 1014 Hz? Give your answer in both J and e. V. © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Short Response 13. What is the energy of a photon of light with frequency f = 2. 80 1014 Hz? Give your answer in both J and e. V. Answer: 1. 86 10– 19 J, 1. 16 e. V © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Short Response, continued 14. Light of wavelength 3. 0 10– 7 m shines on the metals lithium, iron, and mercury, which have work functions of 2. 3 e. V, 3. 9 e. V, and 4. 5 e. V, respectively. Which of these metals will exhibit the photoelectric effect? For each metal that does exhibit the photoelectric effect, what is the maximum kinetic energy of the photoelectrons? © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Short Response, continued 14. Light of wavelength 3. 0 10– 7 m shines on the metals lithium, iron, and mercury, which have work functions of 2. 3 e. V, 3. 9 e. V, and 4. 5 e. V, respectively. Which of these metals will exhibit the photoelectric effect? For each metal that does exhibit the photoelectric effect, what is the maximum kinetic energy of the photoelectrons? Answers: lithium and iron lithium: 1. 8 e. V, iron: 0. 2 e. V © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Short Response, continued 15. Identify the behavior of an electron as primarily like a wave or like a particle in each of the following situations: a. traversing a circular orbit in a magnetic field b. absorbing a photon and being ejected from the surface of a metal c. forming an interference pattern © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Short Response, continued 15. Identify the behavior of an electron as primarily like a wave or like a particle in each of the following situations: a. traversing a circular orbit in a magnetic field b. absorbing a photon and being ejected from the surface of a metal c. forming an interference pattern Answers: a. particle; b. particle; c. wave © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Extended Response 16. Describe Bohr’s model of the atom. Identify the assumptions that Bohr made that were a departure from those of classical physics. Explain how Bohr’s model accounts for atomic spectra. © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Extended Response 16. Describe Bohr’s model of the atom. Identify the assumptions that Bohr made that were a departure from those of classical physics. Explain how Bohr’s model accounts for atomic spectra. Answer: Answers should describe electrons orbiting a nucleus only in discrete energy levels. The model departs from classical physics in that the electrons are only allowed to have certain energies and they do not lose energy simply by moving in an electromagnetic field. Atomic spectra are the result of photons being emitted or absorbed when electrons jump between energy levels. © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Extended Response, continued 17. Electrons are ejected from a surface with speeds ranging up to 4. 6 105 m/s when light with a wavelength of 625 nm is used. a. What is the work function of this surface? b. What is the threshold frequency for this surface? Show all your work. © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Extended Response, continued 17. Electrons are ejected from a surface with speeds ranging up to 4. 6 105 m/s when light with a wavelength of 625 nm is used. a. What is the work function of this surface? b. What is the threshold frequency for this surface? Show all your work. Answers: a. 1. 39 e. V b. 3. 35 1014 Hz © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Extended Response, continued 18. The wave nature of electrons makes an electron microscope, which uses electrons rather than light, possible. The resolving power of any microscope is approximately equal to the wavelength used. A resolution of approximately 1. 0 10– 11 m would be required in order to “see” an atom. a. If electrons were used, what minimum kinetic energy of the electrons (in e. V) would be required to obtain this degree of resolution? b. If photons were used, what minimum photon energy would be required? © Houghton Mifflin Harcourt Publishing Company

Chapter 21 Standardized Test Prep Extended Response, continued 18. The wave nature of electrons makes an electron microscope, which uses electrons rather than light, possible. The resolving power of any microscope is approximately equal to the wavelength used. A resolution of approximately 1. 0 10– 11 m would be required in order to “see” an atom. a. If electrons were used, what minimum kinetic energy of the electrons (in e. V) would be required to obtain this degree of resolution? b. If photons were used, what minimum photon energy would be required? Answers: a. 1. 5 104 e. V; b. 1. 2 105 e. V © Houghton Mifflin Harcourt Publishing Company