Electrochemistry Chapter 21 Electrochemistry and Redox Oxidationreduction Redox

















- Slides: 70

Electrochemistry Chapter 21

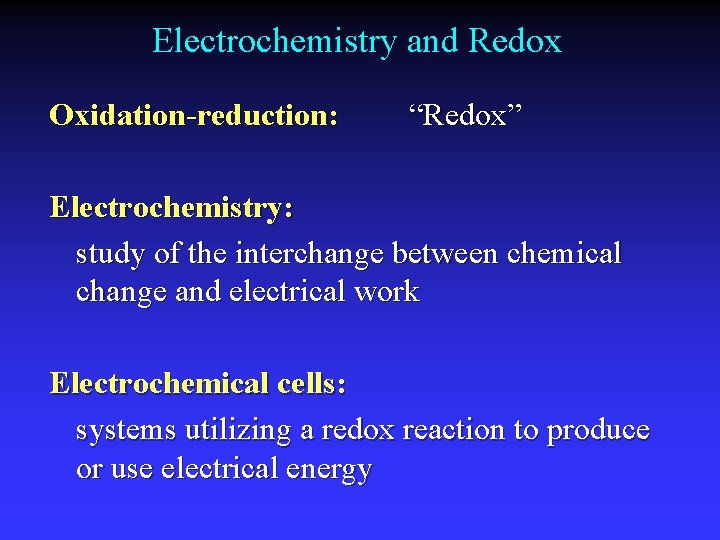
Electrochemistry and Redox Oxidation-reduction: “Redox” Electrochemistry: study of the interchange between chemical change and electrical work Electrochemical cells: systems utilizing a redox reaction to produce or use electrical energy

Redox Review Redox reactions: electron transfer processes Oxidation: Reduction: loss of 1 or more egain of 1 or more e- Oxidation numbers: imaginary charges (Balancing redox reactions)

Oxidation Numbers (O. N. ) 1. Pure element O. N. is zero 2. Monatomic ion O. N. is charge 3. Neutral compound: sum of O. N. is zero Polyatomic ion: sum of O. N. is ion’s charge *Negative O. N. generally assigned to more electronegative element

Oxidation Numbers (O. N. ) 4. Hydrogen assigned +1 (metal hydrides, -1) 5. Oxygen assigned -2 (peroxides, -1; OF 2, +2) 6. Fluorine always -1

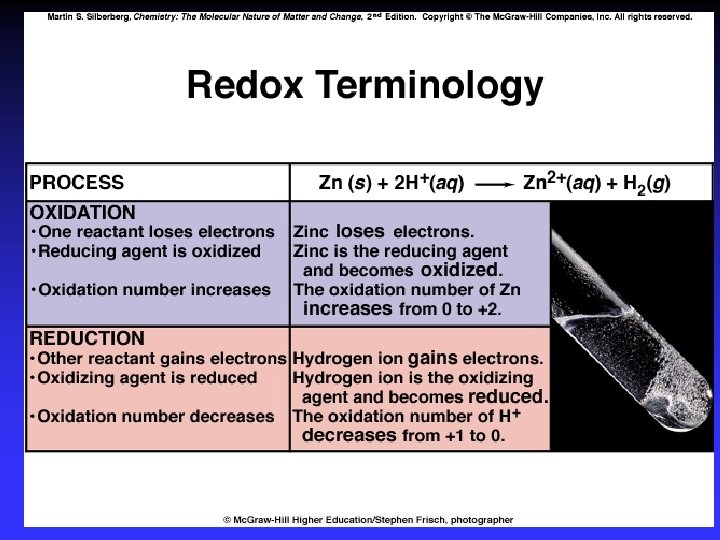
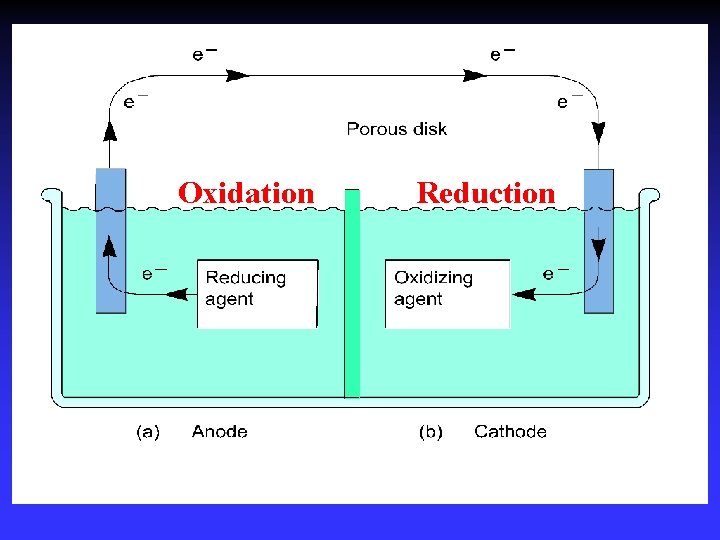
Oxidation-reduction Oxidation is loss of e. O. N. increases (more positive) Reduction is gain of e. O. N. decreases (more negative) Oxidation involves loss Reduction involves gain OIL RIG

Redox Oxidation is loss of ecauses reduction “reducing agent” Reduction is gain of ecauses oxidation “oxidizing agent”


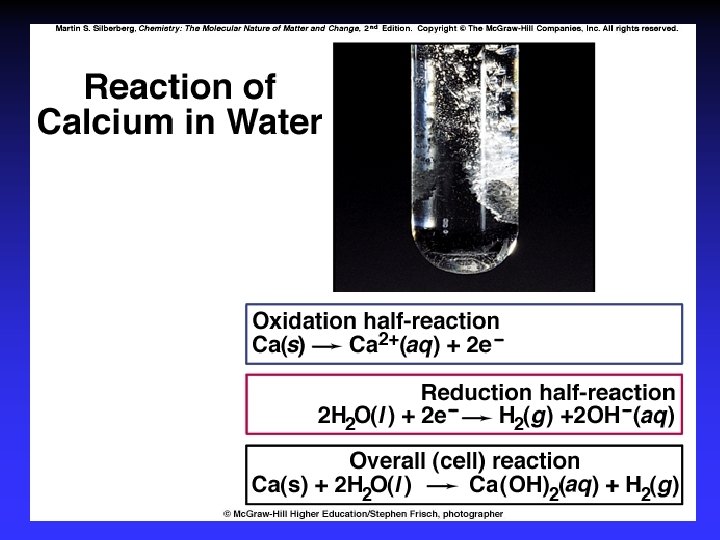
Balancing Redox Reactions 1. Write separate equations (half-reactions) for oxidation and reduction 2. For each half-reaction a. Balance elements involved in e- transfer b. Balance number e- lost and gained 3. To balance emultiply each half-reaction by whole numbers

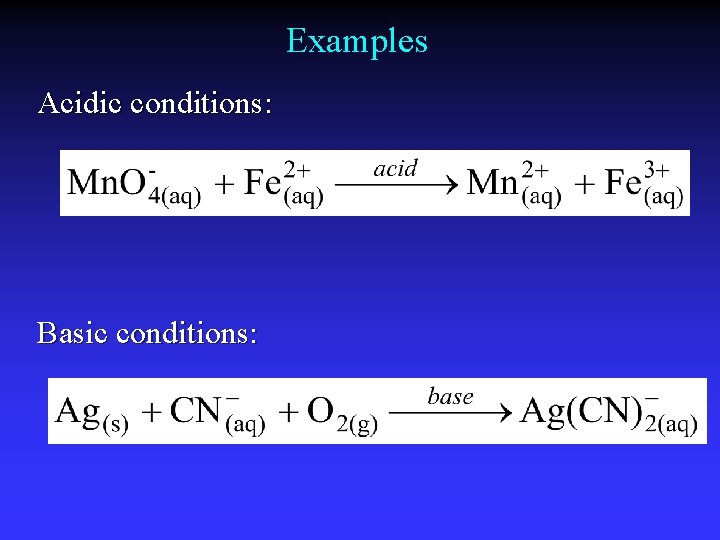
Balancing Redox Reactions: Acidic 4. Add half-reactions/cancel like terms (e-) 5. Acidic conditions: Balance oxygen using H 2 O Balance hydrogen using H+ Basic conditions: Balance oxygen using OHBalance hydrogen using H 2 O 6. Check that all atoms and charges balance

Examples Acidic conditions: Basic conditions:

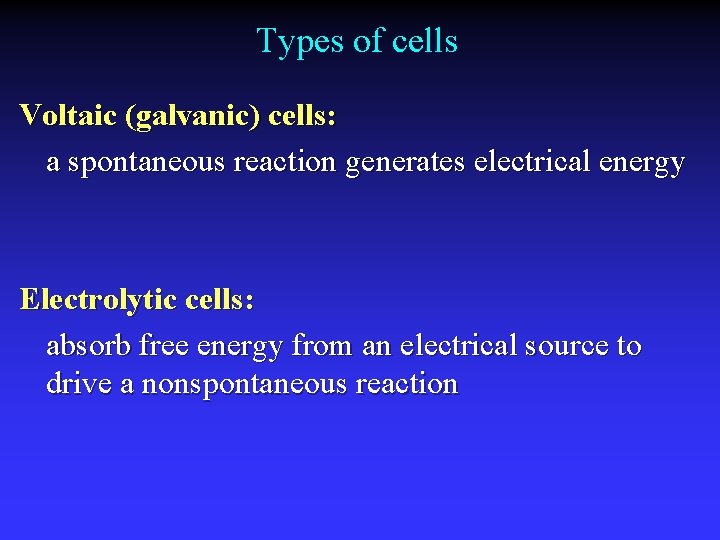
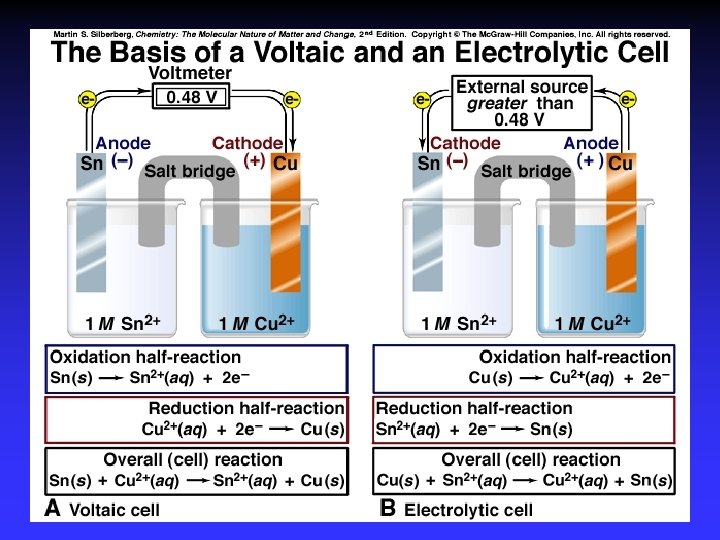
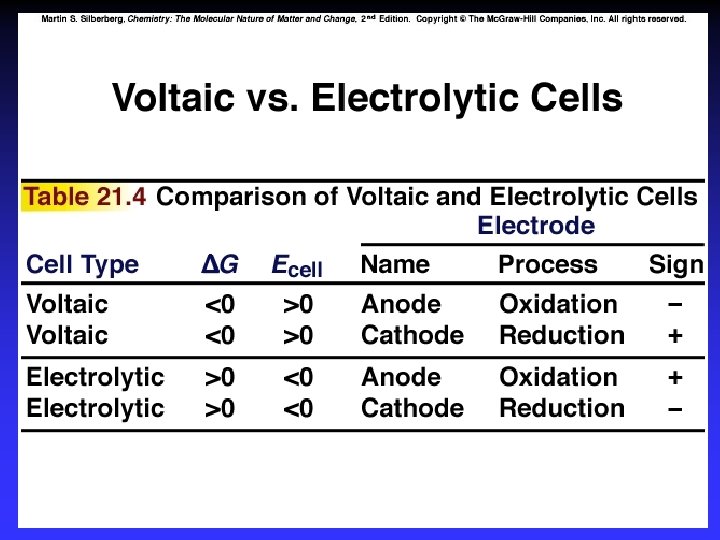
Types of cells Voltaic (galvanic) cells: a spontaneous reaction generates electrical energy Electrolytic cells: absorb free energy from an electrical source to drive a nonspontaneous reaction

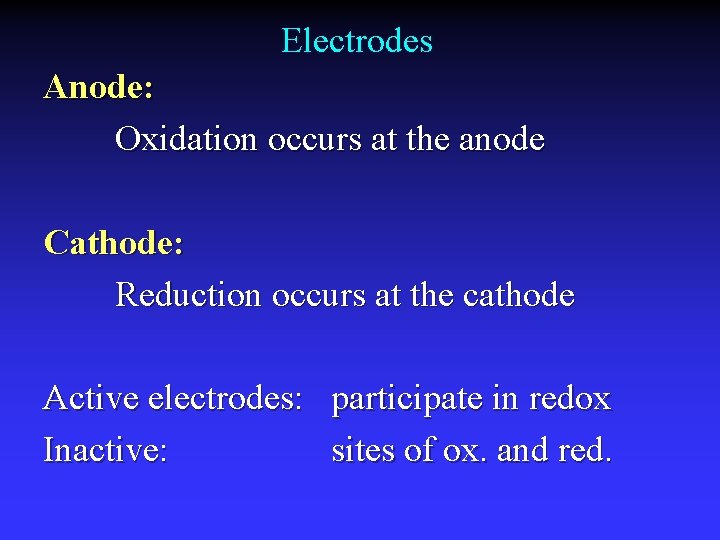
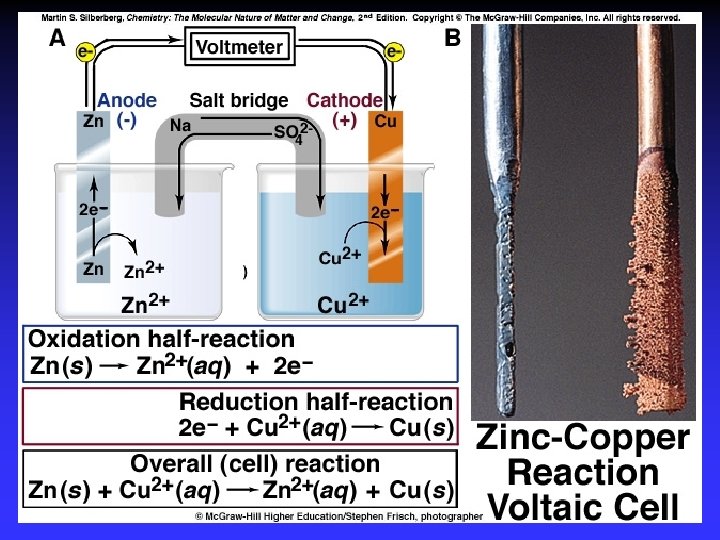
Common Components Electrodes: conduct electricity between cell and surroundings Electrolyte: mixture of ions involved in reaction or carrying charge Salt bridge: completes circuit (provides charge balance)

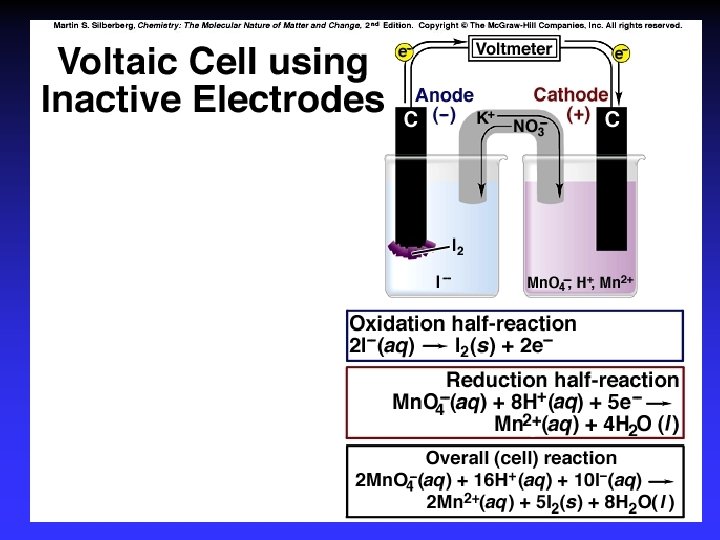
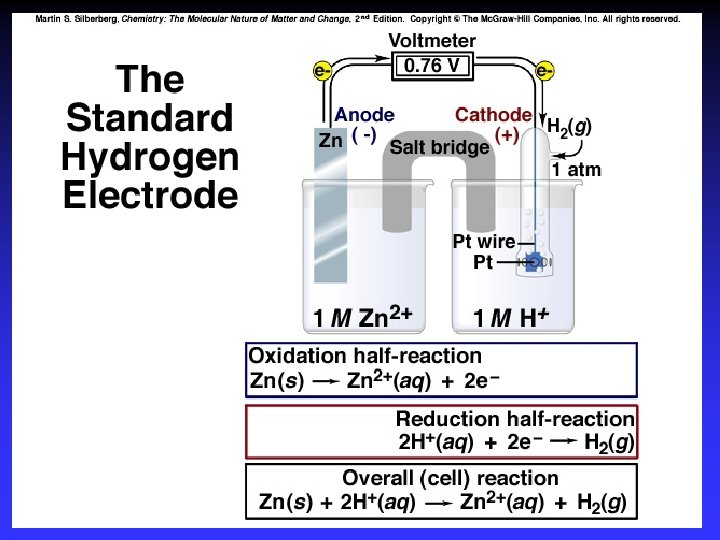
Electrodes Anode: Oxidation occurs at the anode Cathode: Reduction occurs at the cathode Active electrodes: participate in redox Inactive: sites of ox. and red.

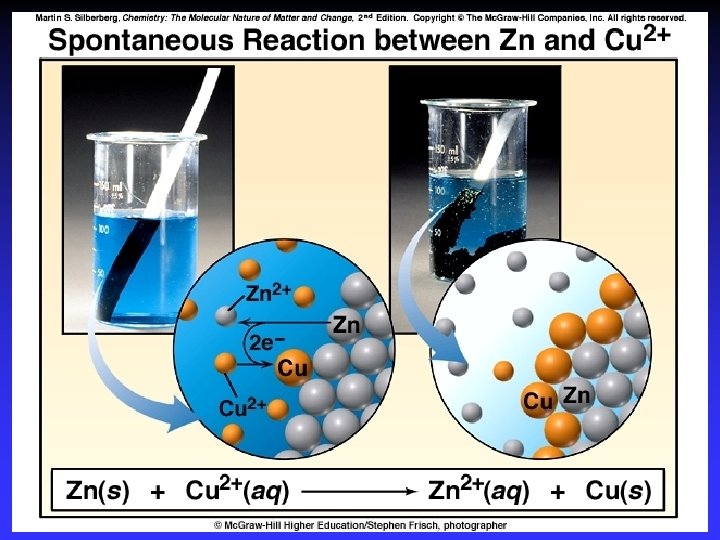
Voltaic (Galvanic) Cells A device in which chemical energy is changed to electrical energy. Uses a spontaneous reaction.

Oxidation Reduction




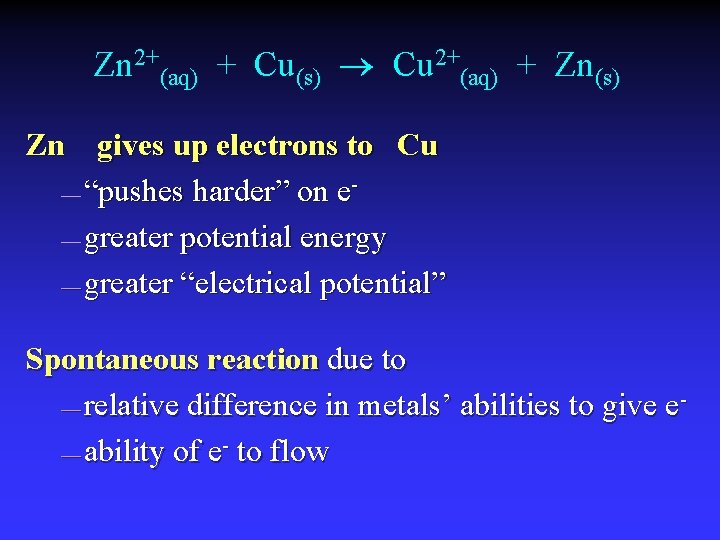
Zn 2+(aq) + Cu(s) Cu 2+(aq) + Zn(s) Zn gives up electrons to Cu — “pushes harder” on e— greater potential energy — greater “electrical potential” Spontaneous reaction due to — relative difference in metals’ abilities to give e — ability of e- to flow

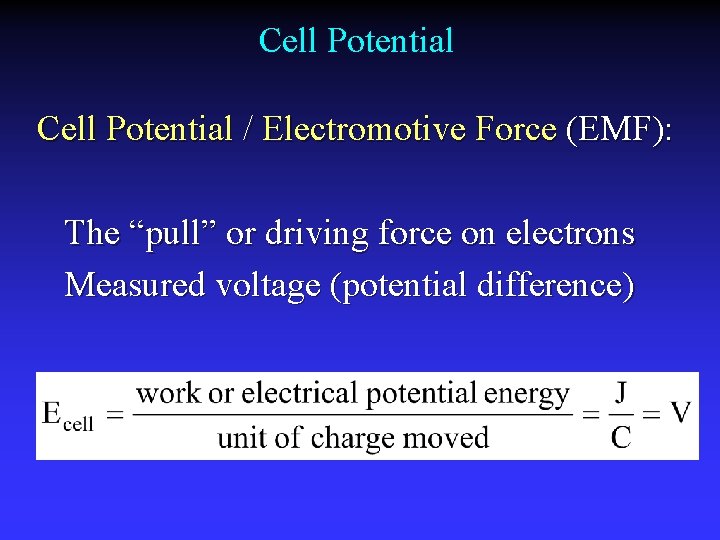
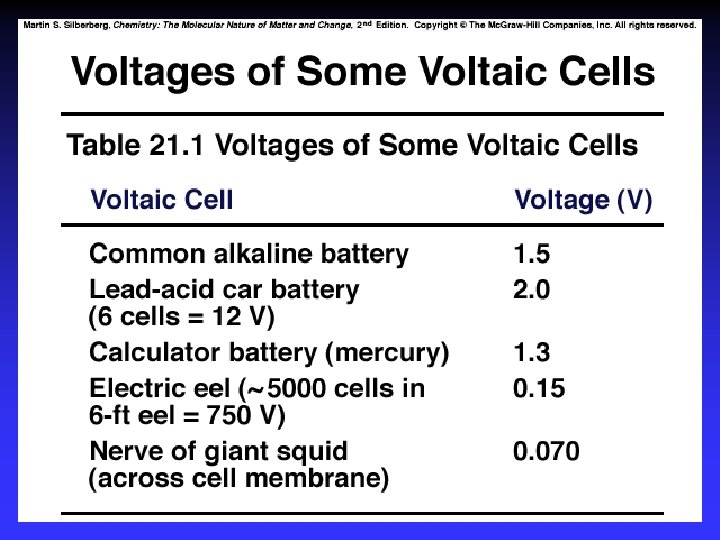
Cell Potential / Electromotive Force (EMF): The “pull” or driving force on electrons Measured voltage (potential difference)

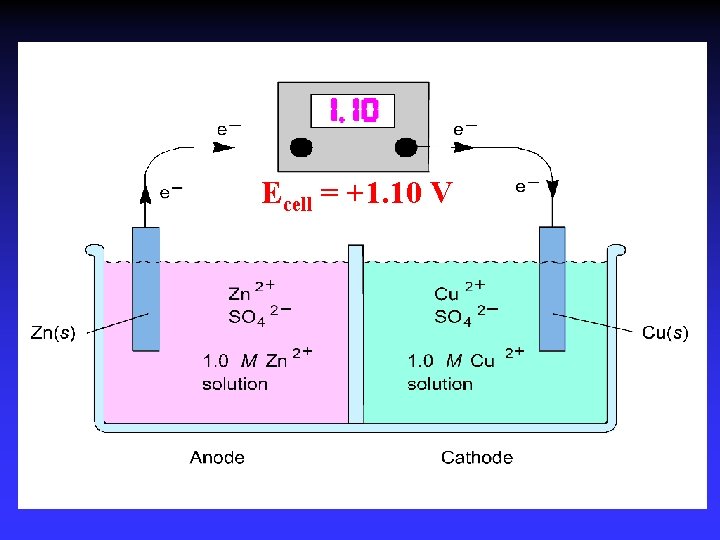
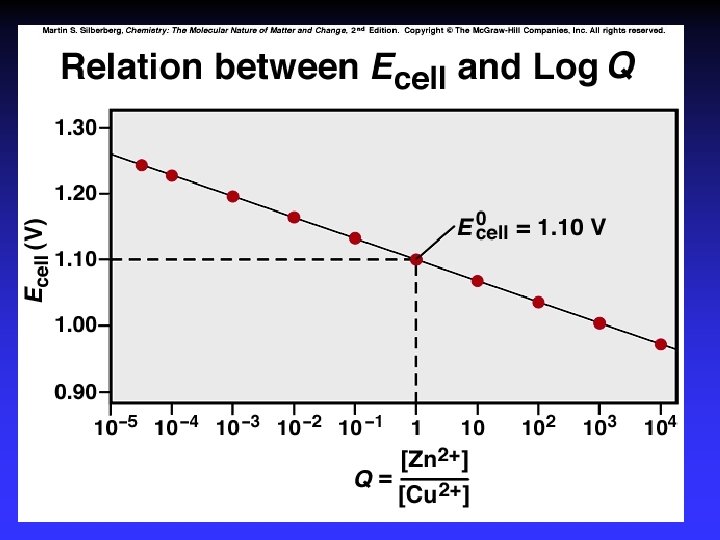
Ecell = +1. 10 V

Cell Potential, E 0 cell potential under standard conditions elements in standard states (298 K) solutions: 1 M gases: 1 atm


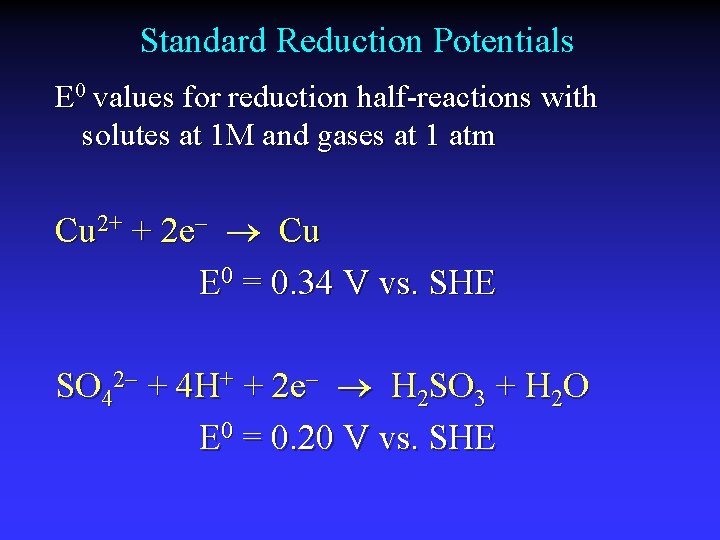
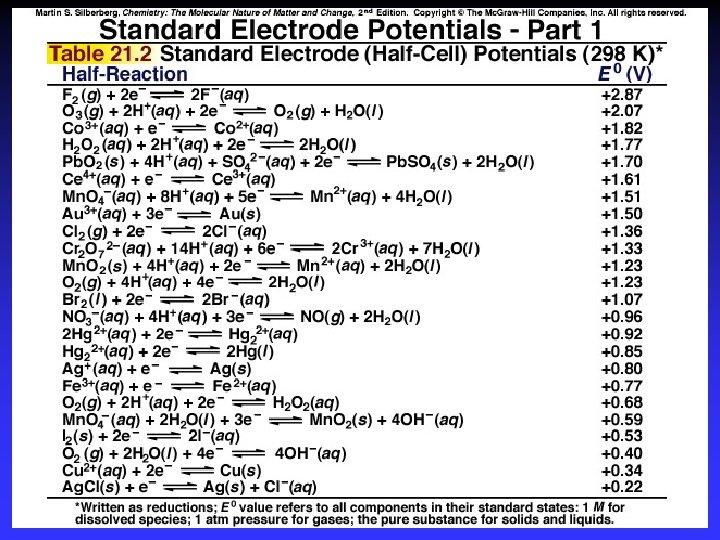
Standard Reduction Potentials E 0 values for reduction half-reactions with solutes at 1 M and gases at 1 atm Cu 2+ + 2 e Cu E 0 = 0. 34 V vs. SHE SO 42 + 4 H+ + 2 e H 2 SO 3 + H 2 O E 0 = 0. 20 V vs. SHE






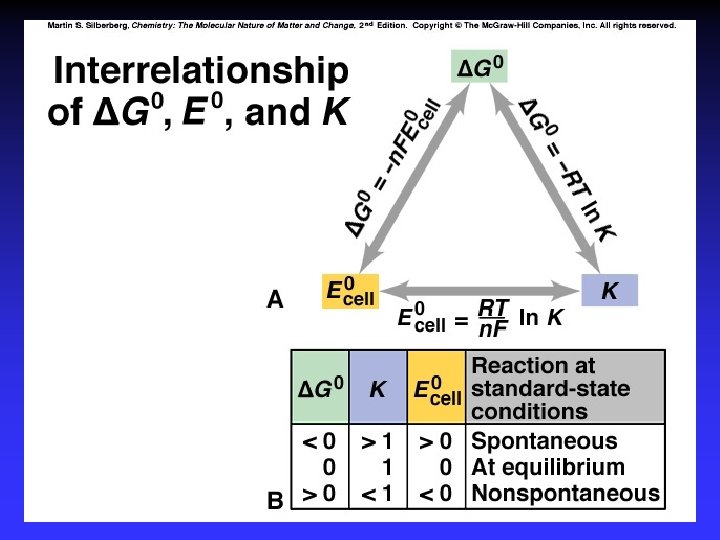
E 0 cell and DG 0 E 0 cell > 0 D G 0 < 0 Spontaneous E 0 cell < 0 D G 0 > 0 Not E 0 cell = 0 D G 0 = 0 Equilibrium

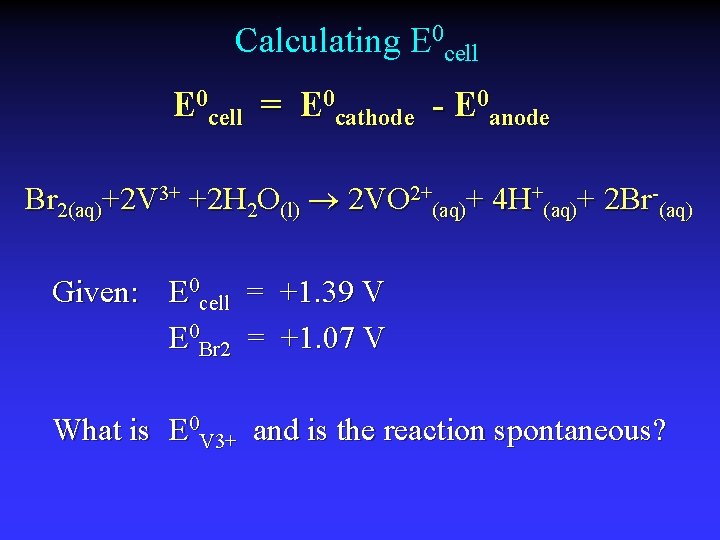
Calculating E 0 cell = E 0 cathode - E 0 anode Br 2(aq)+2 V 3+ +2 H 2 O(l) 2 VO 2+(aq)+ 4 H+(aq)+ 2 Br-(aq) Given: E 0 cell = +1. 39 V E 0 Br 2 = +1. 07 V What is E 0 V 3+ and is the reaction spontaneous?

E 0 values More positive: Stronger oxidizing agent More readily accepts e. More negative: Stronger reducing agent More readily gives e. Stronger R. A. + O. A. Weaker R. A. + O. A.

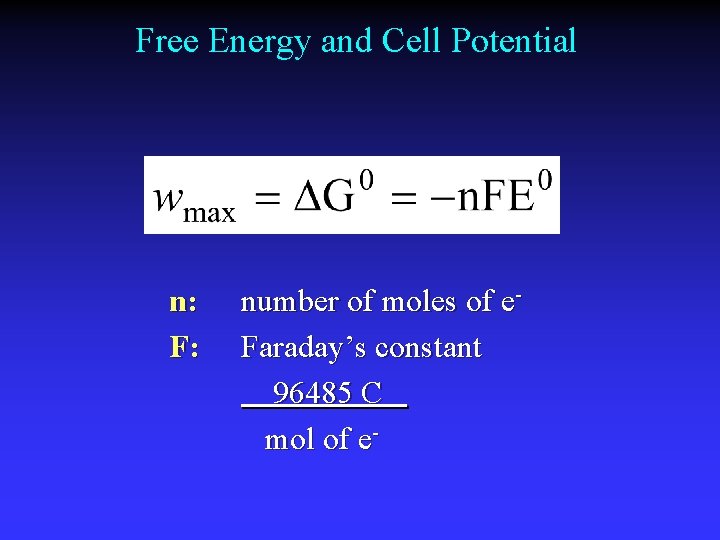
Free Energy and Cell Potential n: F: number of moles of e. Faraday’s constant 96485 C mol of e-

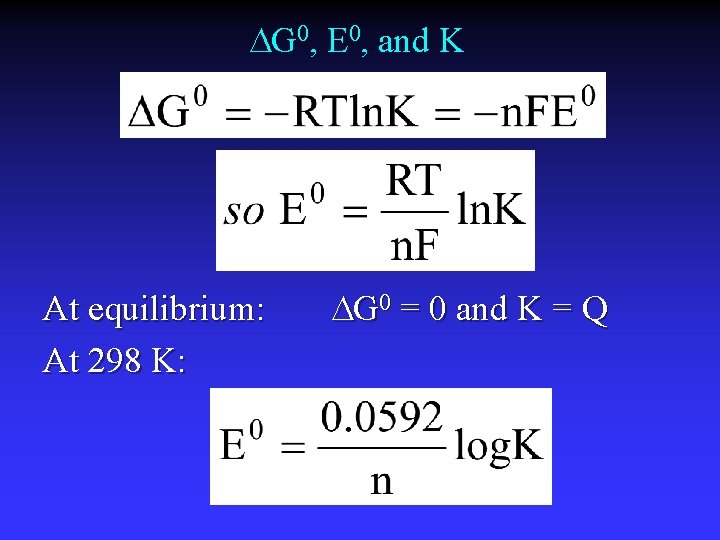
DG 0, E 0, and K At equilibrium: At 298 K: DG 0 = 0 and K = Q


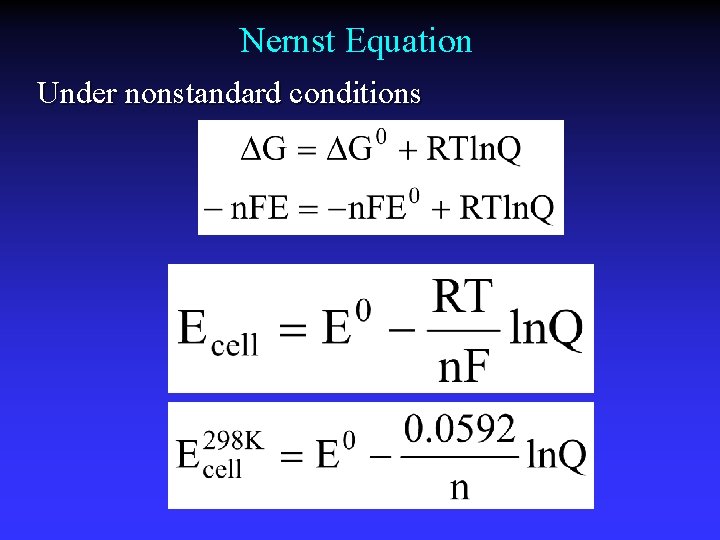
Nernst Equation Under nonstandard conditions


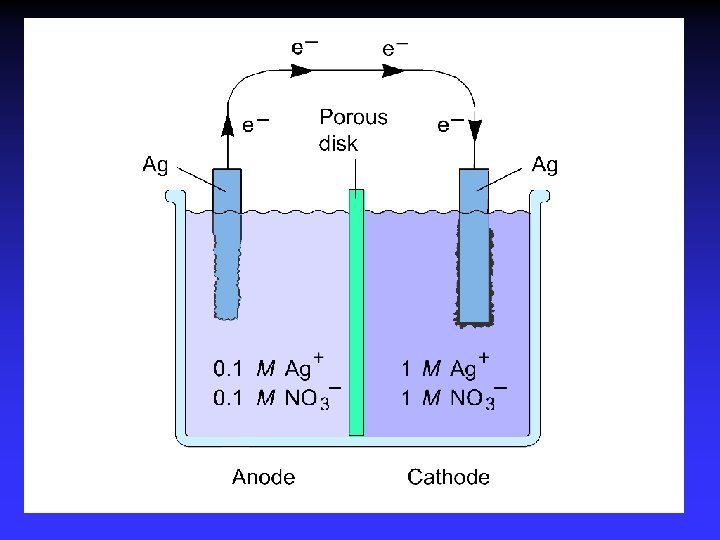
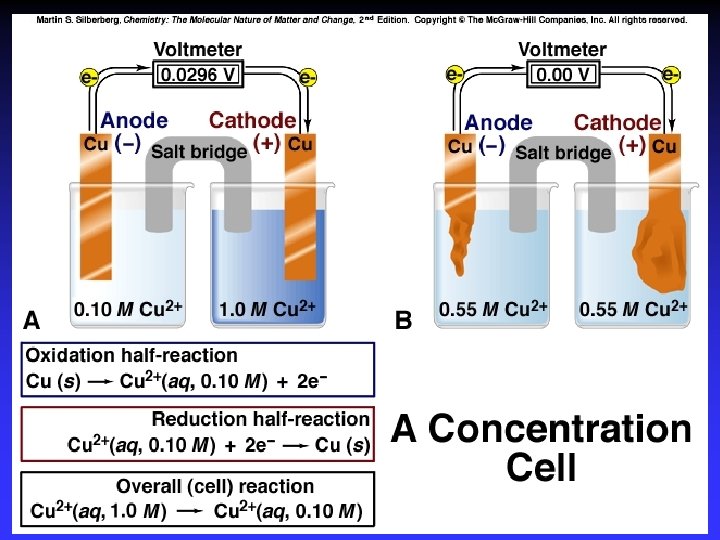
Concentration Cells. . . a cell in which both compartments have the same components but at different concentrations






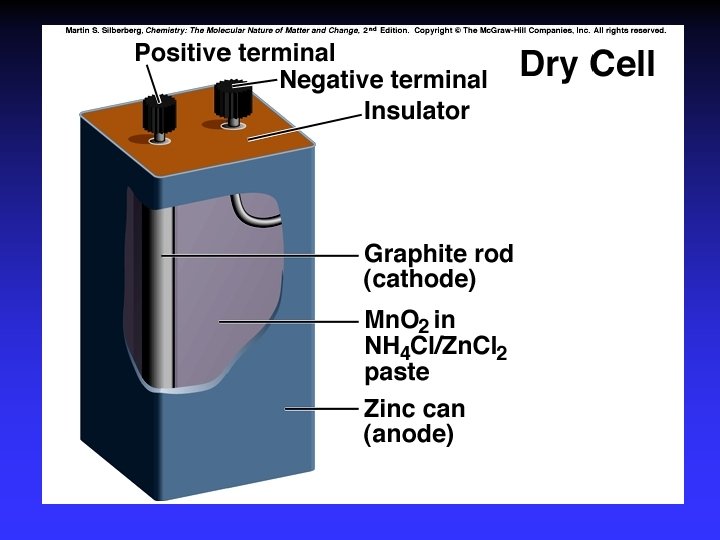
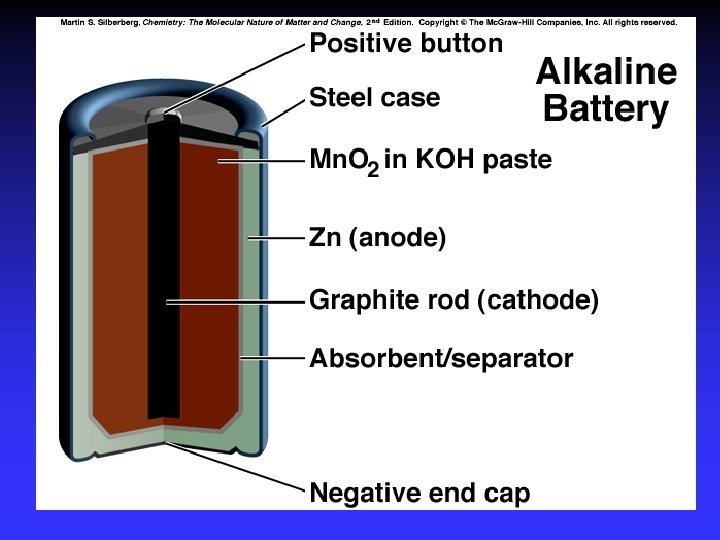
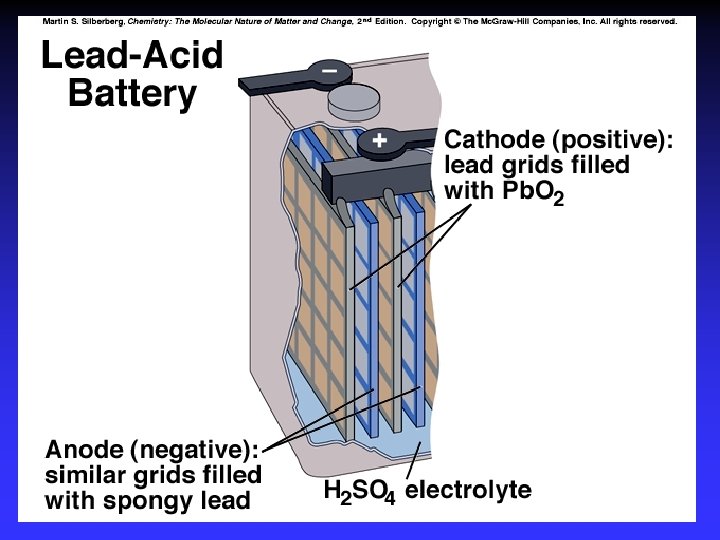
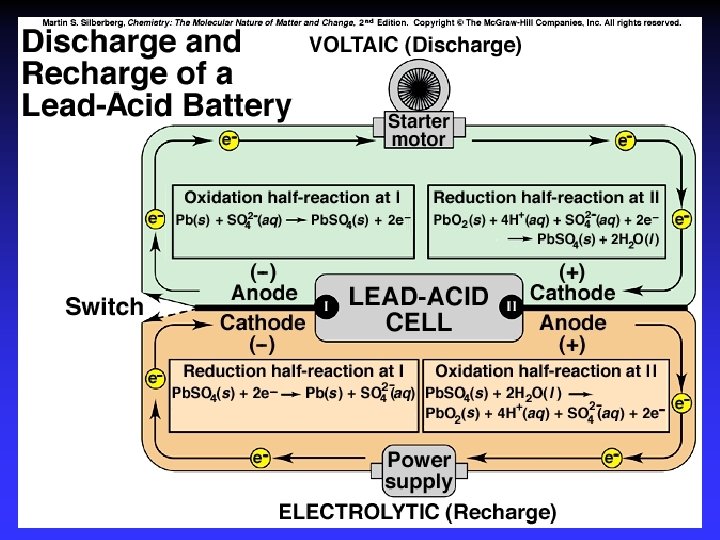
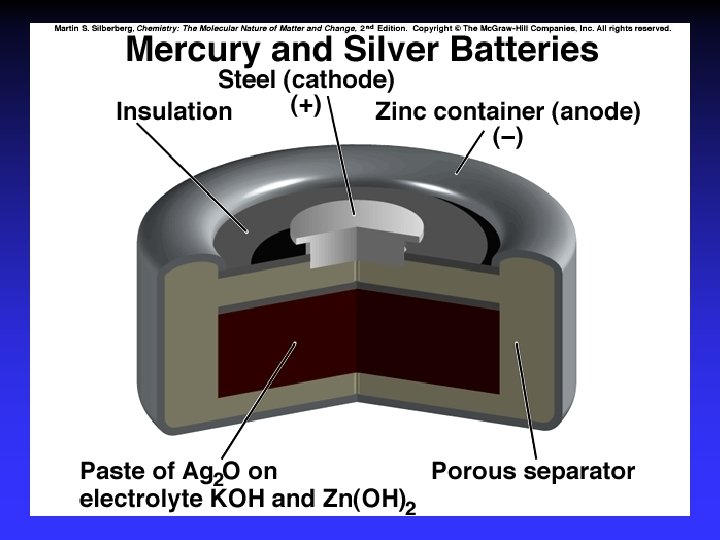
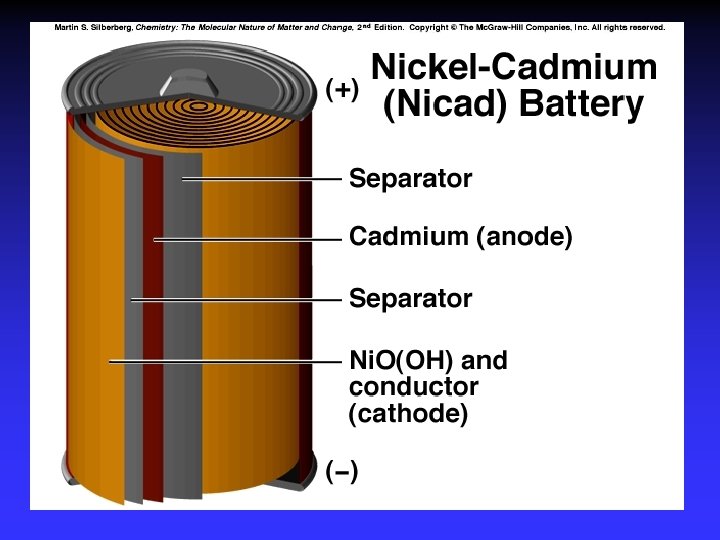
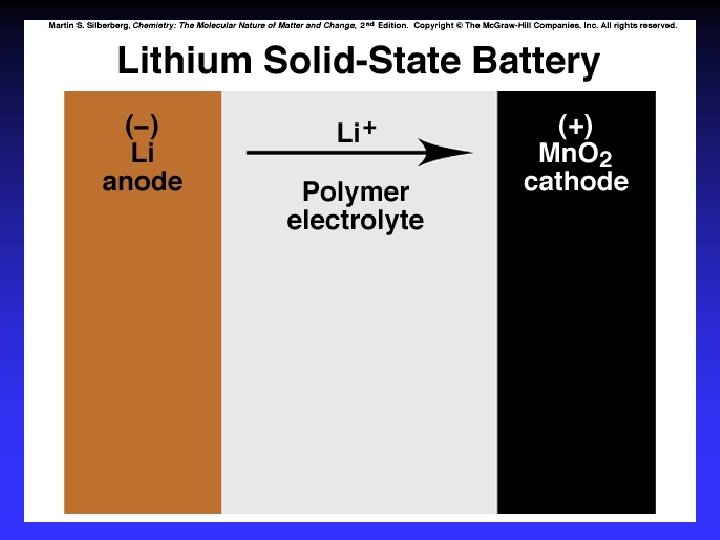
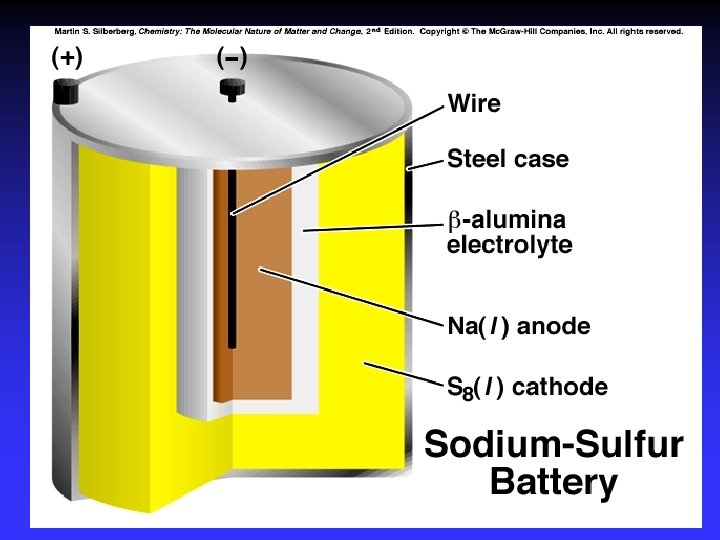
Batteries A battery is a galvanic cell or, more commonly, a group of galvanic cells connected in series.








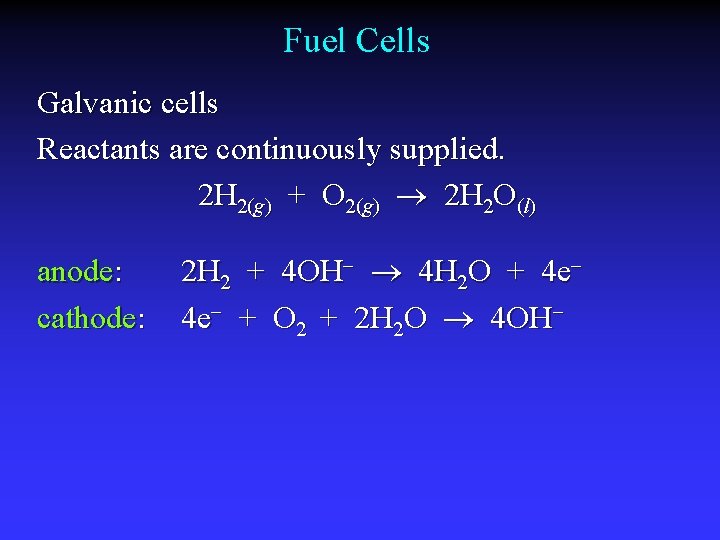
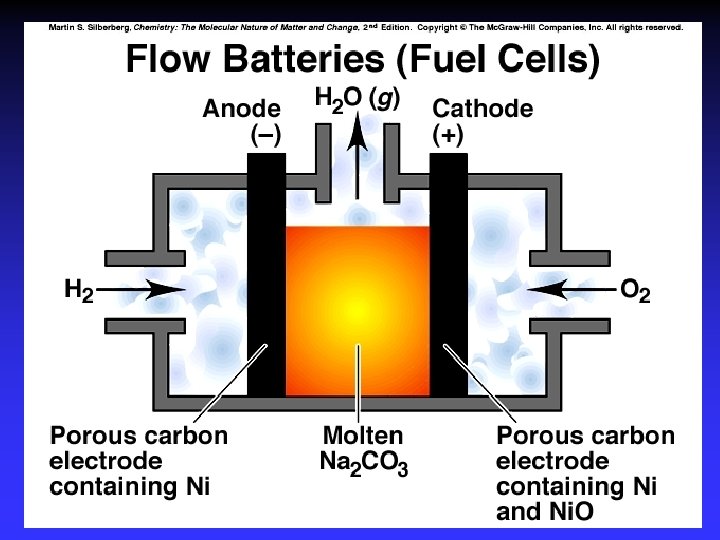
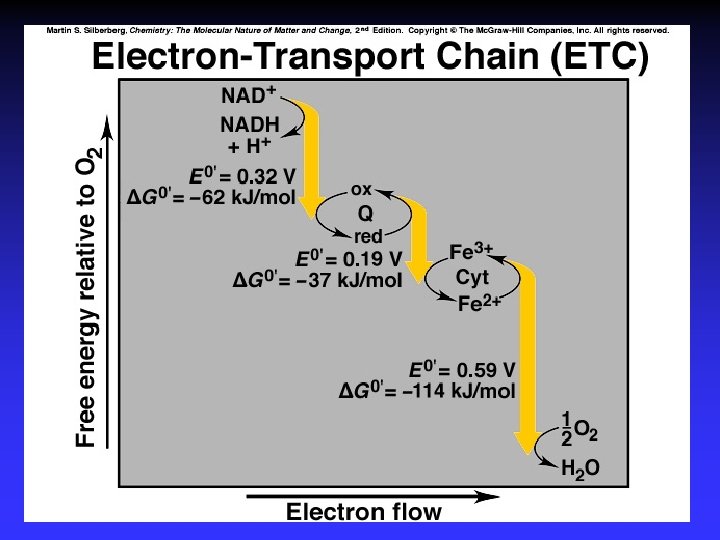
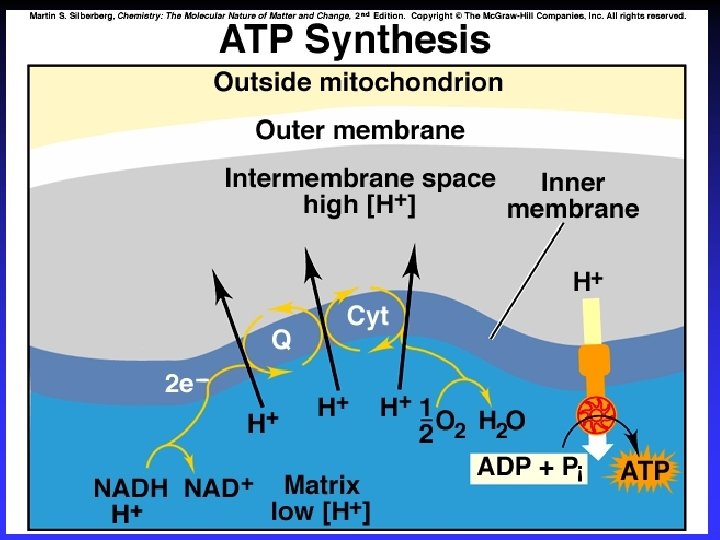
Fuel Cells Galvanic cells Reactants are continuously supplied. 2 H 2(g) + O 2(g) 2 H 2 O(l) anode: cathode: 2 H 2 + 4 OH 4 H 2 O + 4 e 4 e + O 2 + 2 H 2 O 4 OH



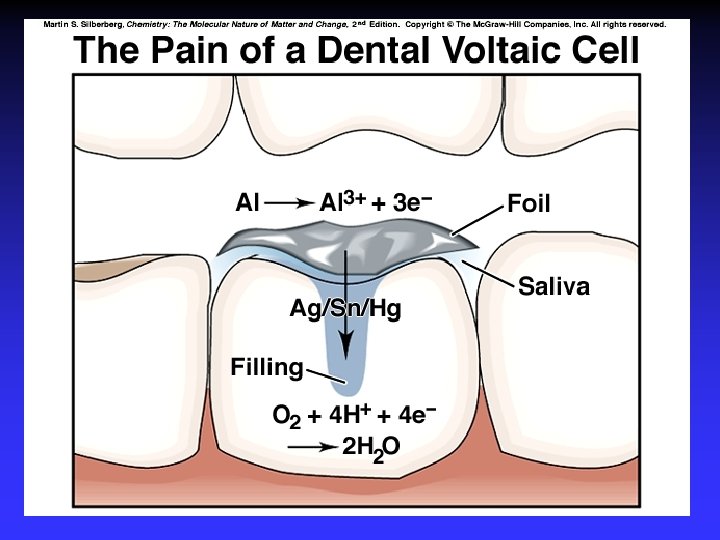
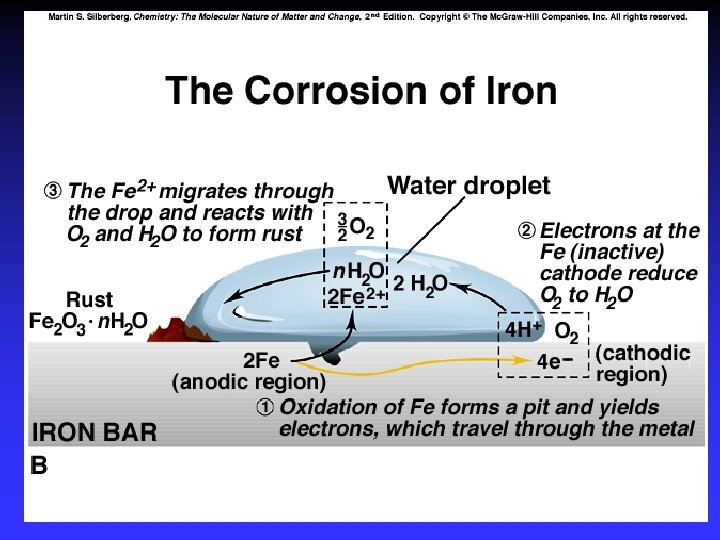
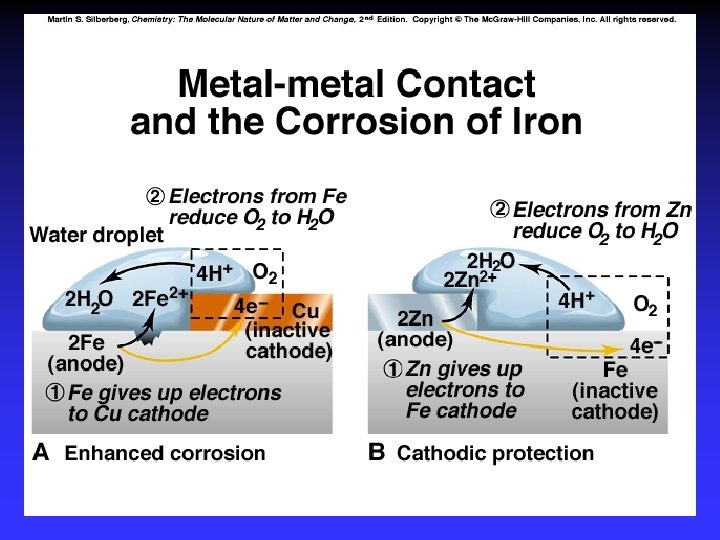
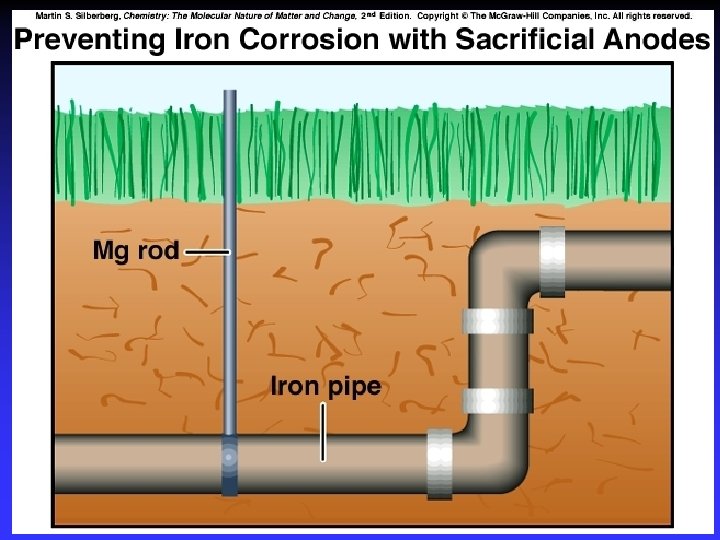
Corrosion Some metals, such as copper, gold, silver and platinum, are relatively difficult to oxidize. These are often called noble metals.




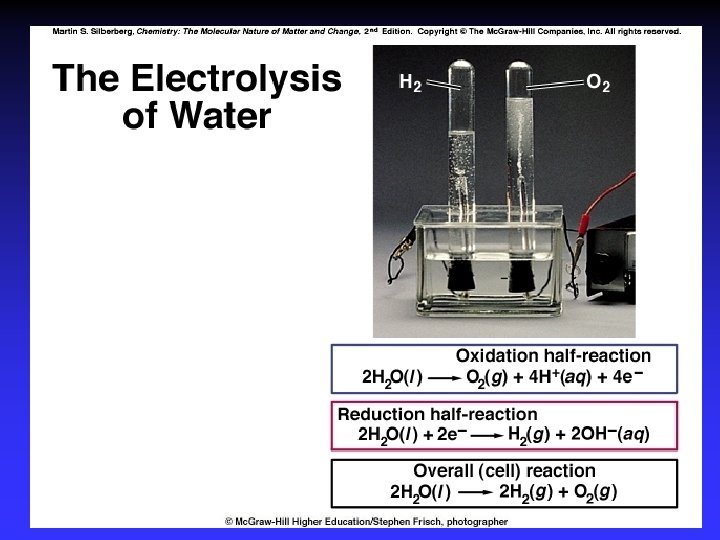
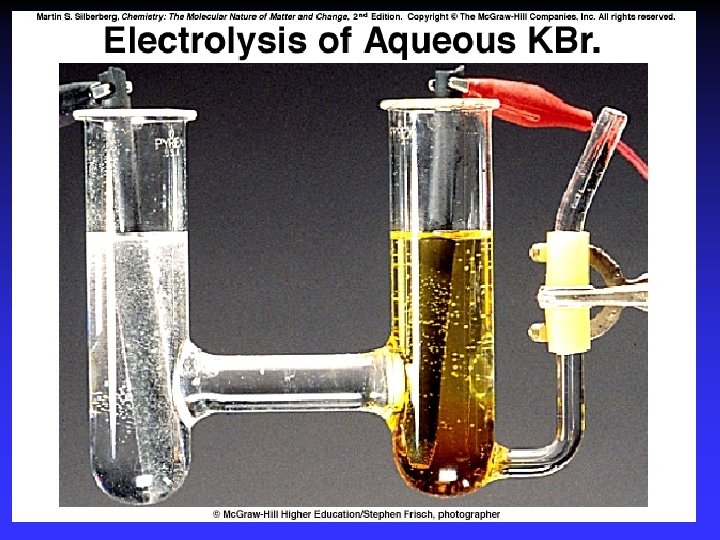
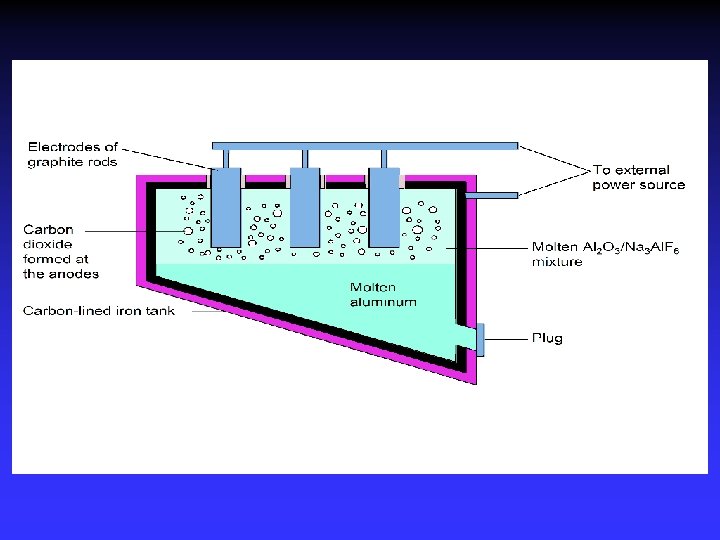
Electrolysis Forcing a current through a cell to produce a chemical change for which the cell potential is negative.






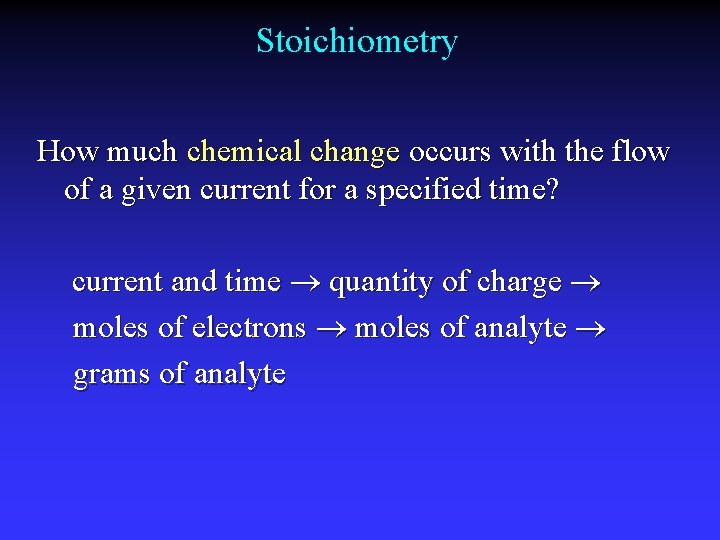
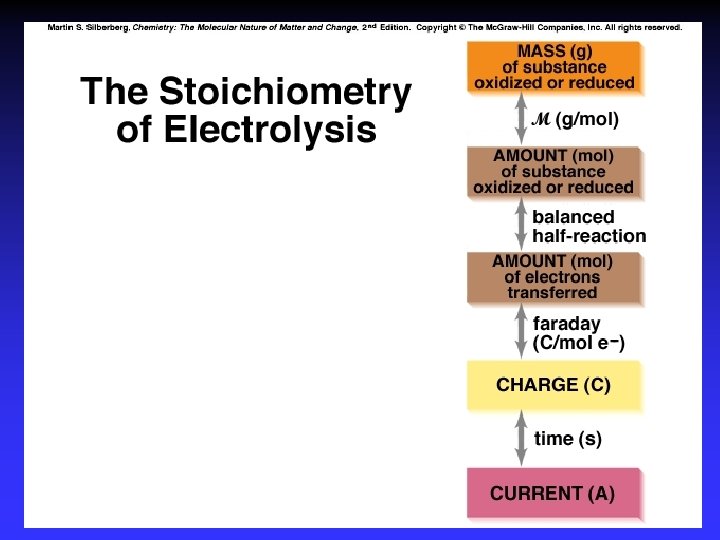
Stoichiometry How much chemical change occurs with the flow of a given current for a specified time? current and time quantity of charge moles of electrons moles of analyte grams of analyte



