OxidationReduction Chapter 17 Hein and Arena Version 1



































- Slides: 74

Oxidation-Reduction Chapter 17 Hein and Arena Version 1. 1 Eugene Passer Chemistry Department 1 College Bronx Community © John Wiley and Sons, Inc.

Chapter Outline 17. 1 Oxidation Number 17. 2 Oxidation-Reduction 17. 3 Balancing Oxidation. Reduction Equations 17. 4 Balancing Ionic Redox Equations 17. 5 Activity Series of Metals 17. 6 Electrolytic and Voltaic Cells 2

Oxidation Number 3

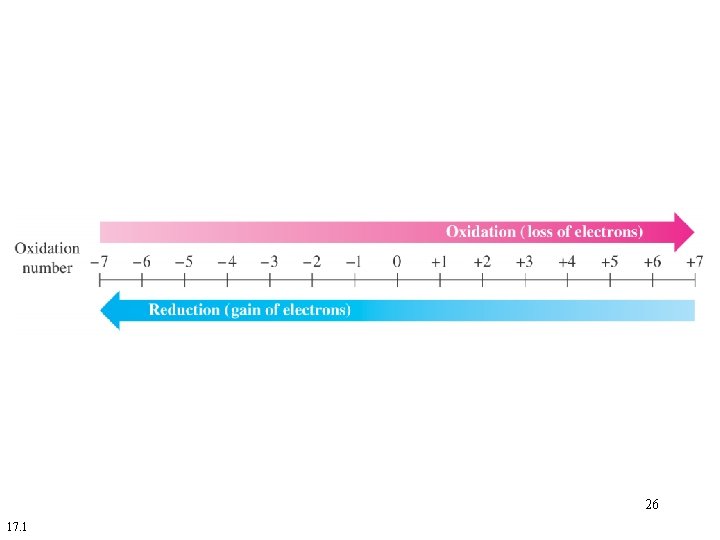
The oxidation number (oxidation state) of an atom represents the number of electrons lost, gained, or unequally shared by an atom. 4

Oxidation numbers can be zero, positive or negative. 5

An oxidation number of zero means the atom has the same number of electrons assigned to it as there are in the free neutral atom. 6

A positive oxidation number means the atom has fewer electrons assigned to it than in the neutral atom. 7

A negative oxidation number means the atom has more electrons assigned to it than in the neutral atom. 8

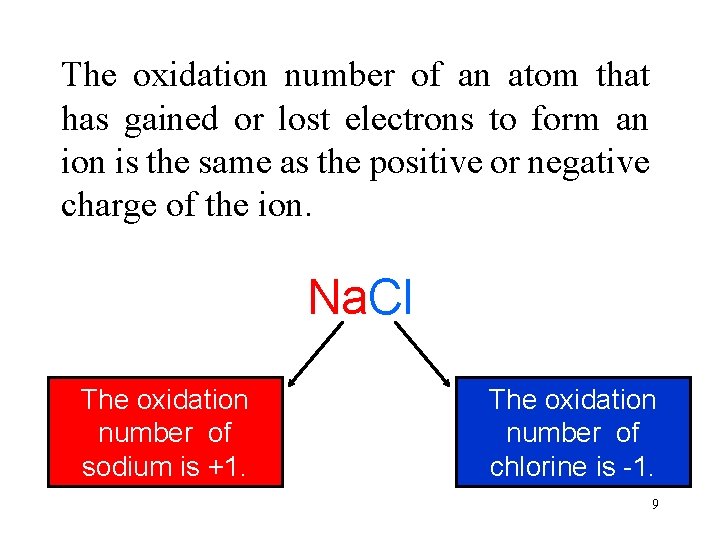
The oxidation number of an atom that has gained or lost electrons to form an ion is the same as the positive or negative charge of the ion. Na. Cl Thecharge oxidation Sodium has lost The on number sodium is +1. an electron. of sodium is +1. The Chlorine oxidation has The charge on number gained an of chlorine is – 1. chlorine electron. is -1. 9

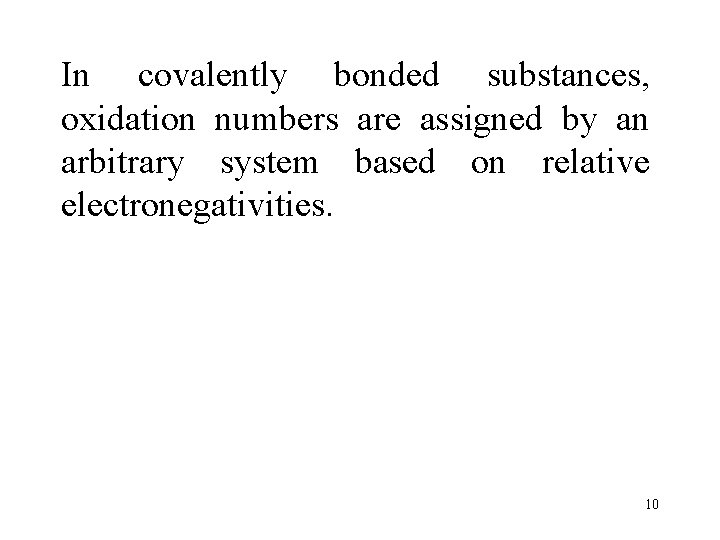
In covalently bonded substances, oxidation numbers are assigned by an arbitrary system based on relative electronegativities. 10

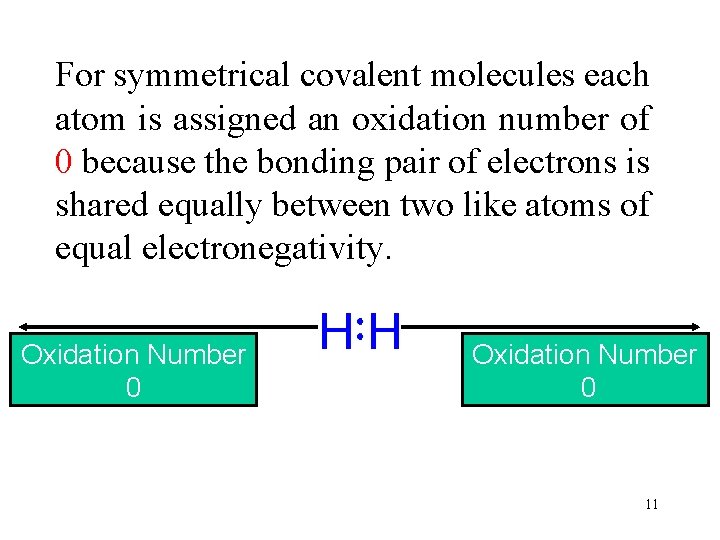
For symmetrical covalent molecules each atom is assigned an oxidation number of 0 because the bonding pair of electrons is shared equally between two like atoms of equal electronegativity. Oxidation Electronegativity Number 2. 1 0 11

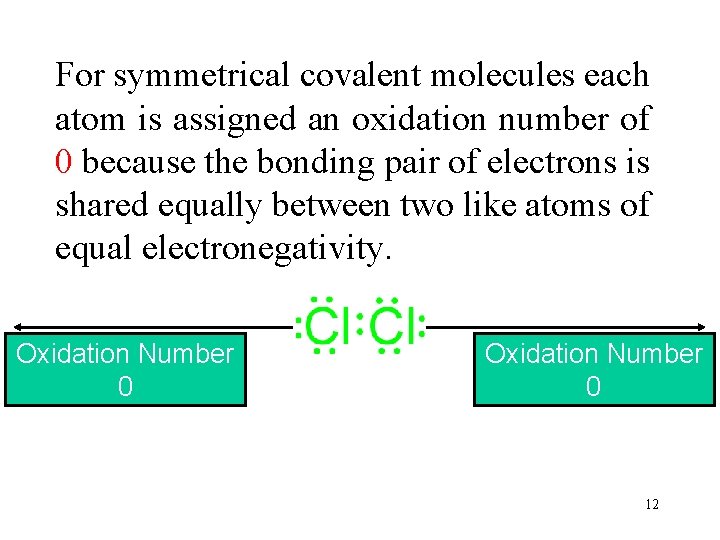
For symmetrical covalent molecules each atom is assigned an oxidation number of 0 because the bonding pair of electrons is shared equally between two like atoms of equal electronegativity. Oxidation Number Electronegativity 0 3. 0 12

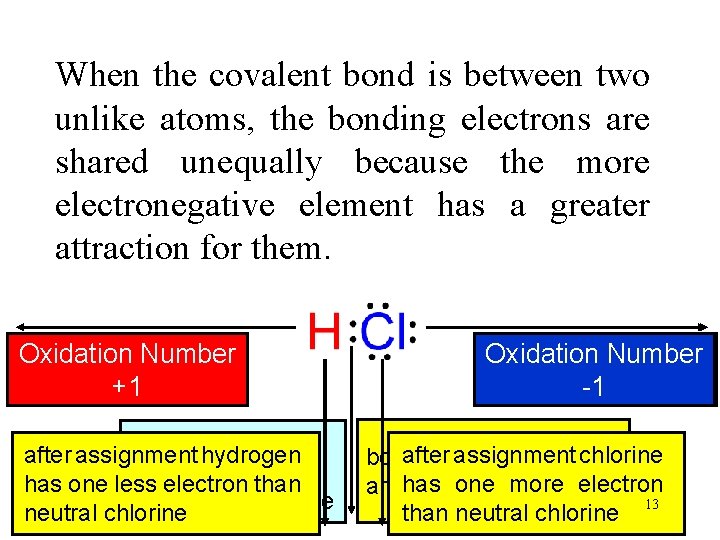
When the covalent bond is between two unlike atoms, the bonding electrons are shared unequally because the more electronegative element has a greater attraction for them. Oxidation Number Electronegativity +1 2. 1 there is a partial after assignment hydrogen unequal shared electron pair of transfer of an has one lesssharing electron than electrons electron to chlorine neutral chlorine Oxidation Number Electronegativity -1 3. 0 assignment chlorine bothafter shared electrons one more electron are has assigned to chlorine than neutral chlorine 13

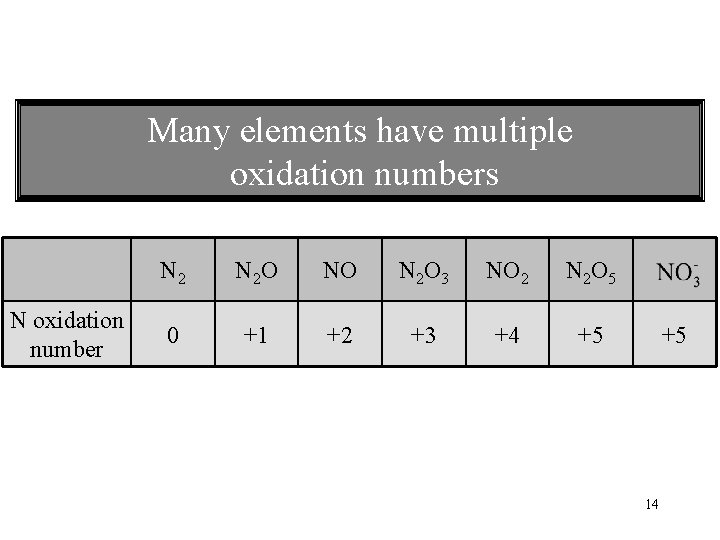
Many elements have multiple oxidation numbers N oxidation number N 2 O NO N 2 O 3 NO 2 N 2 O 5 0 +1 +2 +3 +4 +5 +5 14

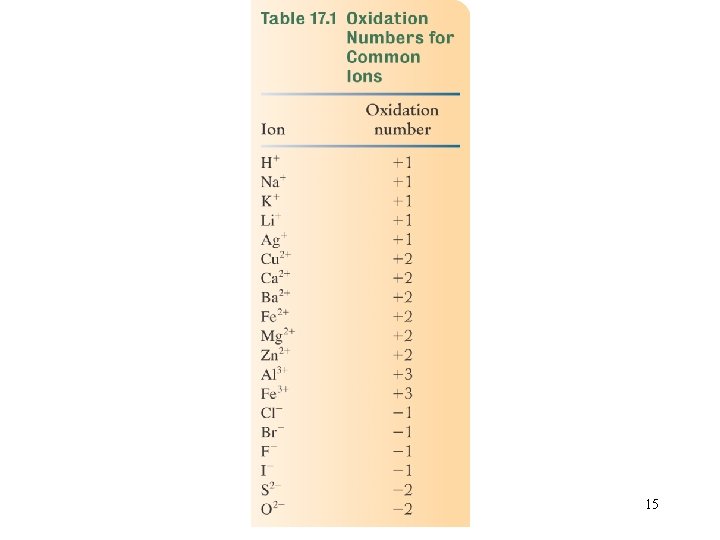
15

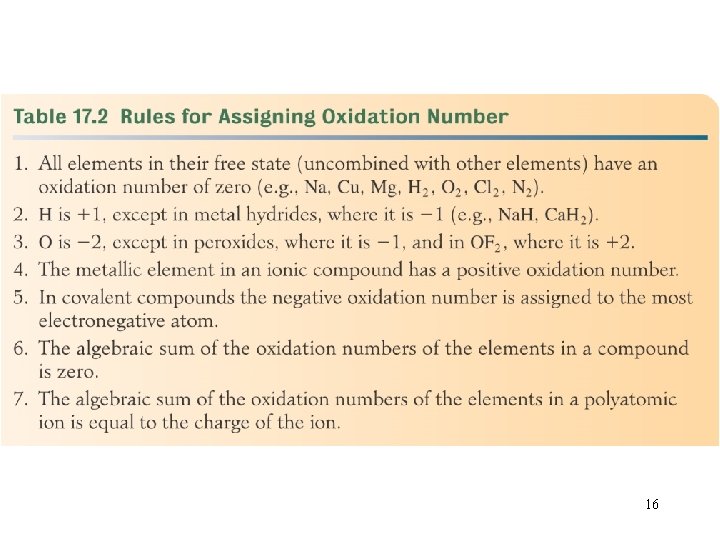
16

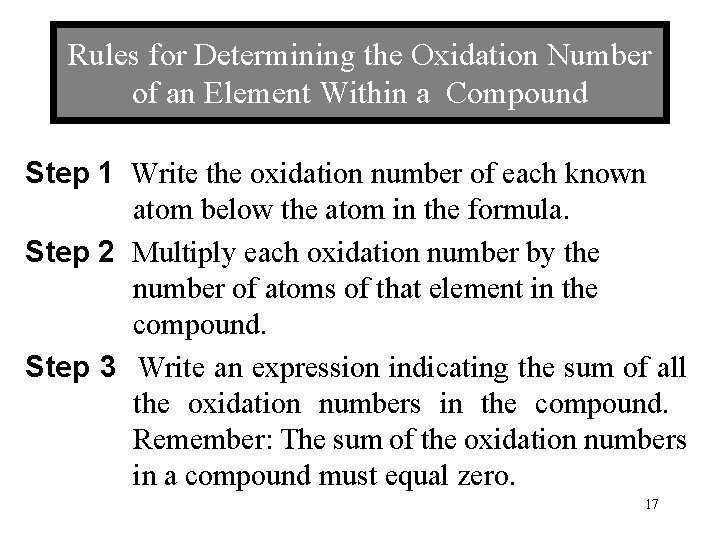
Rules for Determining the Oxidation Number of an Element Within a Compound Step 1 Write the oxidation number of each known atom below the atom in the formula. Step 2 Multiply each oxidation number by the number of atoms of that element in the compound. Step 3 Write an expression indicating the sum of all the oxidation numbers in the compound. Remember: The sum of the oxidation numbers in a compound must equal zero. 17

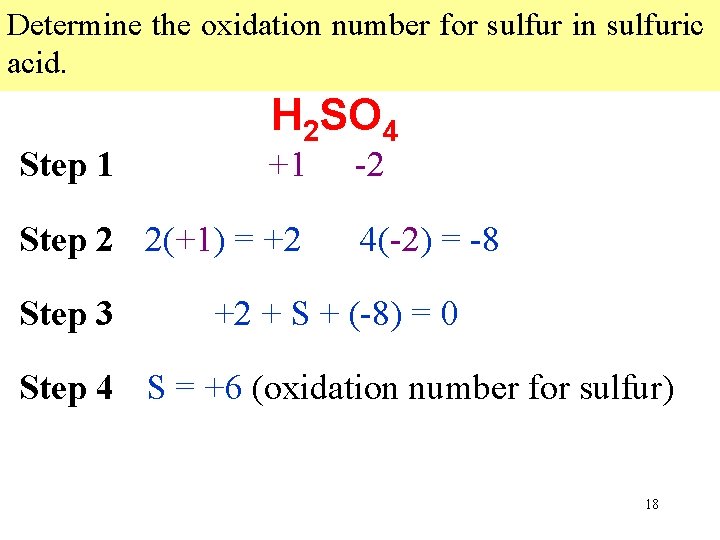
Determine the oxidation number for sulfur in sulfuric acid. Step 1 H 2 SO 4 +1 Step 2 2(+1) = +2 Step 3 -2 4(-2) = -8 +2 + S + (-8) = 0 Step 4 S = +6 (oxidation number for sulfur) Multiply Write each expression oxidation indicating numberthe byeach sum the number of all the of Writeanthe oxidation number of known atoms oxidation that numbers element in in thethe compound. atomofbelow the atom incompound. the formula. 18

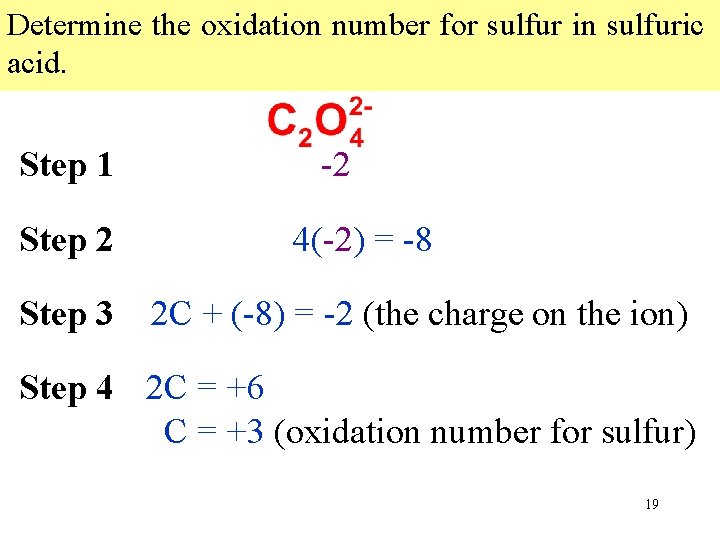
Determine the oxidation number for sulfur in sulfuric acid. Step 1 Step 2 Step 3 -2 4(-2) = -8 2 C + (-8) = -2 (the charge on the ion) Step 4 2 C = +6 C = +3 (oxidation number for sulfur) Multiply Write each expression oxidation indicating numberthe byeach sum the number of all the of Writeanthe oxidation number of known atoms oxidation that numbers element in in thethe compound. atomofbelow the atom incompound. the formula. 19

Oxidation-Reduction 20

Oxidation-reduction (redox) is a chemical process in which the oxidation number of an element is changed. 21

Redox may involve the complete transfer of electrons to form ionic bonds or a partial transfer of electrons to form covalent bonds. 22

• Oxidation occurs when the oxidation number of an element increases as a result of losing electrons. • Reduction occurs when the oxidation number of an element decreases as a result of gaining electrons. • In a redox reaction oxidation and reduction occur simultaneously, one cannot occur in the absence of the other. 23

• Oxidizing agent The substance that causes an increase in the oxidation state of another substance. – The oxidizing agent is reduced in a redox reaction. • Reducing agent The substance that causes a decrease in the oxidation state of another substance. – The reducing agent is oxidized in a redox reaction. 24

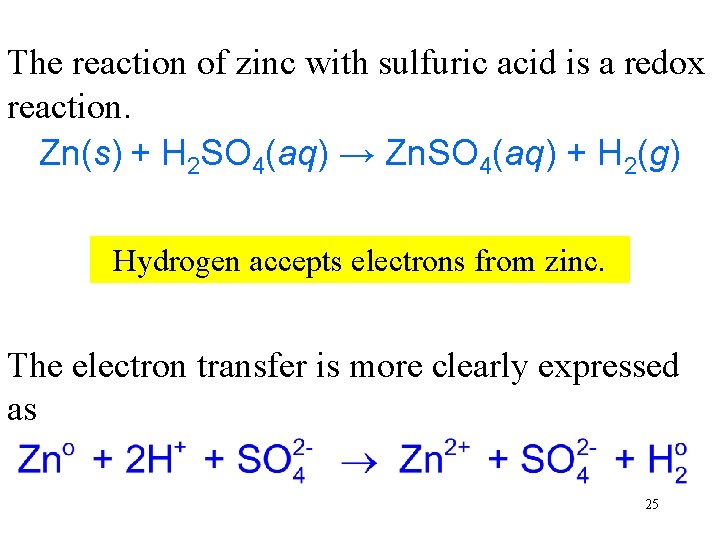
The reaction of zinc with sulfuric acid is a redox reaction. Zn(s) + H 2 SO 4(aq) → Zn. SO 4(aq) + H 2(g) Hydrogen Zinc transfers Hydrogen accepts Zinc is the is electrons the isreducing oxidized. electrons is oxidizing reduced. toagent. hydrogen. from agent. zinc. The electron transfer is more clearly expressed as 25

26 17. 1

Balancing Oxidation. Reduction Equations 27

Change-In-Oxidation Number Method 28

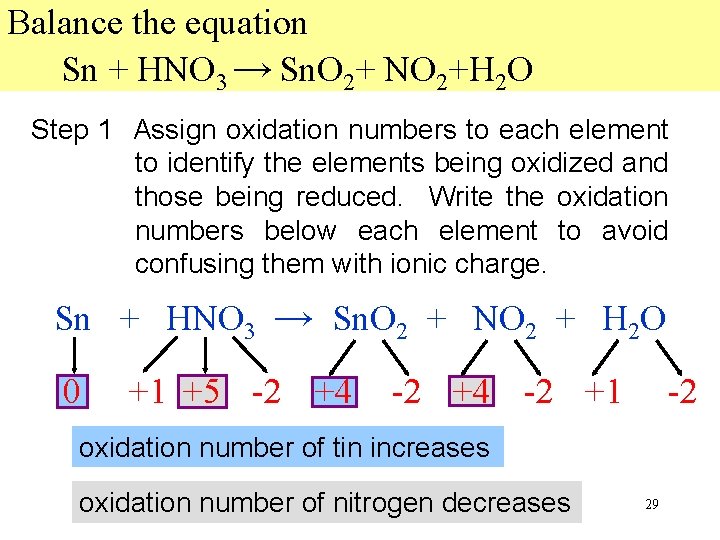
Balance the equation Sn + HNO 3 → Sn. O 2+ NO 2+H 2 O Step 1 Assign oxidation numbers to each element to identify the elements being oxidized and those being reduced. Write the oxidation numbers below each element to avoid confusing them with ionic charge. Sn + HNO 3 → Sn. O 2 + NO 2 + H 2 O 0 +1 +5 -2 +4 -2 +1 -2 oxidation number of tin increases oxidation number of nitrogen decreases 29

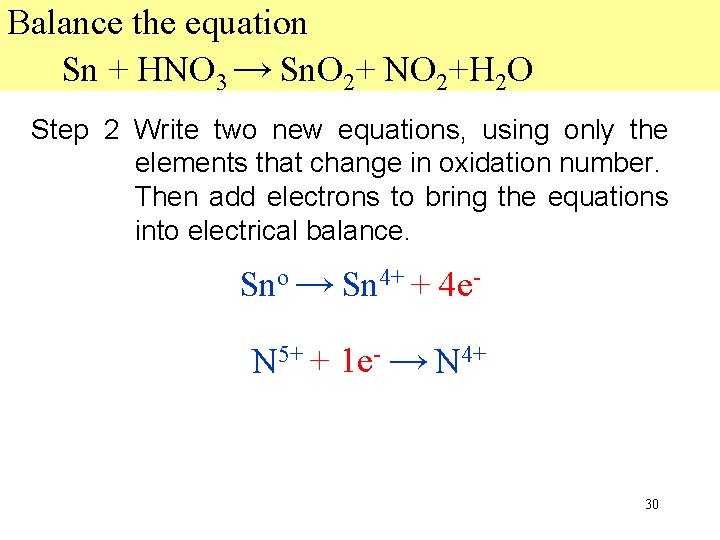
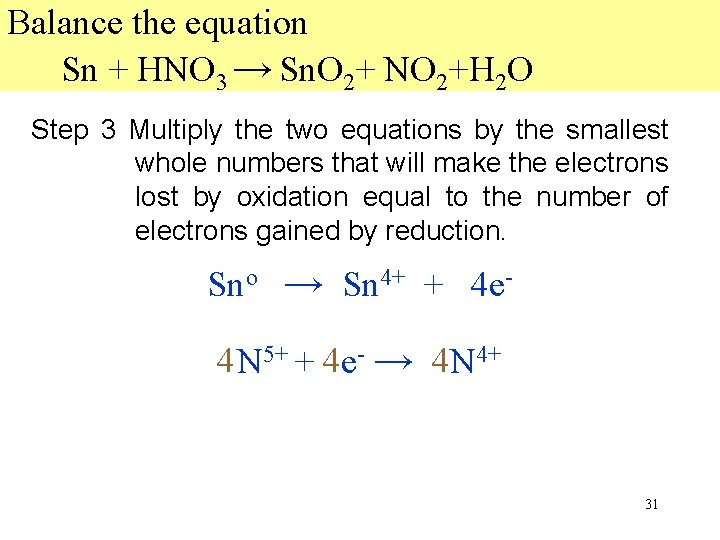
Balance the equation Sn + HNO 3 → Sn. O 2+ NO 2+H 2 O Step 2 Write two new equations, using only the elements that change in oxidation number. Then add electrons to bring the equations into electrical balance. Sno → Sn 4+ + 4 e 4 N 5+ + 1 e- → N 4+ 30

Balance the equation Sn + HNO 3 → Sn. O 2+ NO 2+H 2 O Step 3 Multiply the two equations by the smallest whole numbers that will make the electrons lost by oxidation equal to the number of electrons gained by reduction. Sno → Sn 4+ + 4 e 44 N 5+ + 44 e- → 44 N 4+ 31

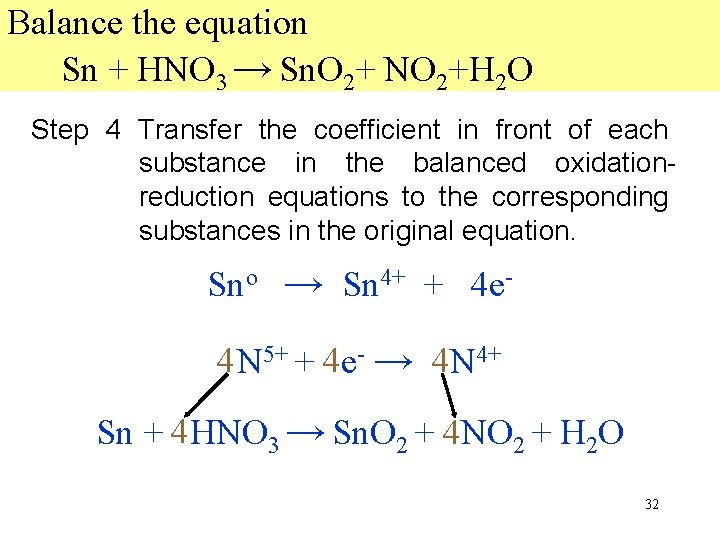
Balance the equation Sn + HNO 3 → Sn. O 2+ NO 2+H 2 O Step 4 Transfer the coefficient in front of each substance in the balanced oxidationreduction equations to the corresponding substances in the original equation. Sno → Sn 4+ + 4 e 44 N 5+ + 44 e- → 44 N 4+ Sn + 44 HNO 3 → Sn. O 2 + 44 NO 2 + H 2 O 32

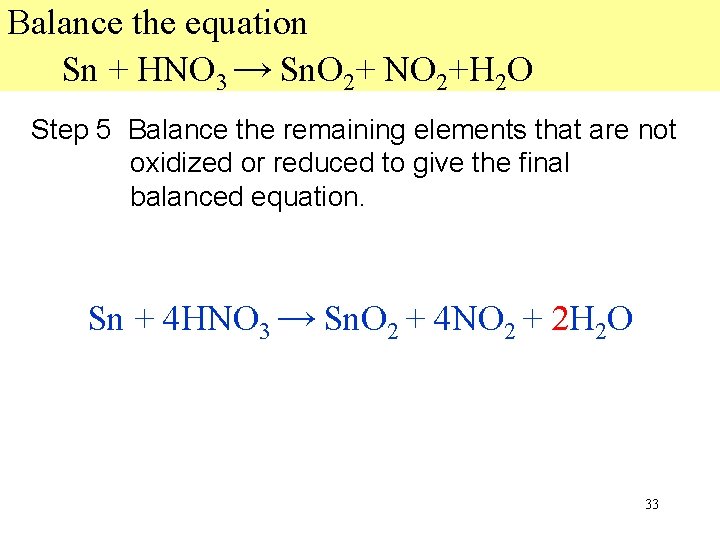
Balance the equation Sn + HNO 3 → Sn. O 2+ NO 2+H 2 O Step 5 Balance the remaining elements that are not oxidized or reduced to give the final balanced equation. 2 2 O Sn + 4 HNO 3 → Sn. O 2 + 4 NO 2 + 2 H 33

Balancing Ionic Redox Equations 34

In ionic redox equations both the numbers of atoms and the charges on both sides of the equation must be the same. 35

The Ion-Electron Method 36

Step 1 Write the two half-reactions that contain the elements being oxidized and reduced. Step 2 Balance the elements other than hydrogen and oxygen. 37

Step 3 Balance hydrogen and oxygen. Acidic solution: • For reactions in acidic solution, use H+ and H 2 O to balance oxygen and hydrogen. • For each oxygen needed use one H 2 O. • Then add H+ as needed to balance the hydrogen atoms. 38

Step 3 Balance hydrogen and oxygen. Basic solution: • For reactions in alkaline solutions, first balance as thought the reactions were in an acid solution, using Steps 1 -3. • Then add as many OH- ions to each side of the equation as there H+ ions in the equation. • Combine OH- ions into water. Example: 4 H+ + 4 OH- → 4 H 2 O • Rewrite the equation, canceling equal numbers of water molecules that appear on opposite 39 side of the equation.

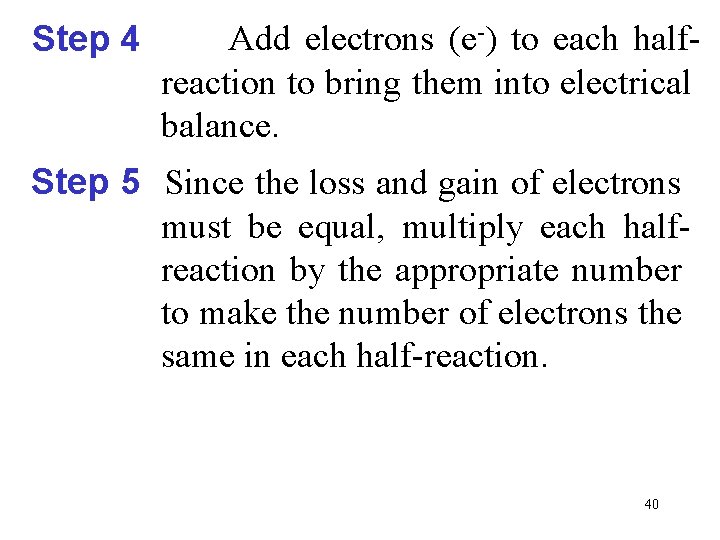
Step 4 Add electrons (e-) to each halfreaction to bring them into electrical balance. Step 5 Since the loss and gain of electrons must be equal, multiply each halfreaction by the appropriate number to make the number of electrons the same in each half-reaction. 40

Step 6 Add the two half-reactions together, canceling electrons and any other identical substances that appear on opposite sides of the equation. 41

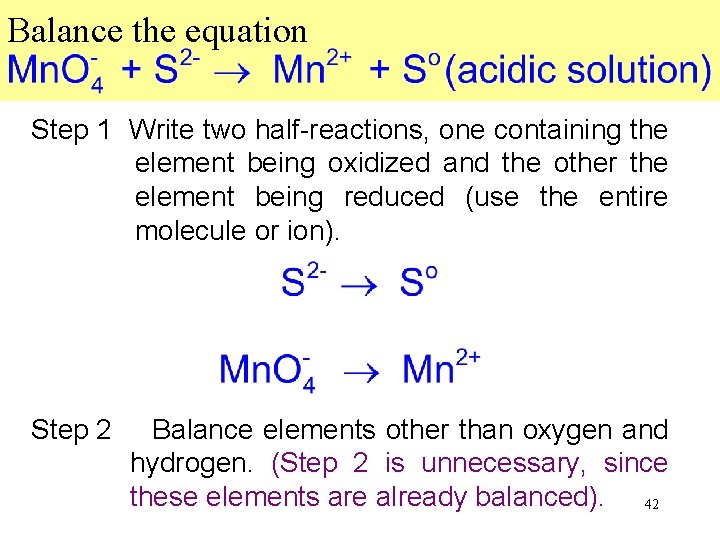
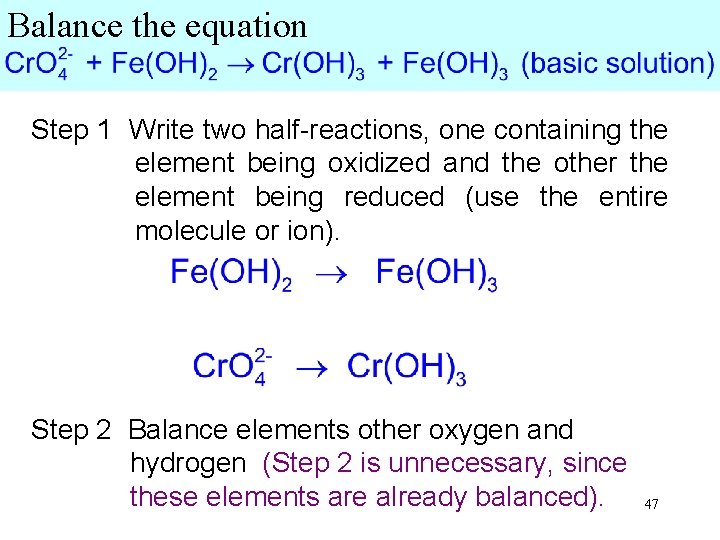
Balance the equation Step 1 Write two half-reactions, one containing the element being oxidized and the other the element being reduced (use the entire molecule or ion). Step 2 Balance elements other than oxygen and hydrogen. (Step 2 is unnecessary, since these elements are already balanced). 42

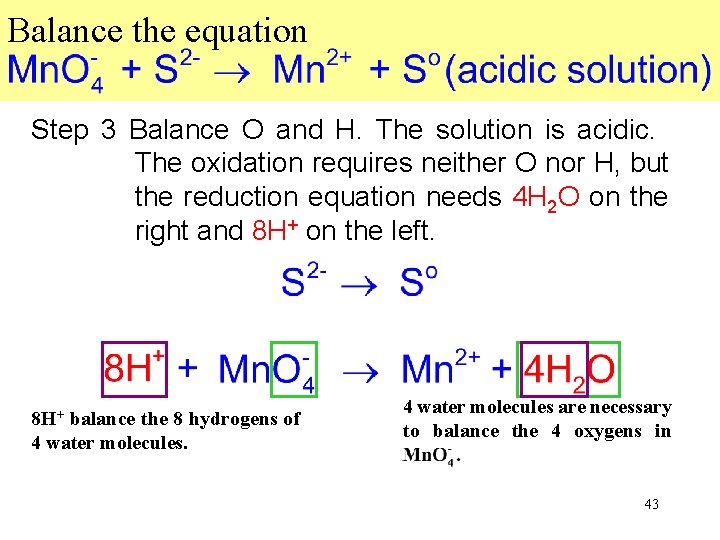
Balance the equation Step 3 Balance O and H. The solution is acidic. The oxidation requires neither O nor H, but the reduction equation needs 4 H 2 O on the right and 8 H+ on the left. 8 H+ balance the 8 hydrogens of 4 water molecules. Mn. O 4. 4 water molecules are necessary to balance the 4 oxygens in Mn. O 4. 43

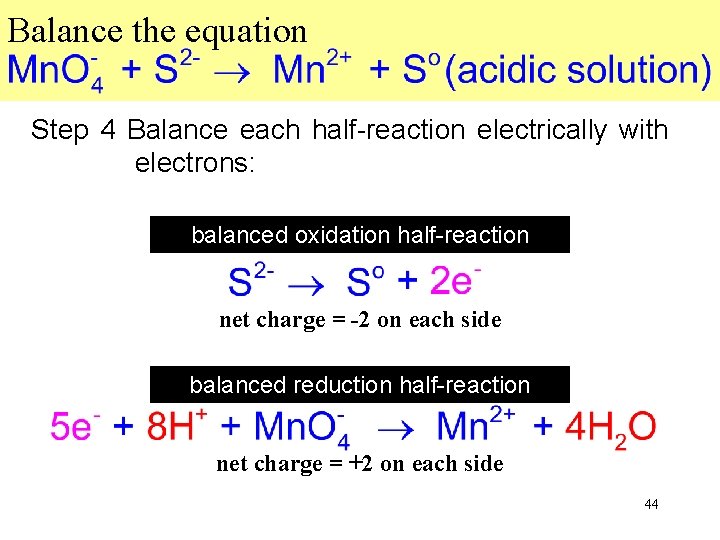
Balance the equation Step 4 Balance each half-reaction electrically with electrons: balanced oxidation half-reaction net charge = -2 on each side balanced reduction half-reaction net charge = +2 on each side 44

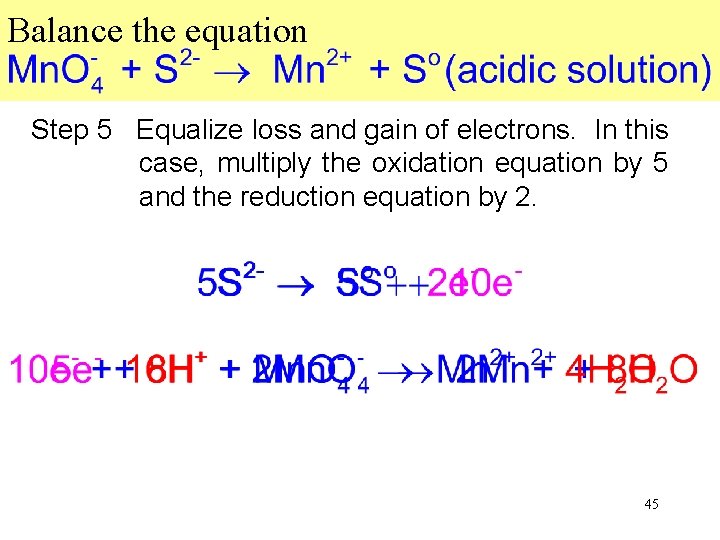
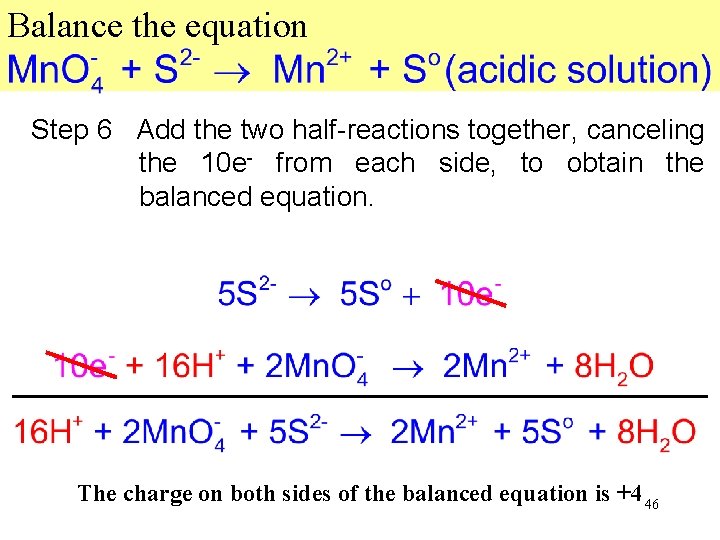
Balance the equation Step 5 Equalize loss and gain of electrons. In this case, multiply the oxidation equation by 5 and the reduction equation by 2. 45

Balance the equation Step 6 Add the two half-reactions together, canceling the 10 e- from each side, to obtain the balanced equation. The charge on both sides of the balanced equation is +4 46

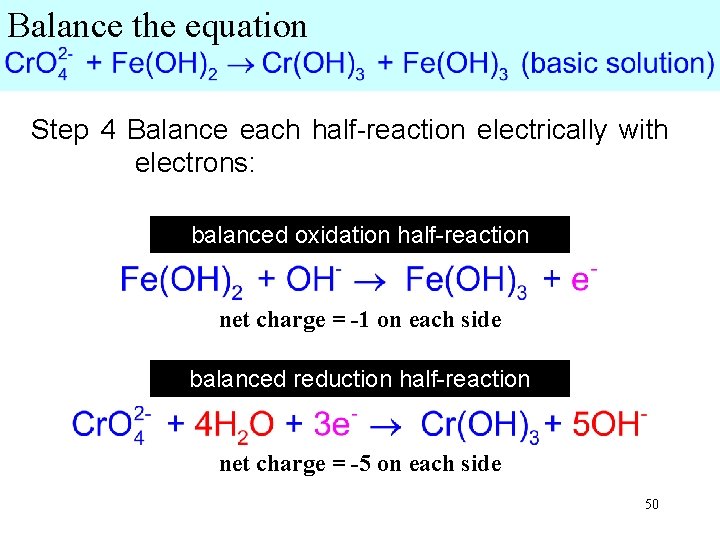
Balance the equation Step 1 Write two half-reactions, one containing the element being oxidized and the other the element being reduced (use the entire molecule or ion). Step 2 Balance elements other oxygen and hydrogen (Step 2 is unnecessary, since these elements are already balanced). 47

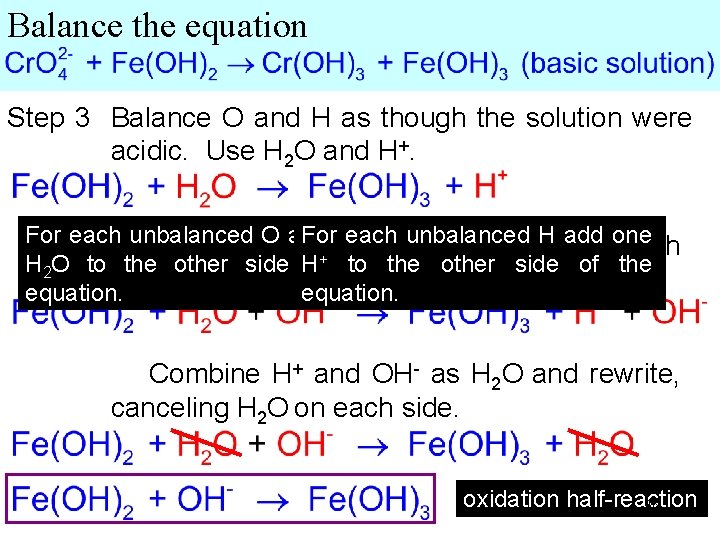
Balance the equation Step 3 Balance O and H as though the solution were acidic. Use H 2 O and H+. For each unbalanced O add Forone each unbalanced H add - toone Since the solution is basic, add 1 OH each + H 2 O to the other side H of the to the other side of the side. equation. Combine H+ and OH- as H 2 O and rewrite, canceling H 2 O on each side. oxidation half-reaction 48

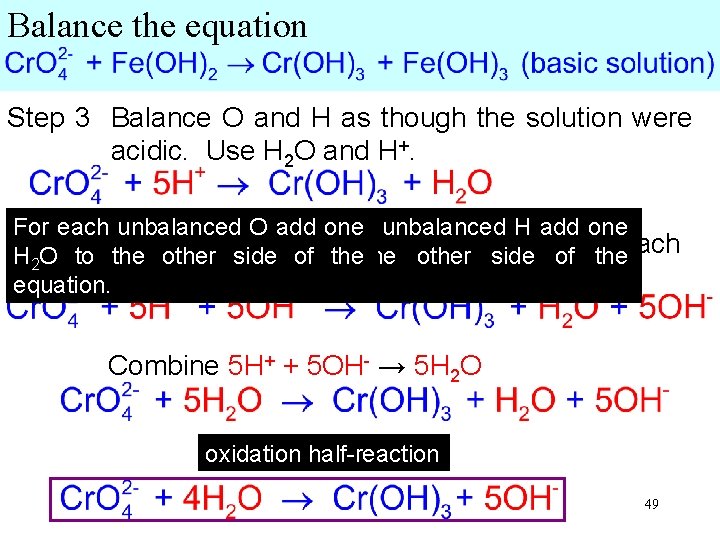
Balance the equation Step 3 Balance O and H as though the solution were acidic. Use H 2 O and H+. For each unbalanced O add For each one unbalanced H add- one Since the side solution isthe basic, addside 5 OH + to H 2 O to the other Hof the other of to theeach equation. side. equation. Combine 5 H+ + 5 OH- → 5 H 2 O half-reaction Rewrite, oxidation canceling 1 H 2 O from each side: 49

Balance the equation Step 4 Balance each half-reaction electrically with electrons: balanced oxidation half-reaction net charge = -1 on each side balanced reduction half-reaction net charge = -5 on each side 50

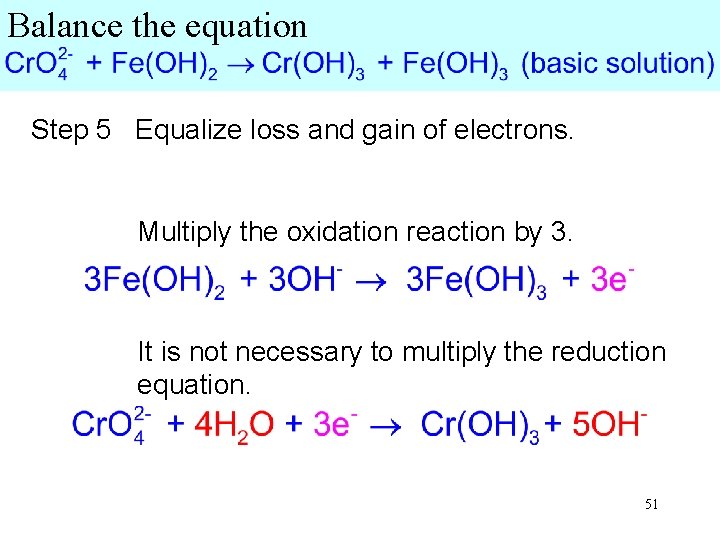
Balance the equation Step 5 Equalize loss and gain of electrons. Multiply the oxidation reaction by 3. It is not necessary to multiply the reduction equation. 51

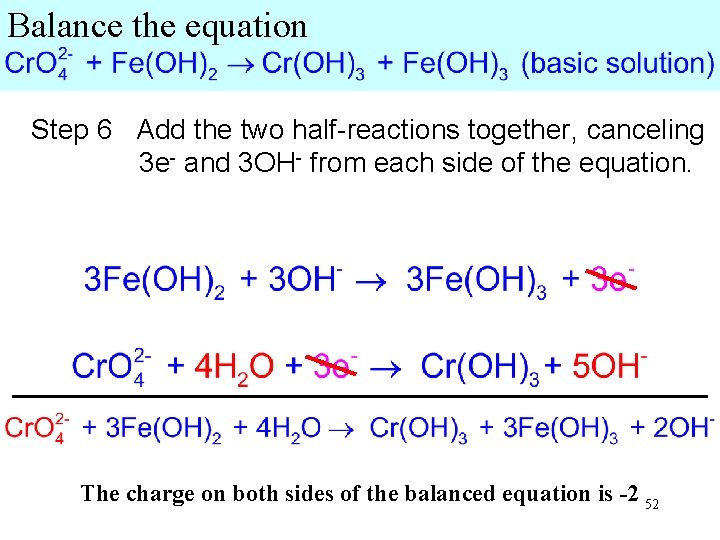
Balance the equation Step 6 Add the two half-reactions together, canceling 3 e- and 3 OH- from each side of the equation. The charge on both sides of the balanced equation is -2 52

Activity Series of Metals 53

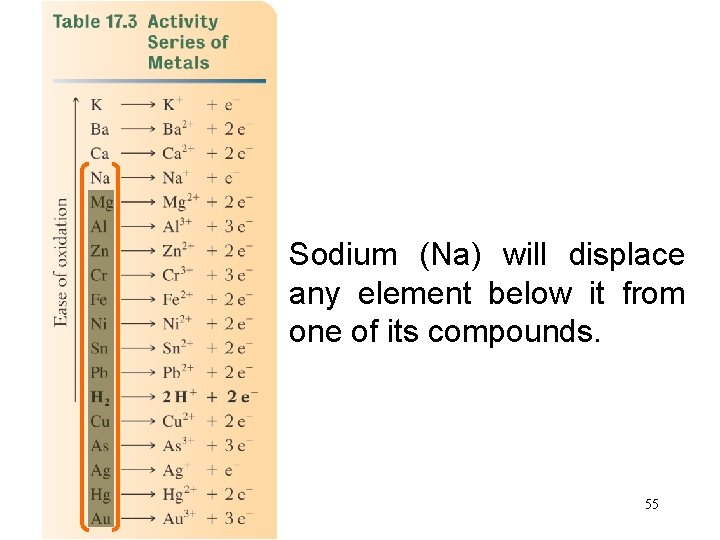
activity series A listing of metallic elements in descending order of reactivity. 54

Sodium (Na) will displace any element below it from one of its compounds. 55

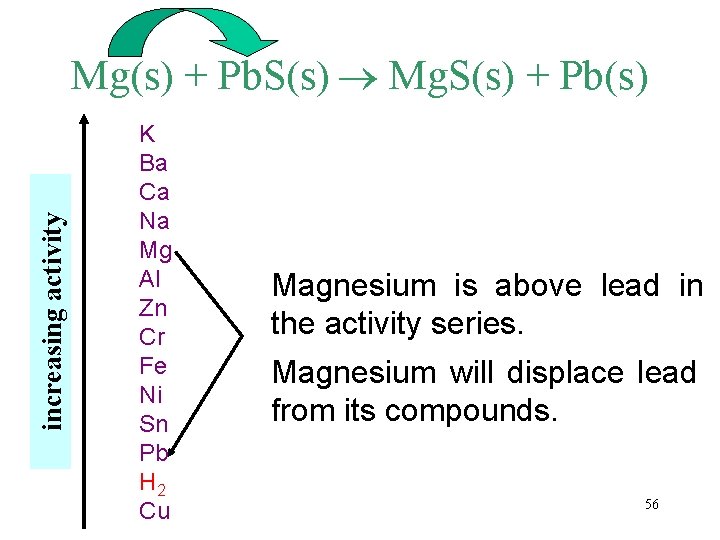
increasing activity Mg(s) + Pb. S(s) Mg. S(s) + Pb(s) K Ba Ca Na Mg Al Zn Cr Fe Ni Sn Pb H 2 Cu Magnesium is above lead in the activity series. Magnesium will displace lead from its compounds. 56

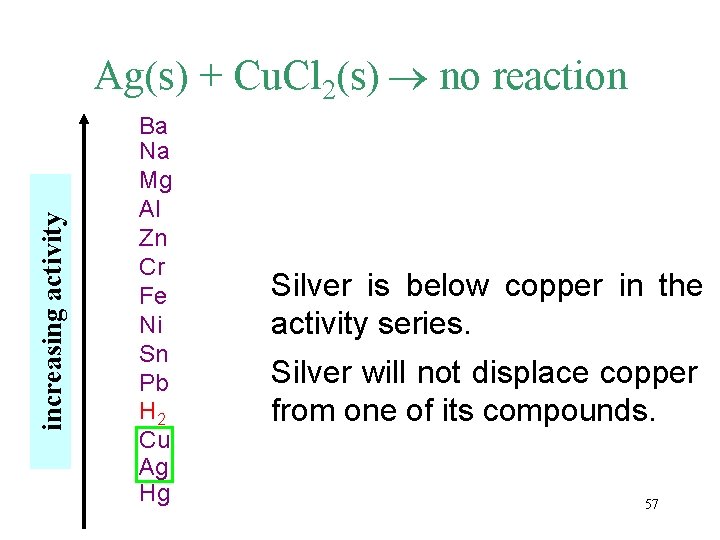
increasing activity Ag(s) + Cu. Cl 2(s) no reaction Ba Na Mg Al Zn Cr Fe Ni Sn Pb H 2 Cu Ag Hg Silver is below copper in the activity series. Silver will not displace copper from one of its compounds. 57

Electrolytic and Voltaic Cells 58

electrolysis cell The. An process whereby electrical electrolytic electrolysis apparatus in energy electrical which is used toenergy bring about from aanchemical outside source change. is used to produce a chemical change. 59

anode positive electrode. cathode. The negative electrode. 60

Electrolysis of Hydrochloric Acid 61

In an electrolytic cell electrical energy from the voltage source is used to bring about nonspontaneous redox reactions. 62

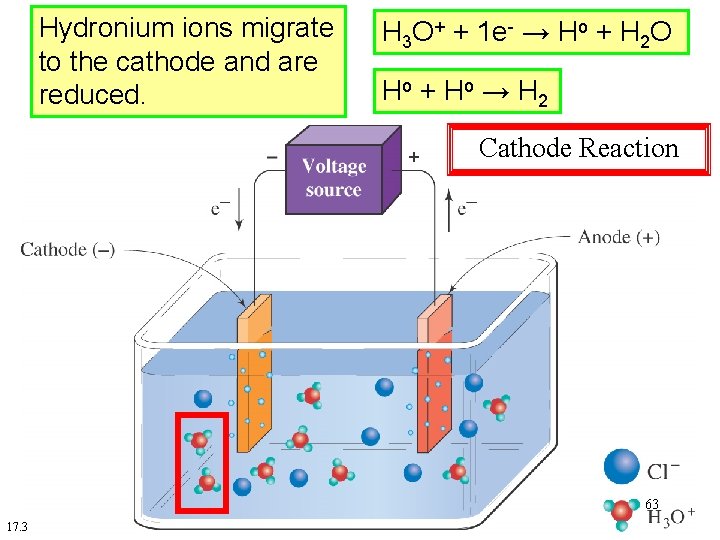
Hydronium ions migrate to the cathode and are reduced. H 3 O+ + 1 e- → Ho + H 2 O Ho + H o → H 2 Cathode Reaction 63 17. 3

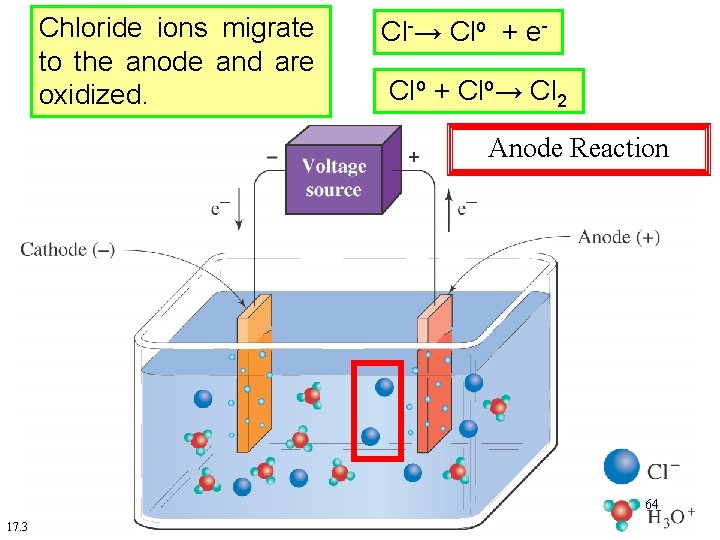
Chloride ions migrate to the anode and are oxidized. Cl-→ Clo + e. Clo + Clo→ Cl 2 Anode Reaction 64 17. 3

2 HCl(aq) electrolysis H 2(g) + Cl 2(g) The hydrogen and chlorine produced when HCl is electrolyzed have more potential energy than was present in the hydrochloric acid before electrolysis. 65

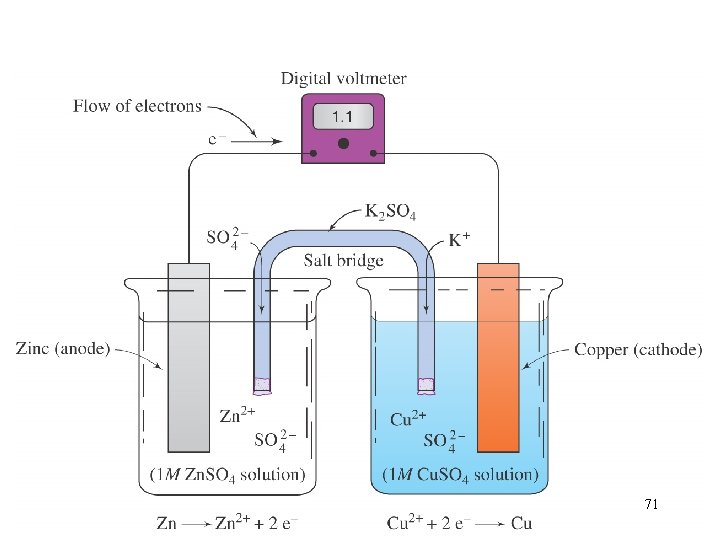
The Zinc-Copper Voltaic Cell 66

voltaic cell A cell that produces electrical energy from a spontaneous chemical reaction. (Also known as a galvanic cell). 67

When a piece of zinc is put in a copper(II) sulfate solution, the zinc quickly becomes coated with metallic copper. This occurs because zinc is above copper in the activity series. 68

increasing activity Zn(s) + Cu. SO 4(s) Zn. SO 4(s) + Cu(s) K Ba Ca Na Mg Al Zn Cr Fe Ni Sn Pb H 2 Cu Zinc is above copper in the activity series. Zinc will displace copper from one of its compounds. 69

If this reaction is carried out in a voltaic cell, an electric current is produced. 70

71

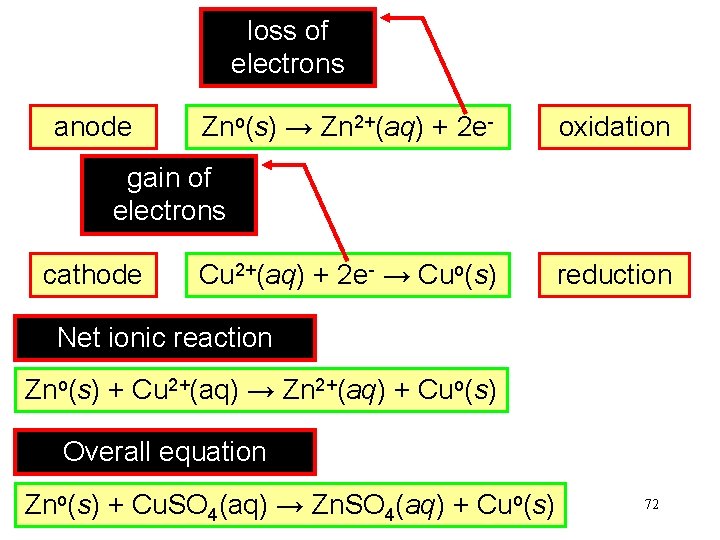
loss of electrons anode Zno(s) → Zn 2+(aq) + 2 e- oxidation gain of electrons cathode Cu 2+(aq) + 2 e- → Cuo(s) reduction Net ionic reaction Zno(s) + Cu 2+(aq) → Zn 2+(aq) + Cuo(s) Overall equation Zno(s) + Cu. SO 4(aq) → Zn. SO 4(aq) + Cuo(s) 72

Spontaneity The Spontaneous Reactions reactions thatreactions isofoccur the zinc-copper incrucial occur electrolytic in difference allcell voltaic cells are spontaneous. cells. are between nonspontaneous. all voltaic and electrolytic cells. 73

Key Concepts 17. 1 Oxidation Number 17. 2 Oxidation-Reduction 17. 3 Balancing Oxidation. Reduction Equations 17. 4 Balancing Ionic Redox Equations 17. 5 Activity Series of Metals 17. 6 Electrolytic and Voltaic Cells 74