The Gaseous State of Matter Chapter 12 Hein


















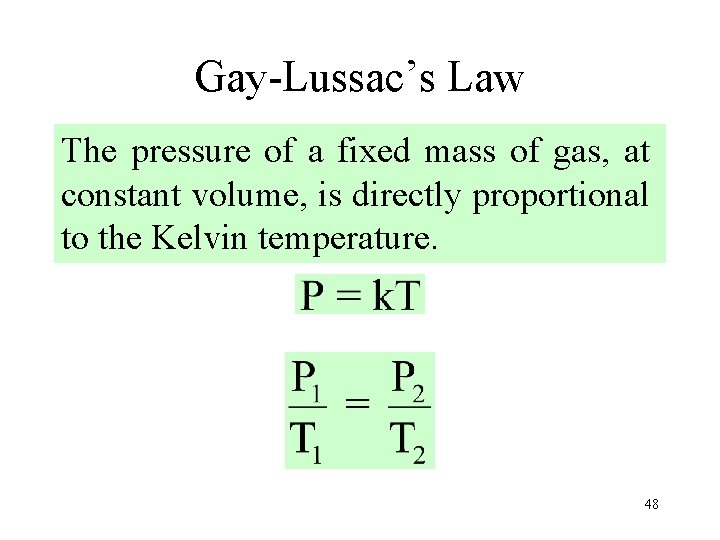



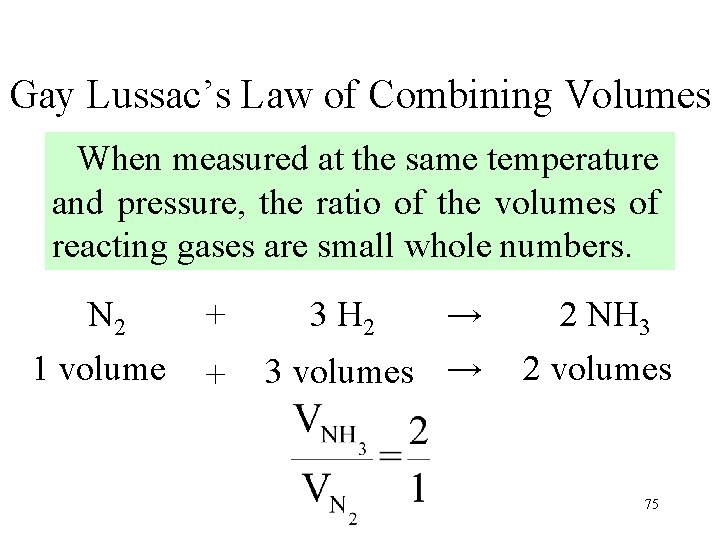
















- Slides: 116

The Gaseous State of Matter Chapter 12 Hein and Arena Version 2. 0 12 th Edition Eugene Passer Chemistry Department Bronx Community 1 College © John Wiley and Sons, Inc

Chapter Outline 12. 2 The Kinetic Molecular Theory 12. 9 Dalton’s Law of Partial Pressures 12. 3 Measurement of Pressure of 12. 10 Avogadro’s Law Gases 12. 4 Dependence of Pressure on 12. 11 Mole-Mass-Volume Relationships of Gases Number of Molecules and Temperature 12. 5 Boyle’s Law 12. 6 Charles’ Law 12. 12 Density of Gases 12. 13 Ideal Gas Law 12. 7 Gay-Lussac’s Law 12. 14 Gas Stoichiometry 12. 8 Combined Gas Laws 12. 15 Real Gases 2

12. 2 The Kinetic. Molecular Theory 3

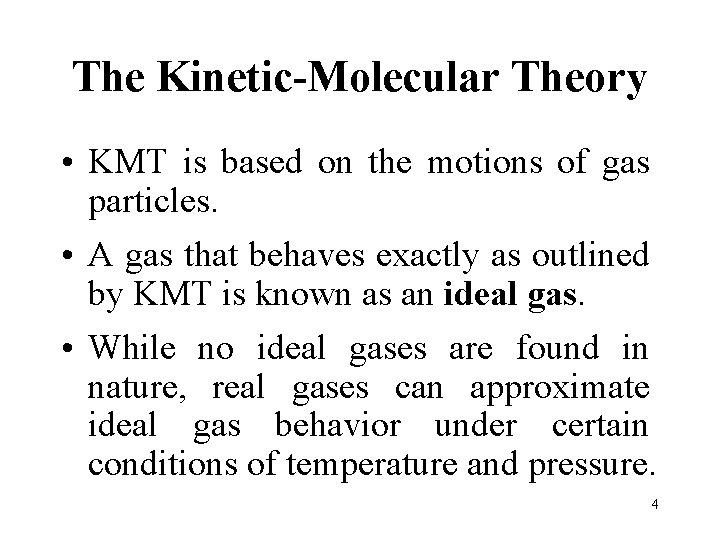
The Kinetic-Molecular Theory • KMT is based on the motions of gas particles. • A gas that behaves exactly as outlined by KMT is known as an ideal gas. • While no ideal gases are found in nature, real gases can approximate ideal gas behavior under certain conditions of temperature and pressure. 4

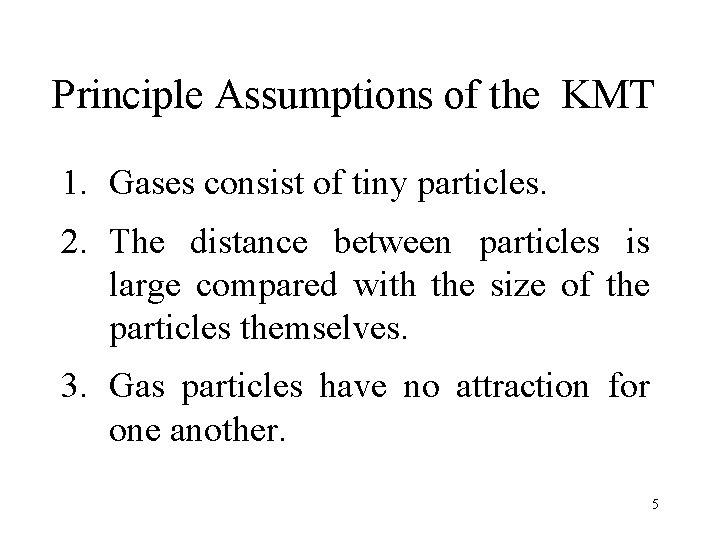
Principle Assumptions of the KMT 1. Gases consist of tiny particles. 2. The distance between particles is large compared with the size of the particles themselves. 3. Gas particles have no attraction for one another. 5

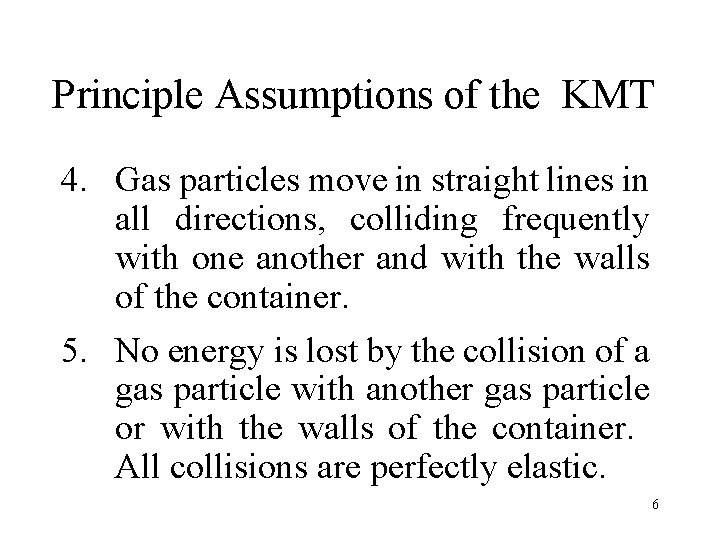
Principle Assumptions of the KMT 4. Gas particles move in straight lines in all directions, colliding frequently with one another and with the walls of the container. 5. No energy is lost by the collision of a gas particle with another gas particle or with the walls of the container. All collisions are perfectly elastic. 6

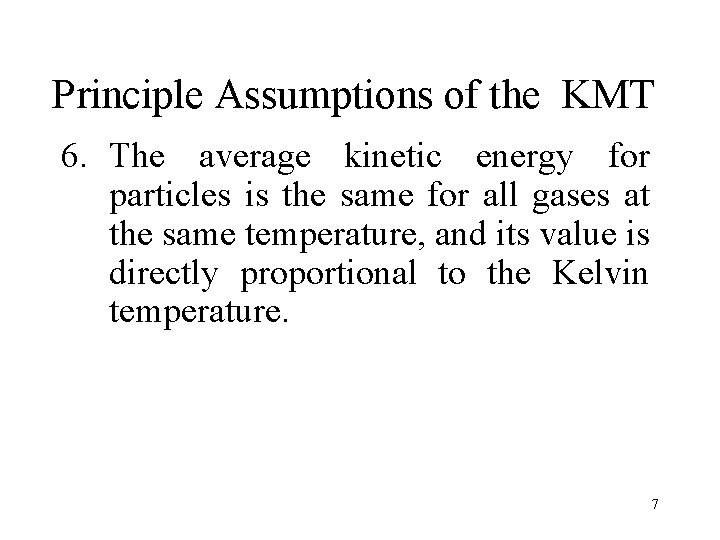
Principle Assumptions of the KMT 6. The average kinetic energy for particles is the same for all gases at the same temperature, and its value is directly proportional to the Kelvin temperature. 7

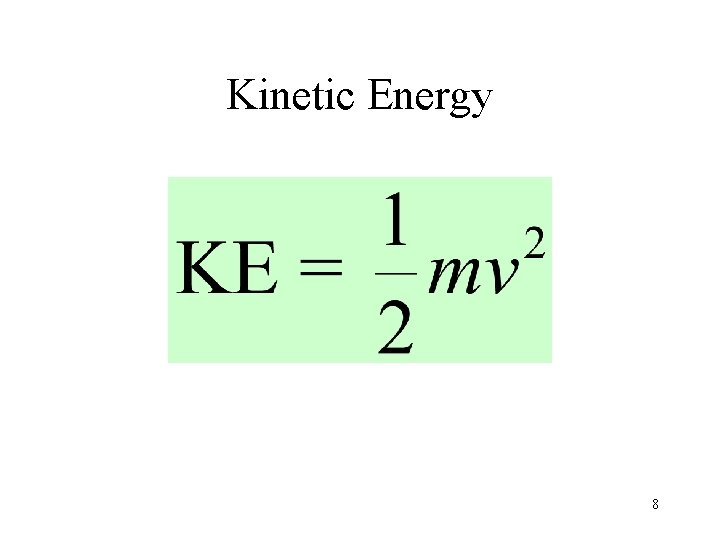
Kinetic Energy 8

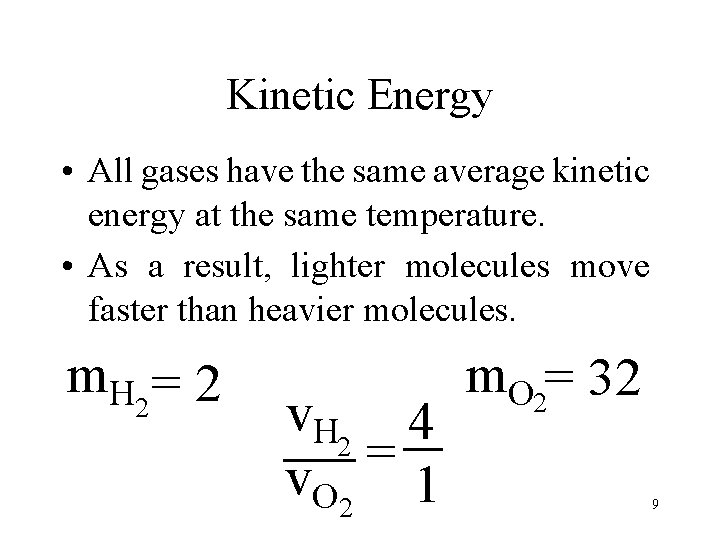
Kinetic Energy • All gases have the same average kinetic energy at the same temperature. • As a result, lighter molecules move faster than heavier molecules. m. H 2 = 2 v. H 2 4 = v. O 2 1 m. O 2= 32 9

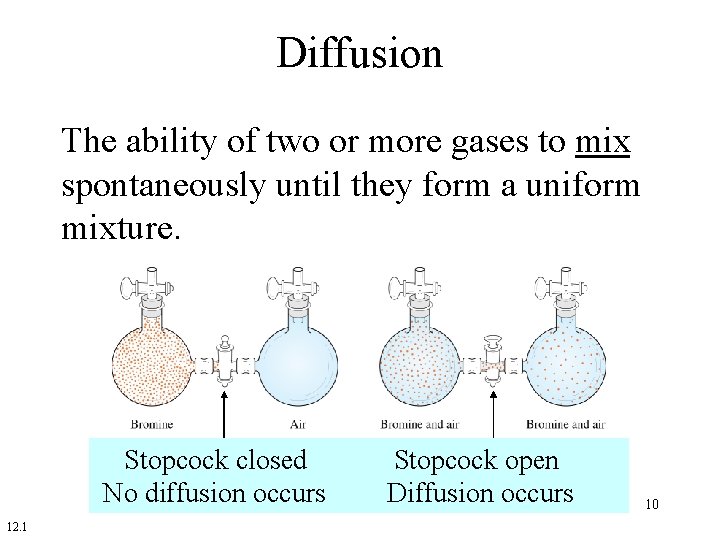
Diffusion The ability of two or more gases to mix spontaneously until they form a uniform mixture. Stopcock closed No diffusion occurs 12. 1 Stopcock open Diffusion occurs 10

Effusion A process by which gas molecules pass through a very small orifice from a container at higher pressure to one at lower pressure. 11

Graham’s Law of Effusion The rates of effusion of two gases at the same temperature and pressure are inversely proportional to the square roots of their densities, or molar masses. 12

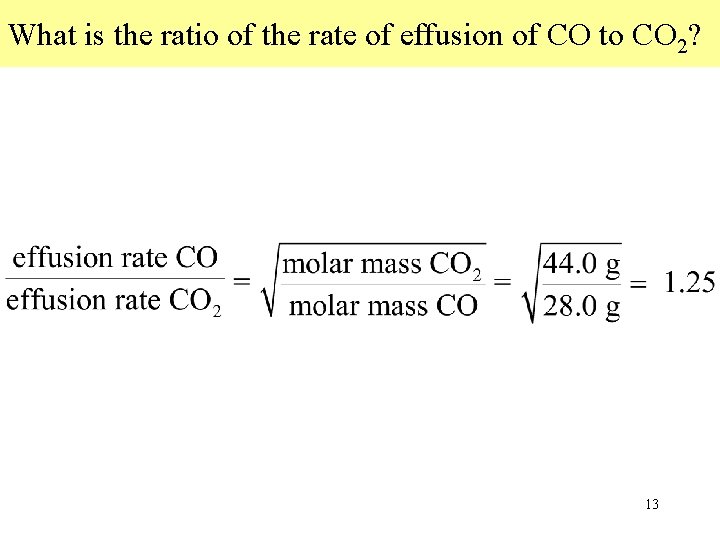
What is the ratio of the rate of effusion of CO to CO 2? 13

12. 3 Measurement of Pressure of Gases 14

Pressure equals force per unit area. 15

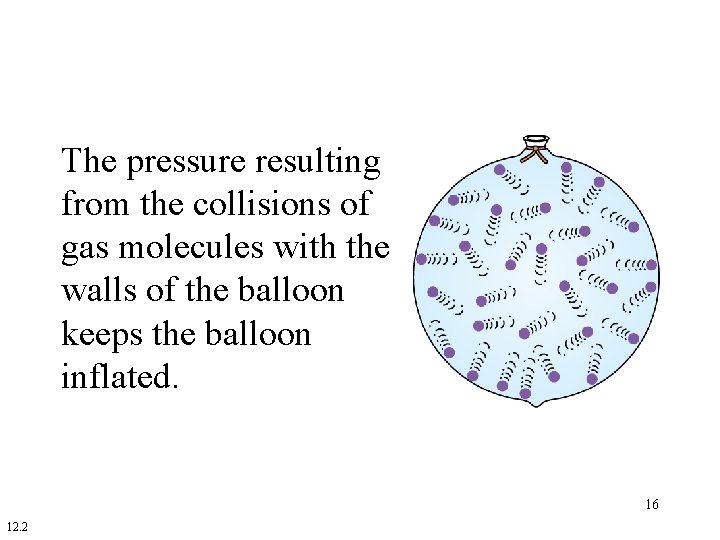
The pressure resulting from the collisions of gas molecules with the walls of the balloon keeps the balloon inflated. 16 12. 2

The pressure exerted by a gas depends on the • Number of gas molecules present. • Temperature of the gas. • Volume in which the gas is confined. 17

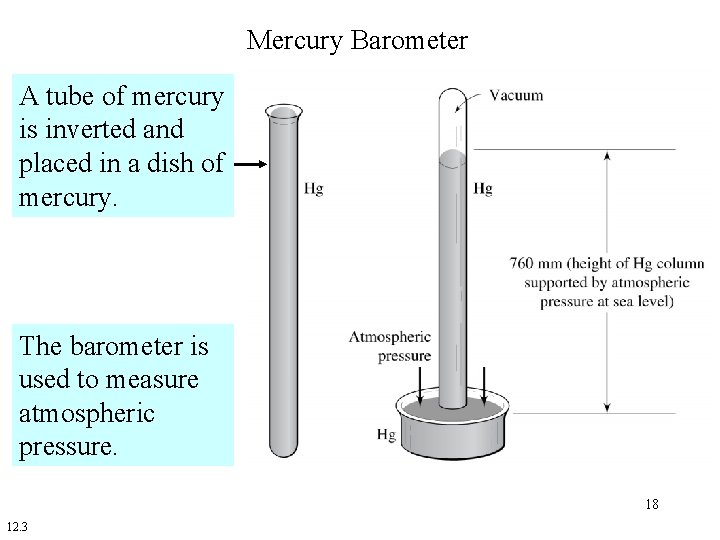
Mercury Barometer A tube of mercury is inverted and placed in a dish of mercury. The barometer is used to measure atmospheric pressure. 18 12. 3

19

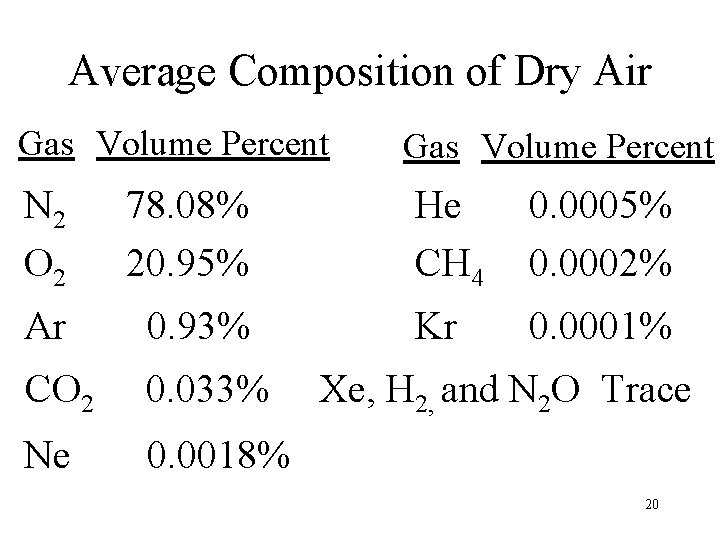
Average Composition of Dry Air Gas Volume Percent N 2 O 2 78. 08% 20. 95% Ar 0. 93% CO 2 0. 033% Ne 0. 0018% Gas Volume Percent He CH 4 0. 0005% 0. 0002% Kr 0. 0001% Xe, H 2, and N 2 O Trace 20

12. 4 Dependence of Pressure on Number of Molecules and Temperature 21

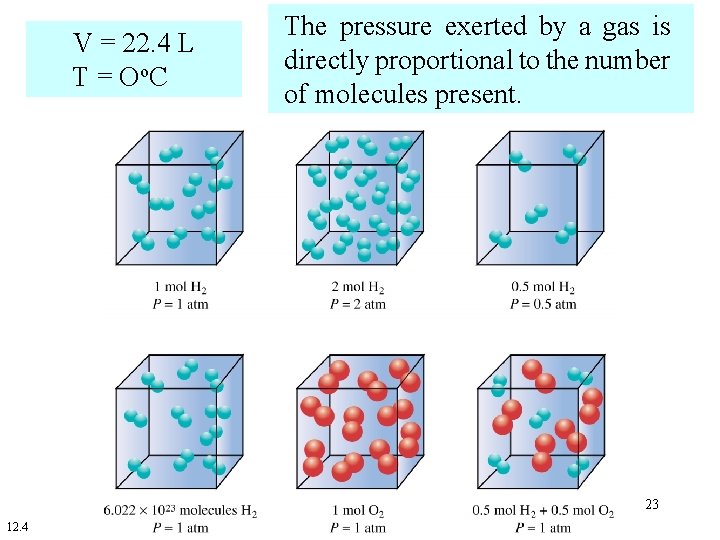
• Pressure is produced by gas molecules colliding with the walls of a container. • At a specific temperature and volume, the number of collisions depends on the number of gas molecules present. • For an ideal gas the number of collisions is directly proportional to the number of gas molecules present. 22

V = 22. 4 L T = Oo. C The pressure exerted by a gas is directly proportional to the number of molecules present. 23 12. 4

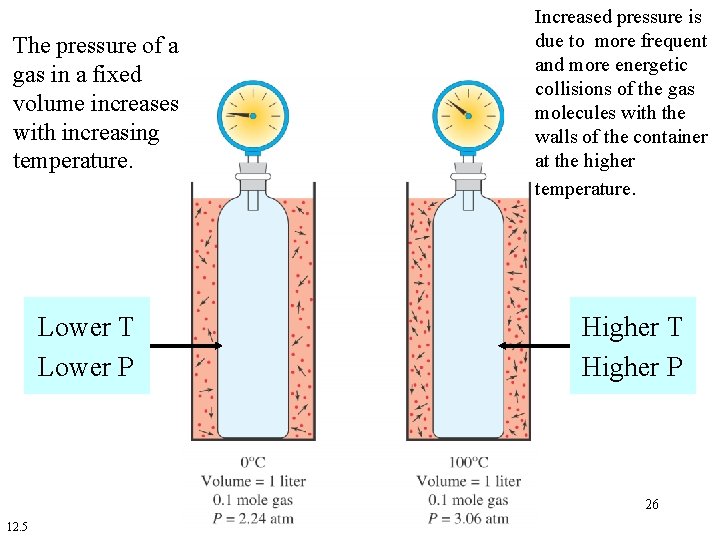
Dependence of Pressure on Temperature • The pressure of a gas in a fixed volume increases with increasing temperature. • When the pressure of a gas increases, its kinetic energy increases. • The increased kinetic energy of the gas results in more frequent and energetic collisions of the molecules with the walls of the container. 24

The pressure of a gas in a fixed volume increases with increasing temperature. Lower T Lower P Increased pressure is due to more frequent and more energetic collisions of the gas molecules with the walls of the container at the higher temperature. Higher T Higher P 26 12. 5

12. 5 Boyle’s Law 27

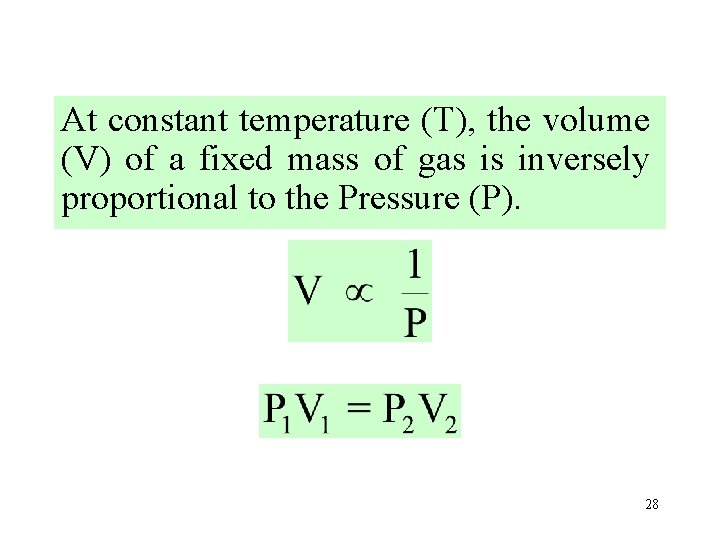
At constant temperature (T), the volume (V) of a fixed mass of gas is inversely proportional to the Pressure (P). 28

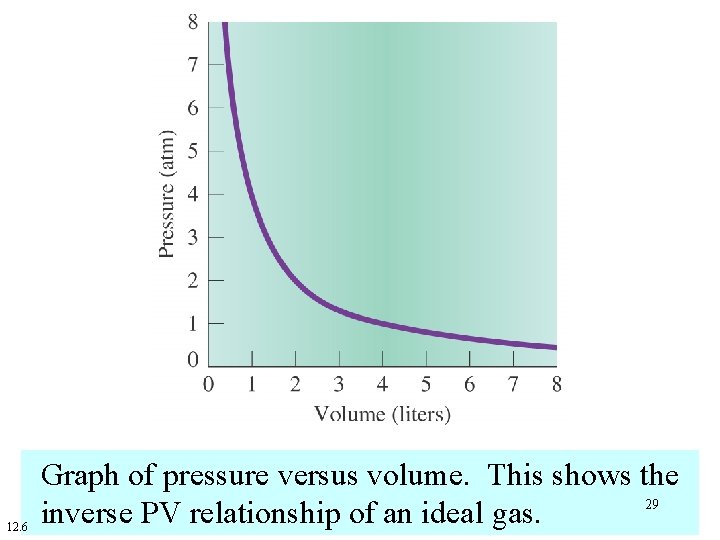
12. 6 Graph of pressure versus volume. This shows the 29 inverse PV relationship of an ideal gas.

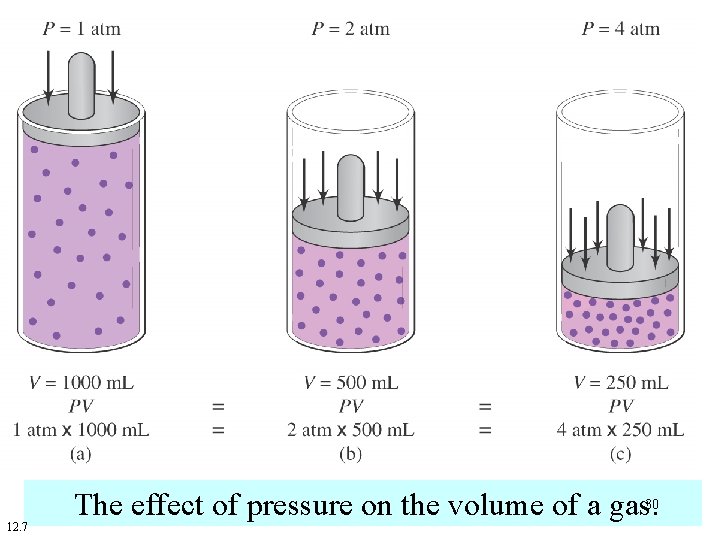
12. 7 The effect of pressure on the volume of a gas. 30

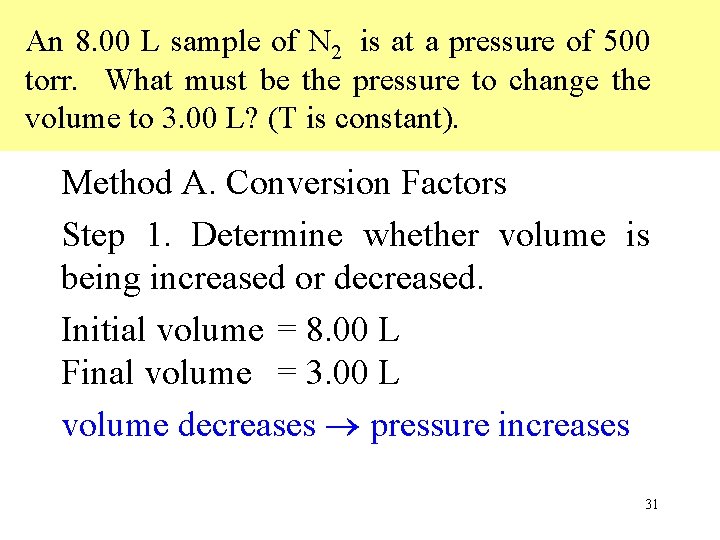
An 8. 00 L sample of N 2 is at a pressure of 500 torr. What must be the pressure to change the volume to 3. 00 L? (T is constant). Method A. Conversion Factors Step 1. Determine whether volume is being increased or decreased. Initial volume = 8. 00 L Final volume = 3. 00 L volume decreases pressure increases 31

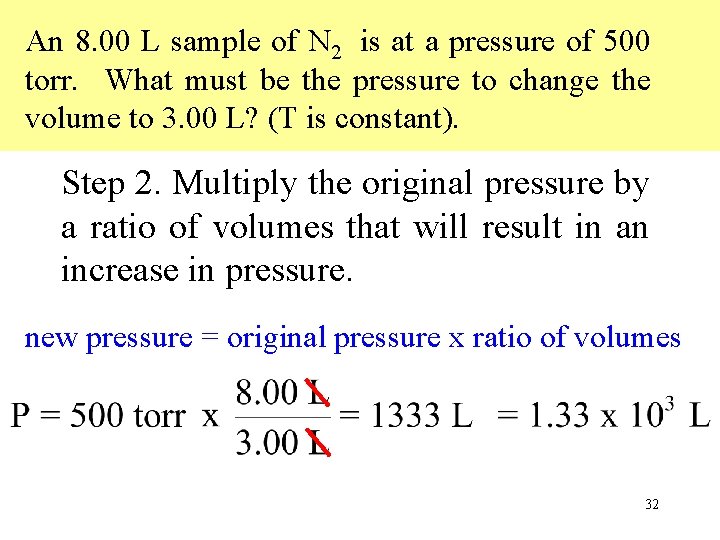
An 8. 00 L sample of N 2 is at a pressure of 500 torr. What must be the pressure to change the volume to 3. 00 L? (T is constant). Step 2. Multiply the original pressure by a ratio of volumes that will result in an increase in pressure. new pressure = original pressure x ratio of volumes 32

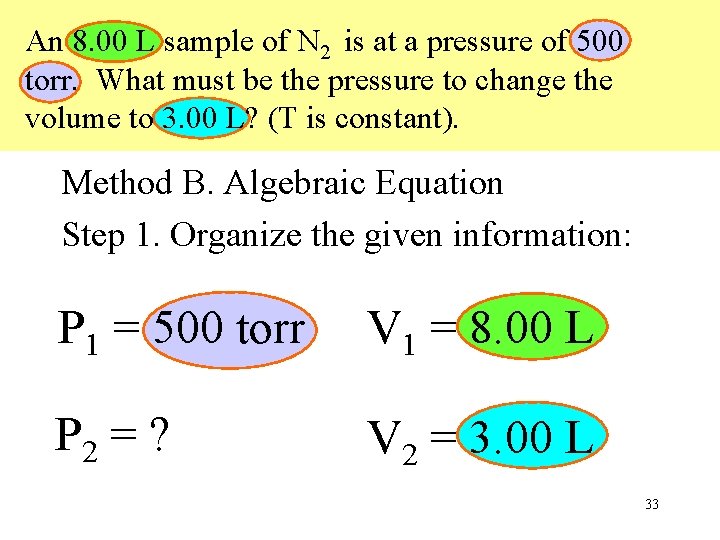
An 8. 00 L sample of N 2 is at a pressure of 500 torr. What must be the pressure to change the volume to 3. 00 L? (T is constant). Method B. Algebraic Equation Step 1. Organize the given information: P 1 = 500 torr V 1 = 8. 00 L P 2 = ? V 2 = 3. 00 L 33

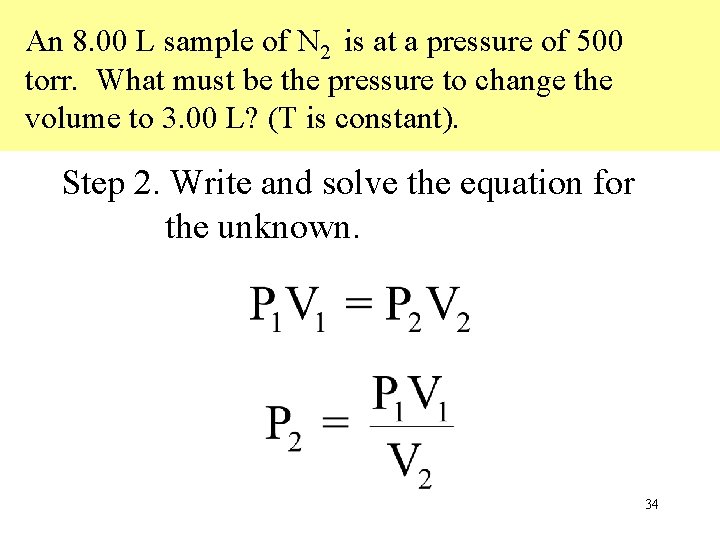
An 8. 00 L sample of N 2 is at a pressure of 500 torr. What must be the pressure to change the volume to 3. 00 L? (T is constant). Step 2. Write and solve the equation for the unknown. 34

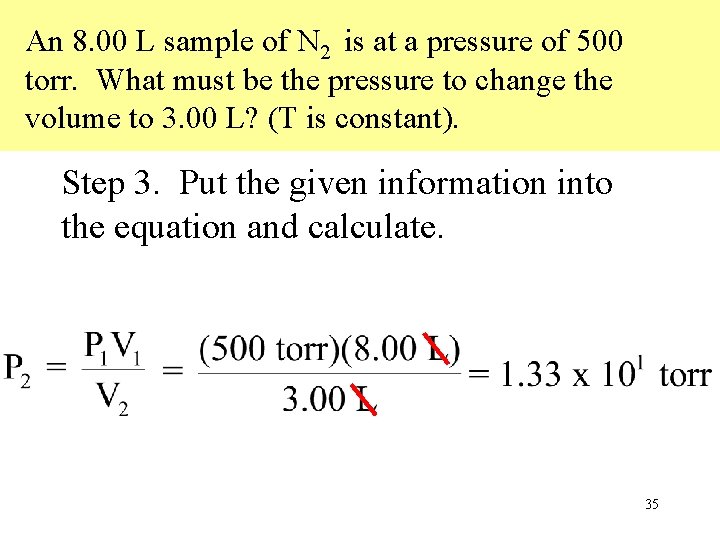
An 8. 00 L sample of N 2 is at a pressure of 500 torr. What must be the pressure to change the volume to 3. 00 L? (T is constant). Step 3. Put the given information into the equation and calculate. 35

12. 6 Charles’ Law 36

Absolute Zero on the Kelvin Scale • If a given volume of any gas at 0 o. C is cooled by 1 o. C the volume of the gas decreases by. • If a given volume of any gas at 0 o. C is cooled by 20 o. C the volume of the gas decreases by. 37

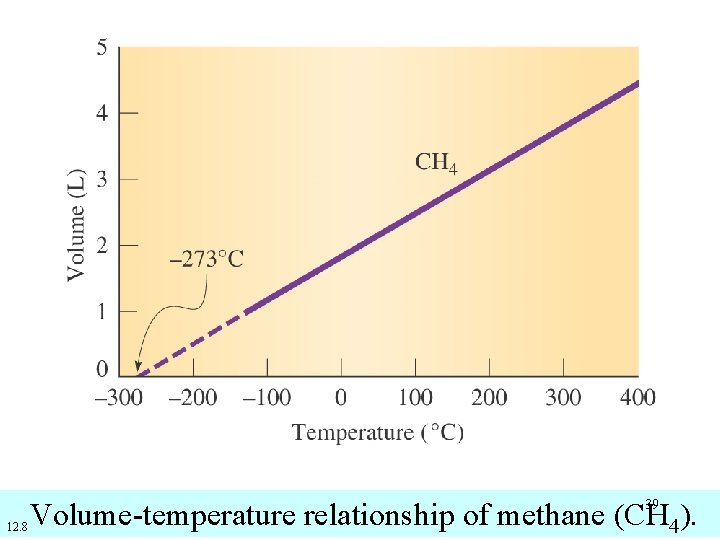
Absolute Zero on the Kelvin Scale • If a given volume of any gas at 0 o. C is cooled by 273 o. C the volume of the gas decreases by. • -273 o. C (more precisely – 273. 15 o. C) is the zero point on the Kelvin scale. It is the temperature at which an ideal gas would have 0 volume. 38

39 12. 8 Volume-temperature relationship of methane (CH 4).

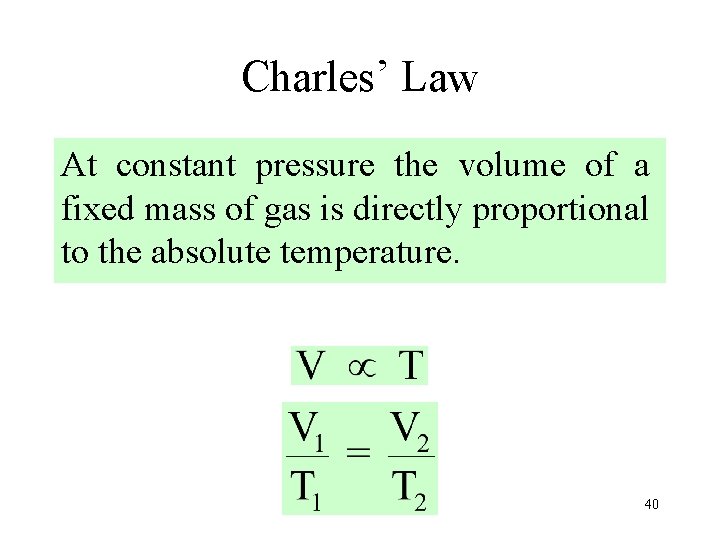
Charles’ Law At constant pressure the volume of a fixed mass of gas is directly proportional to the absolute temperature. 40

12. 9 Effect of temperature on the volume of a gas. Pressure is constant at 1 atm. When temperature increases at 41 constant pressure, the volume of the gas increases.

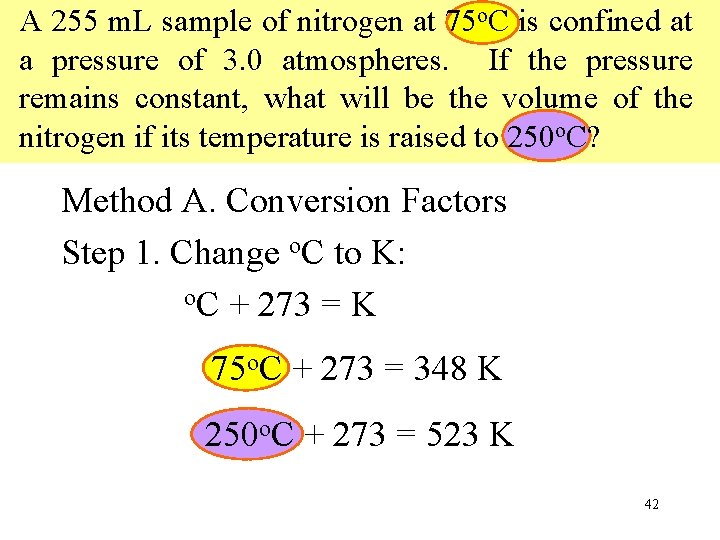
A 255 m. L sample of nitrogen at 75 o. C is confined at a pressure of 3. 0 atmospheres. If the pressure remains constant, what will be the volume of the nitrogen if its temperature is raised to 250 o. C? Method A. Conversion Factors Step 1. Change o. C to K: o. C + 273 = K 75 o. C + 273 = 348 K 250 o. C + 273 = 523 K 42

A 255 m. L sample of nitrogen at 75 o. C is confined at a pressure of 3. 0 atmospheres. If the pressure remains constant, what will be the volume of the nitrogen if its temperature is raised to 250 o. C? Step 2: Multiply the original volume by a ratio of Kelvin temperatures that will result in an increase in volume: 43

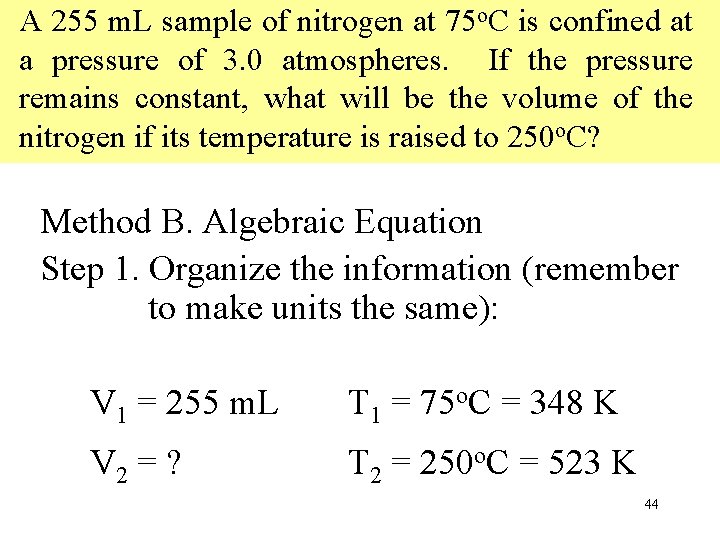
A 255 m. L sample of nitrogen at 75 o. C is confined at a pressure of 3. 0 atmospheres. If the pressure remains constant, what will be the volume of the nitrogen if its temperature is raised to 250 o. C? Method B. Algebraic Equation Step 1. Organize the information (remember to make units the same): V 1 = 255 m. L T 1 = 75 o. C = 348 K V 2 = ? T 2 = 250 o. C = 523 K 44

A 255 m. L sample of nitrogen at 75 o. C is confined at a pressure of 3. 0 atmospheres. If the pressure remains constant, what will be the volume of the nitrogen if its temperature is raised to 250 o. C? Step 2. Write and solve the equation for the unknown: 45

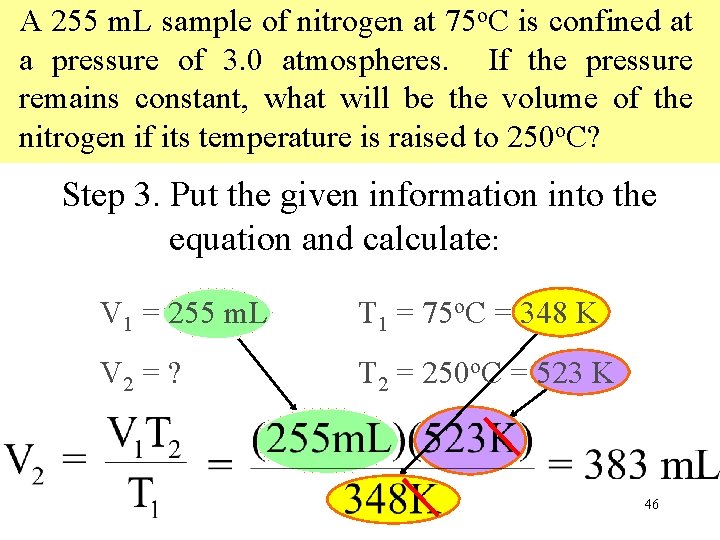
A 255 m. L sample of nitrogen at 75 o. C is confined at a pressure of 3. 0 atmospheres. If the pressure remains constant, what will be the volume of the nitrogen if its temperature is raised to 250 o. C? Step 3. Put the given information into the equation and calculate: V 1 = 255 m. L T 1 = 75 o. C = 348 K V 2 = ? T 2 = 250 o. C = 523 K 46
12. 7 Gay-Lussac’s Law 47
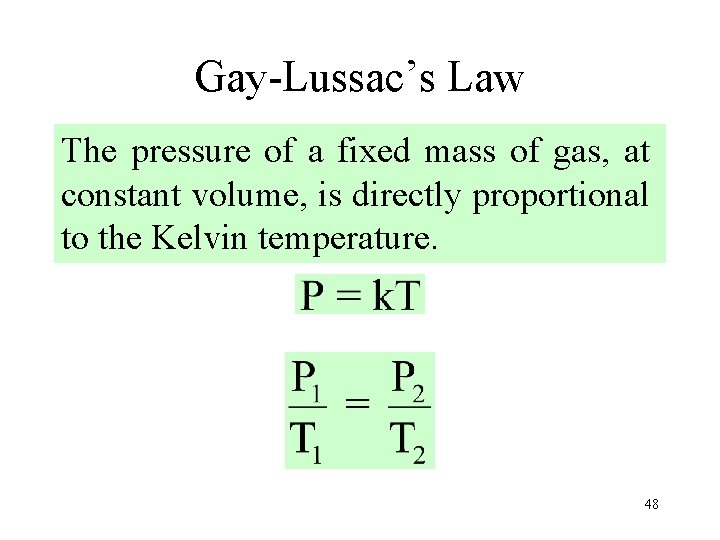
Gay-Lussac’s Law The pressure of a fixed mass of gas, at constant volume, is directly proportional to the Kelvin temperature. 48

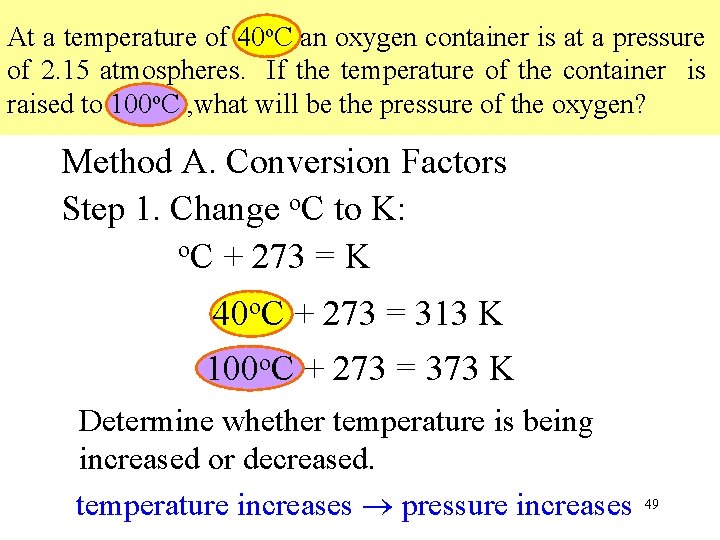
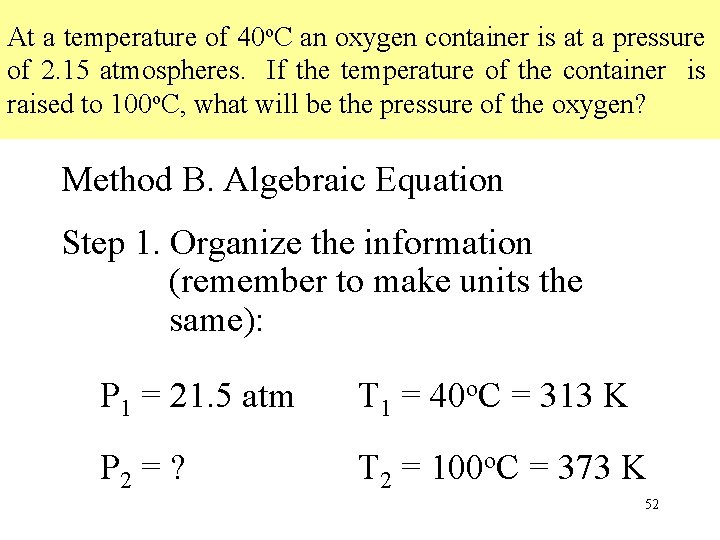
At a temperature of 40 o. C an oxygen container is at a pressure of 2. 15 atmospheres. If the temperature of the container is raised to 100 o. C , what will be the pressure of the oxygen? Method A. Conversion Factors Step 1. Change o. C to K: o. C + 273 = K 40 o. C + 273 = 313 K 100 o. C + 273 = 373 K Determine whether temperature is being increased or decreased. temperature increases pressure increases 49

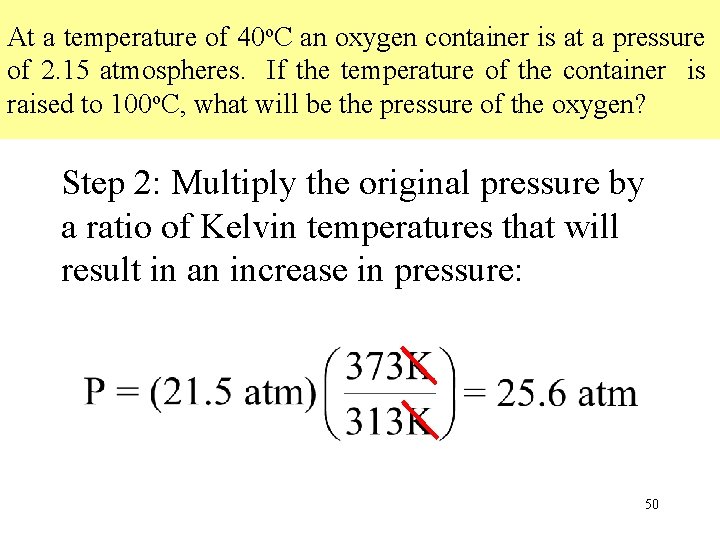
At a temperature of 40 o. C an oxygen container is at a pressure of 2. 15 atmospheres. If the temperature of the container is raised to 100 o. C, what will be the pressure of the oxygen? Step 2: Multiply the original pressure by a ratio of Kelvin temperatures that will result in an increase in pressure: 50

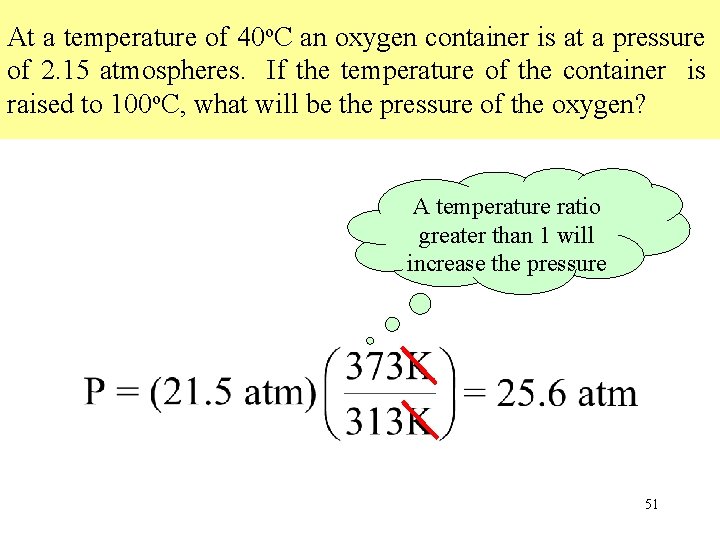
At a temperature of 40 o. C an oxygen container is at a pressure of 2. 15 atmospheres. If the temperature of the container is raised to 100 o. C, what will be the pressure of the oxygen? A temperature ratio greater than 1 will increase the pressure 51

At a temperature of 40 o. C an oxygen container is at a pressure of 2. 15 atmospheres. If the temperature of the container is raised to 100 o. C, what will be the pressure of the oxygen? Method B. Algebraic Equation Step 1. Organize the information (remember to make units the same): P 1 = 21. 5 atm T 1 = 40 o. C = 313 K P 2 = ? T 2 = 100 o. C = 373 K 52

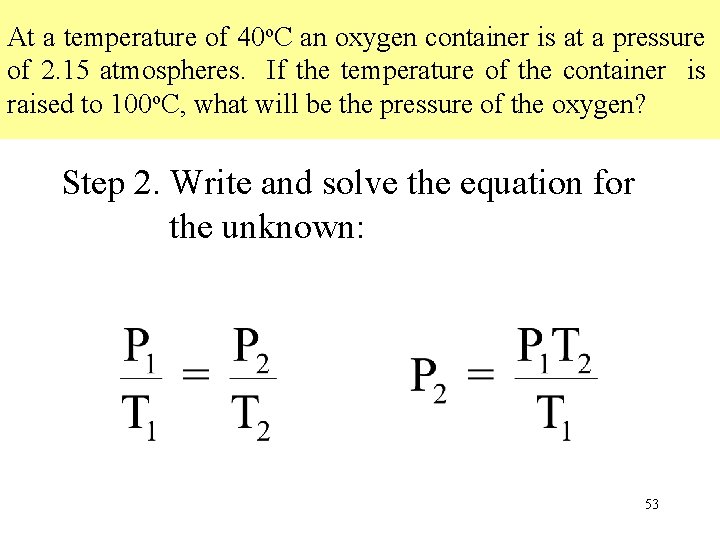
At a temperature of 40 o. C an oxygen container is at a pressure of 2. 15 atmospheres. If the temperature of the container is raised to 100 o. C, what will be the pressure of the oxygen? Step 2. Write and solve the equation for the unknown: 53

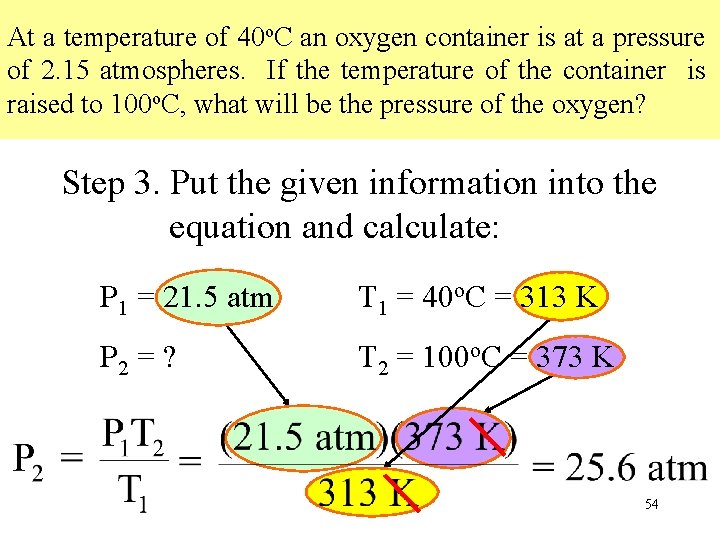
At a temperature of 40 o. C an oxygen container is at a pressure of 2. 15 atmospheres. If the temperature of the container is raised to 100 o. C, what will be the pressure of the oxygen? Step 3. Put the given information into the equation and calculate: P 1 = 21. 5 atm T 1 = 40 o. C = 313 K P 2 = ? T 2 = 100 o. C = 373 K 54

12. 8 Combined Gas Laws 55

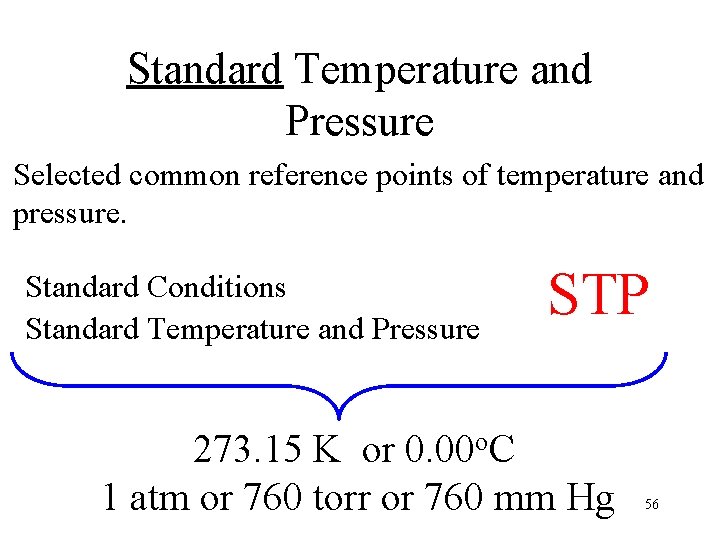
Standard Temperature and Pressure Selected common reference points of temperature and pressure. Standard Conditions Standard Temperature and Pressure STP 273. 15 K or 0. 00 o. C 1 atm or 760 torr or 760 mm Hg 56

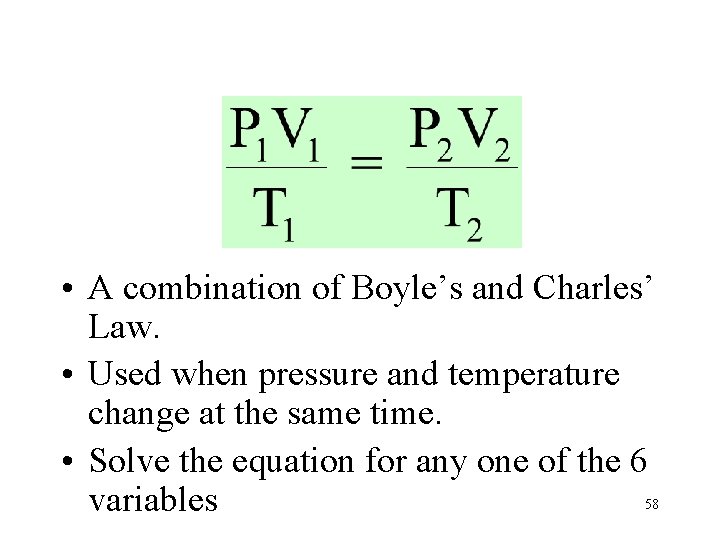
• A combination of Boyle’s and Charles’ Law. • Used when pressure and temperature change at the same time. • Solve the equation for any one of the 6 variables 58

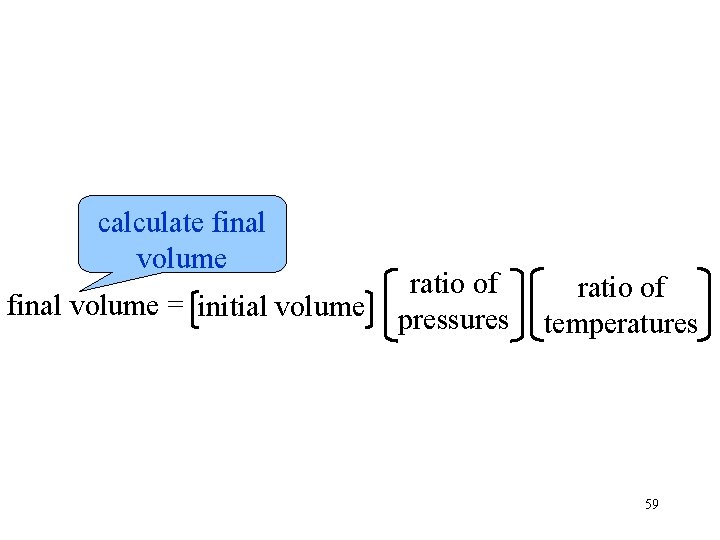
calculate final volume = initial volume ratio of pressures ratio of temperatures 59

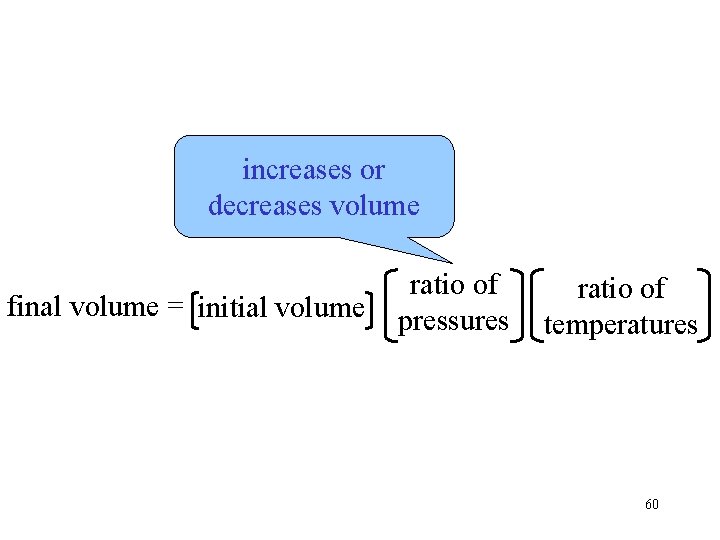
increases or decreases volume final volume = initial volume ratio of pressures ratio of temperatures 60

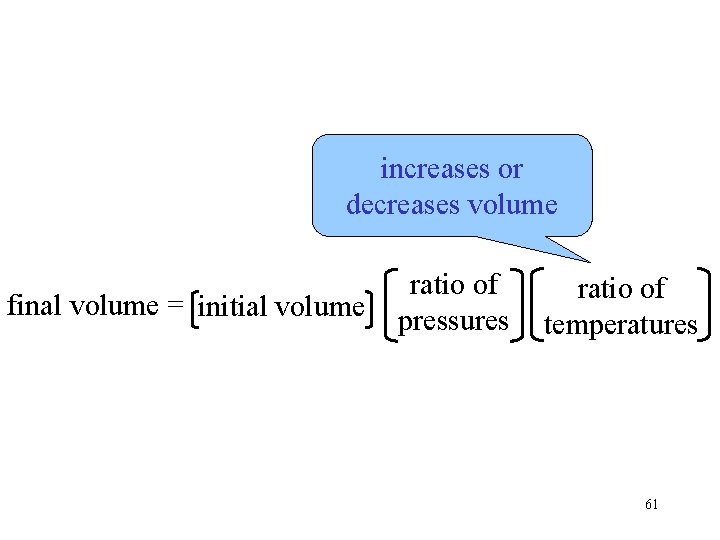
increases or decreases volume final volume = initial volume ratio of pressures ratio of temperatures 61

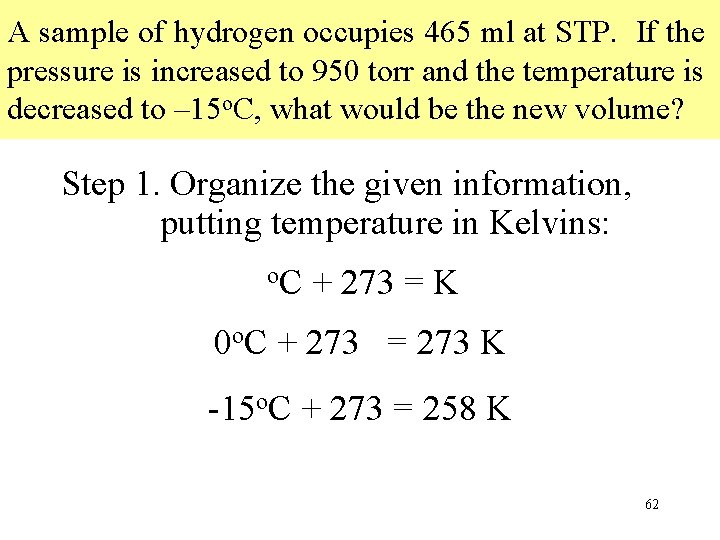
A sample of hydrogen occupies 465 ml at STP. If the pressure is increased to 950 torr and the temperature is decreased to – 15 o. C, what would be the new volume? Step 1. Organize the given information, putting temperature in Kelvins: o. C + 273 = K 0 o. C + 273 = 273 K -15 o. C + 273 = 258 K 62

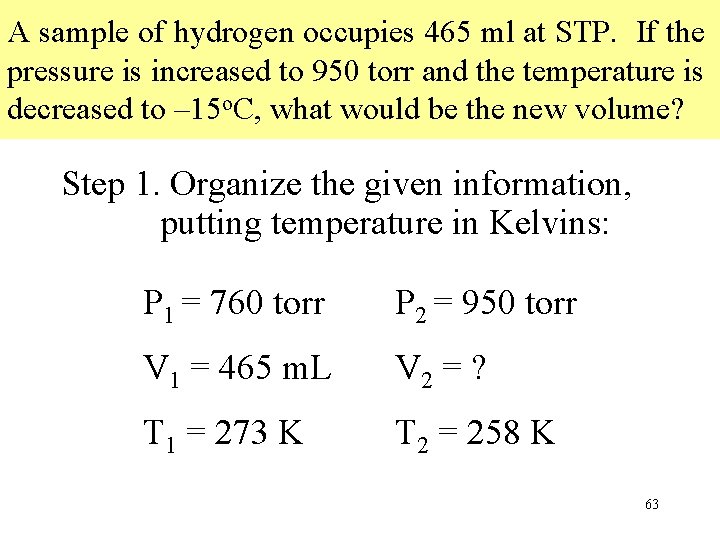
A sample of hydrogen occupies 465 ml at STP. If the pressure is increased to 950 torr and the temperature is decreased to – 15 o. C, what would be the new volume? Step 1. Organize the given information, putting temperature in Kelvins: P 1 = 760 torr P 2 = 950 torr V 1 = 465 m. L V 2 = ? T 1 = 273 K T 2 = 258 K 63

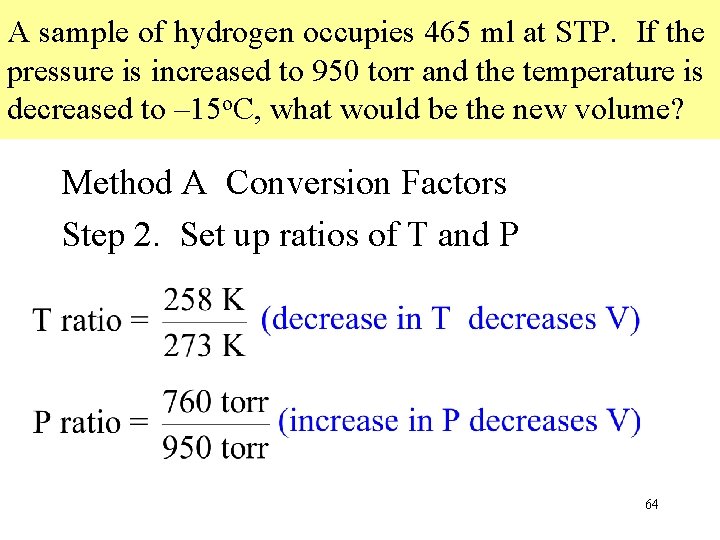
A sample of hydrogen occupies 465 ml at STP. If the pressure is increased to 950 torr and the temperature is decreased to – 15 o. C, what would be the new volume? Method A Conversion Factors Step 2. Set up ratios of T and P 64

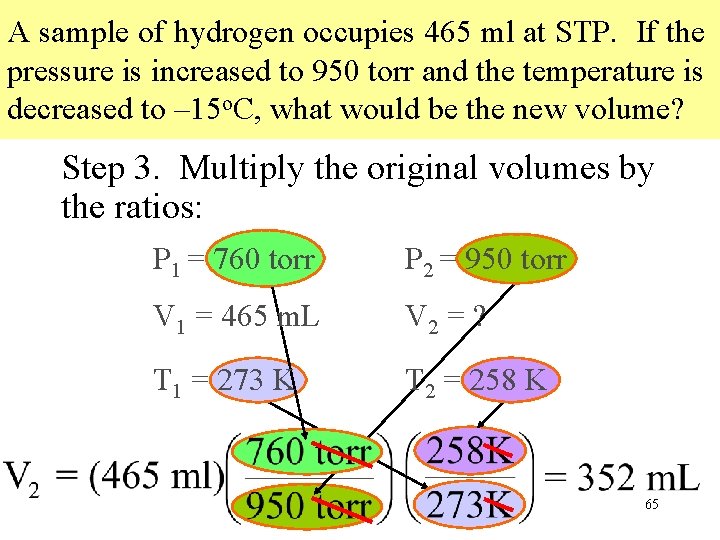
A sample of hydrogen occupies 465 ml at STP. If the pressure is increased to 950 torr and the temperature is decreased to – 15 o. C, what would be the new volume? Step 3. Multiply the original volumes by the ratios: P 1 = 760 torr P 2 = 950 torr V 1 = 465 m. L V 2 = ? T 1 = 273 K T 2 = 258 K 65

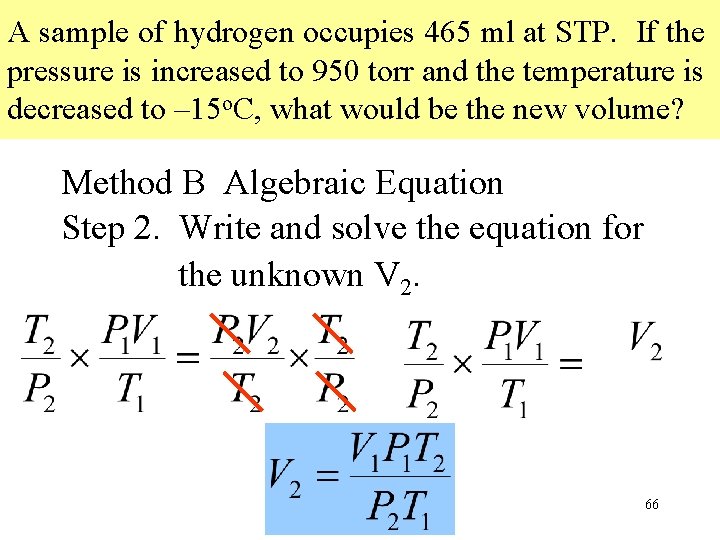
A sample of hydrogen occupies 465 ml at STP. If the pressure is increased to 950 torr and the temperature is decreased to – 15 o. C, what would be the new volume? Method B Algebraic Equation Step 2. Write and solve the equation for the unknown V 2. 66

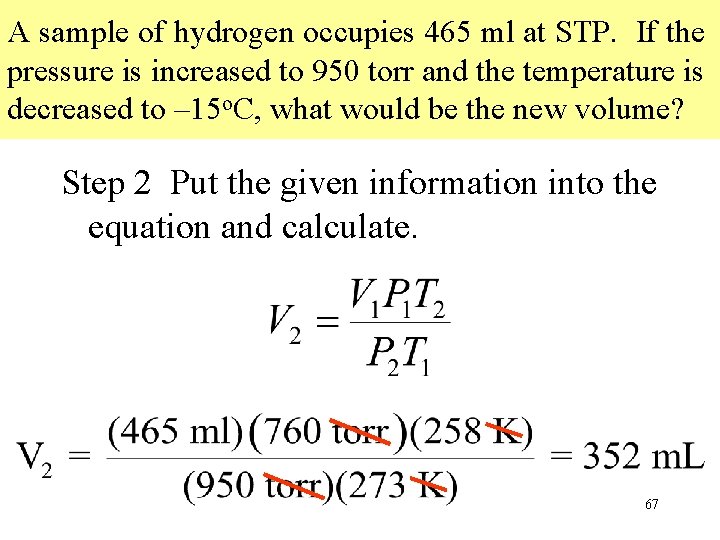
A sample of hydrogen occupies 465 ml at STP. If the pressure is increased to 950 torr and the temperature is decreased to – 15 o. C, what would be the new volume? Step 2 Put the given information into the equation and calculate. 67

12. 9 Dalton’s Law of Partial Pressures 68

Each gas in a mixture exerts a pressure that is independent of the other gases present. 69

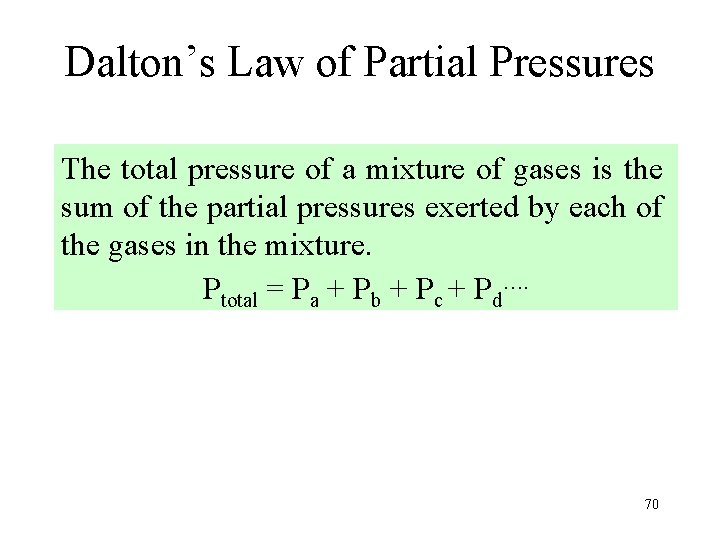
Dalton’s Law of Partial Pressures The total pressure of a mixture of gases is the sum of the partial pressures exerted by each of the gases in the mixture. Ptotal = Pa + Pb + Pc + Pd…. 70

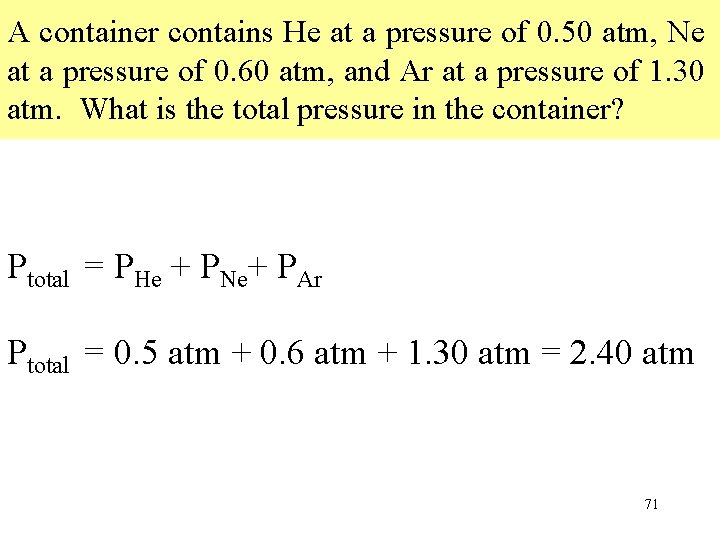
A container contains He at a pressure of 0. 50 atm, Ne at a pressure of 0. 60 atm, and Ar at a pressure of 1. 30 atm. What is the total pressure in the container? Ptotal = PHe + PNe+ PAr Ptotal = 0. 5 atm + 0. 6 atm + 1. 30 atm = 2. 40 atm 71

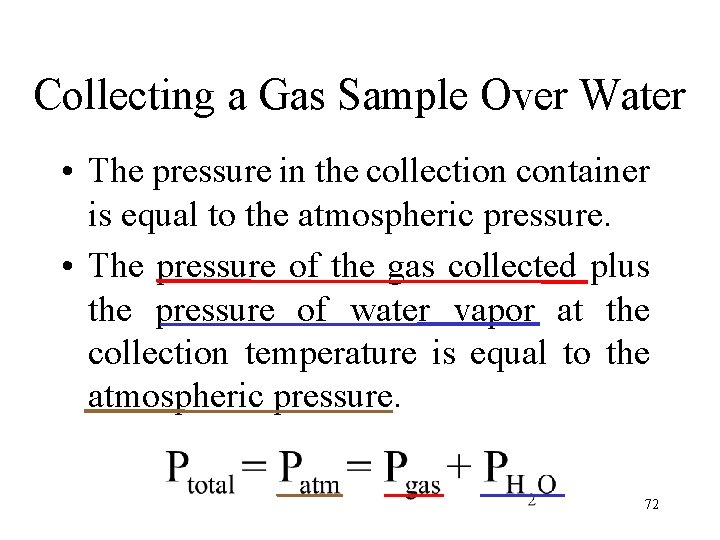
Collecting a Gas Sample Over Water • The pressure in the collection container is equal to the atmospheric pressure. • The pressure of the gas collected plus the pressure of water vapor at the collection temperature is equal to the atmospheric pressure. 72

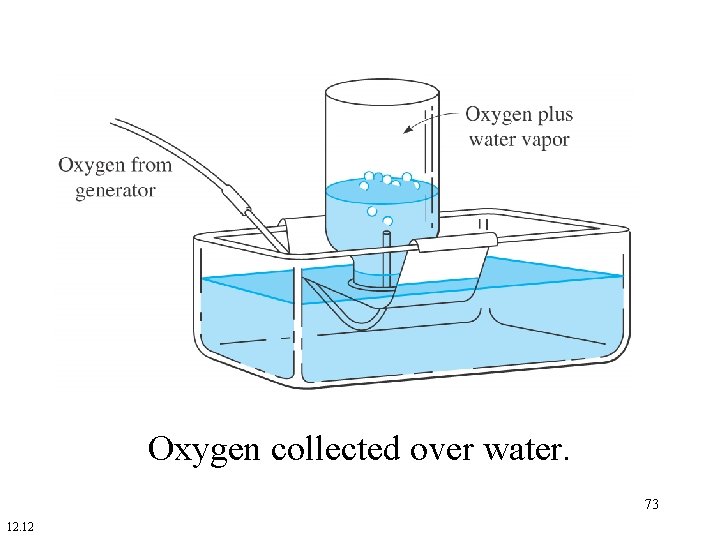
Oxygen collected over water. 73 12. 12

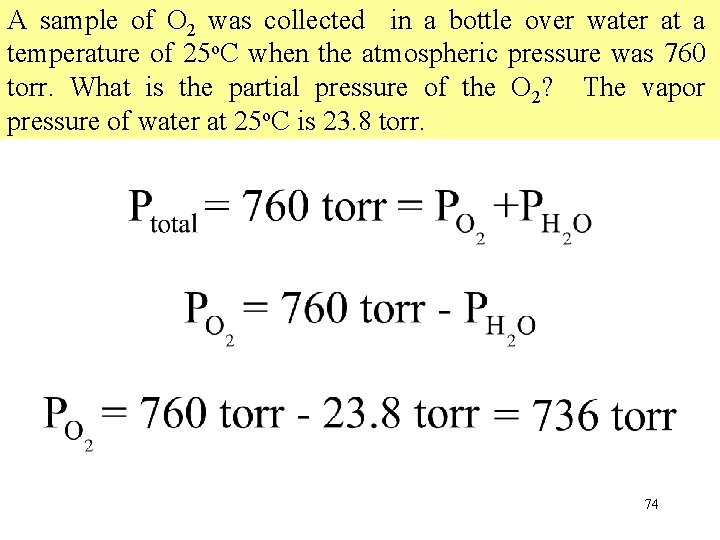
A sample of O 2 was collected in a bottle over water at a temperature of 25 o. C when the atmospheric pressure was 760 torr. What is the partial pressure of the O 2? The vapor pressure of water at 25 o. C is 23. 8 torr. 74
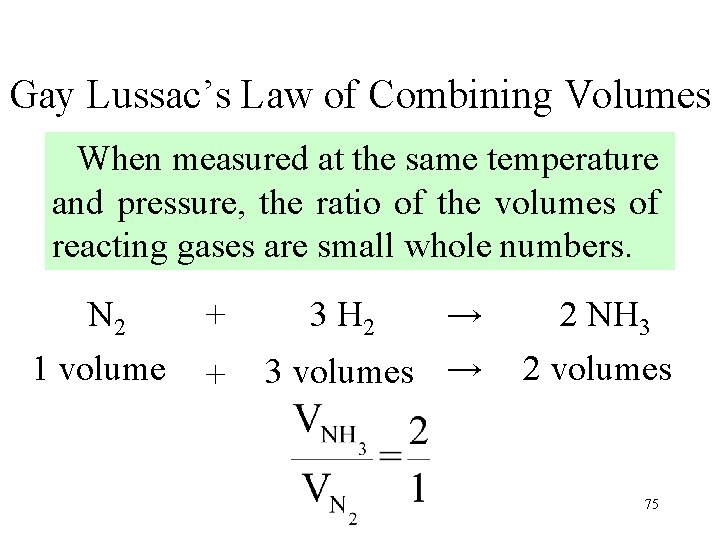
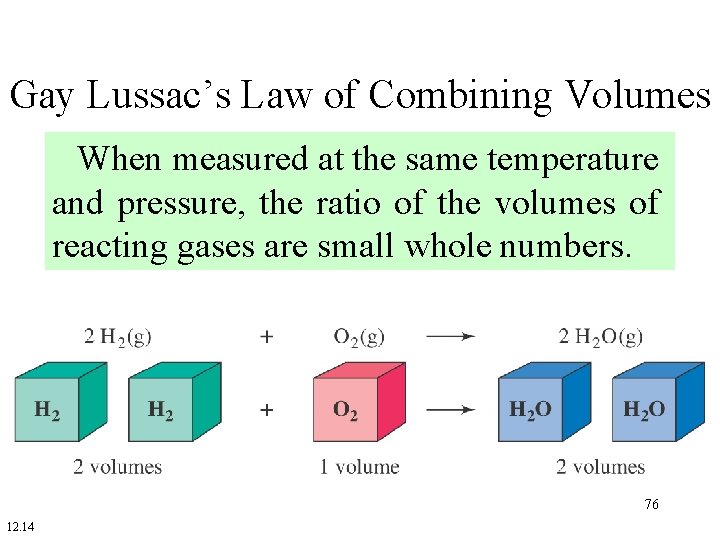
Gay Lussac’s Law of Combining Volumes When measured at the same temperature and pressure, the ratio of the volumes of reacting gases are small whole numbers. N 2 1 volume + + 3 H 2 → 3 volumes → 2 NH 3 2 volumes 75

Gay Lussac’s Law of Combining Volumes When measured at the same temperature and pressure, the ratio of the volumes of reacting gases are small whole numbers. 76 12. 14

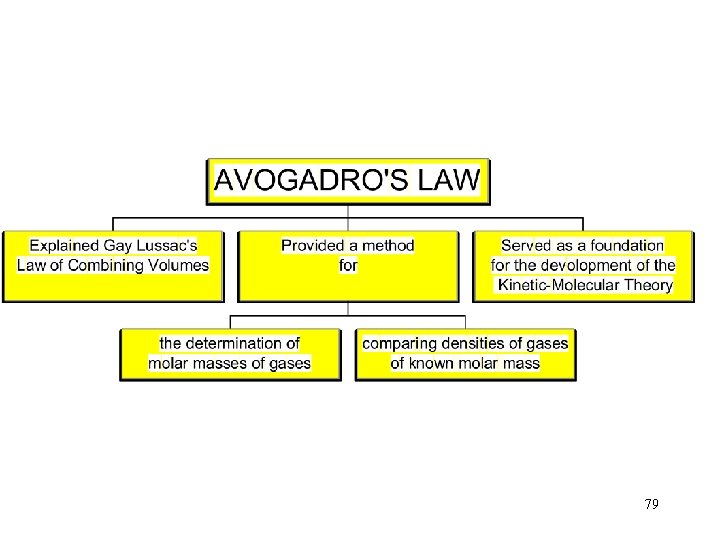
12. 10 Avogadro’s Law 77

Avogadro’s Law Equal volumes of different gases at the same temperature and pressure contain the same number of molecules. 78

79

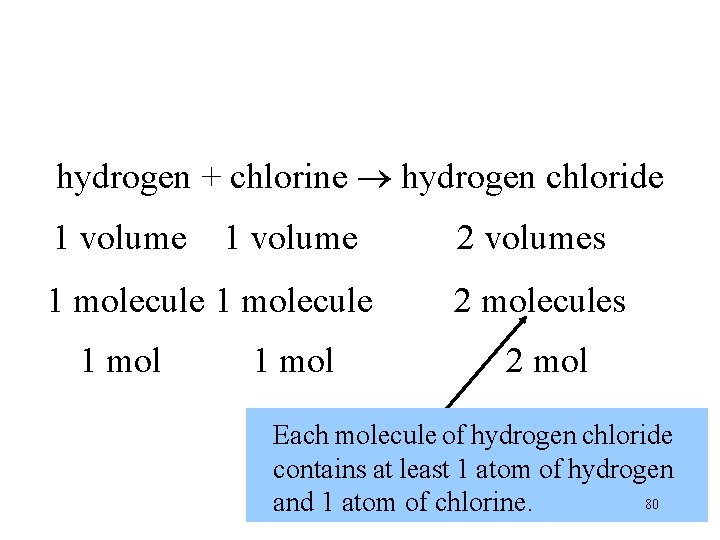
hydrogen + chlorine hydrogen chloride 1 volume 1 molecule 1 mol 2 volumes 2 molecules 2 mol Theremolecule are 2 molecules of hydrogen Each of hydrogen chloride. at least 1 atom of hydrogen contains 80 and 1 atom of chlorine.

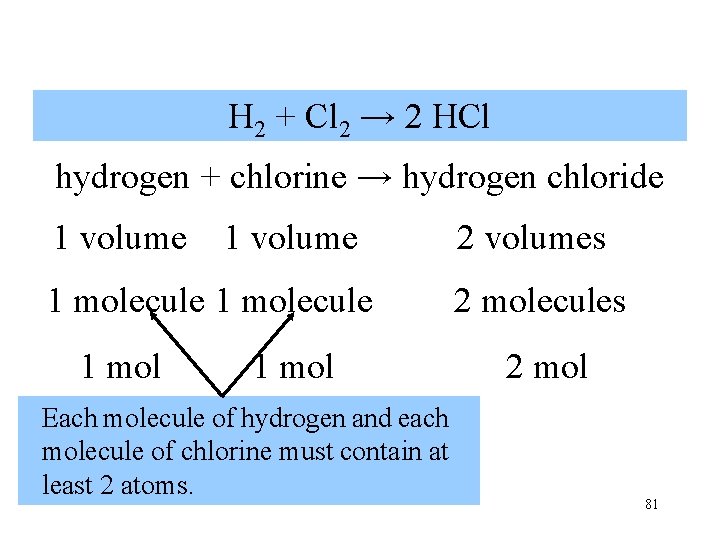
H 2 + Cl 2 → 2 HCl hydrogen + chlorine → hydrogen chloride 1 volume 2 volumes 1 molecule 2 molecules 1 mol Each molecule of hydrogen and each molecule of chlorine must contain at least 2 atoms. 2 mol 81

12. 11 Mole-Mass-Volume Relationships of Gases 82

• Volume of one mole of any gas at STP = 22. 4 L. • 22. 4 L at STP is known as the molar volume of any gas. 83

84 12. 13

The density of neon at STP is 0. 900 g/L. What is the molar mass of neon? 85

12. 12 Density of Gases 86

87

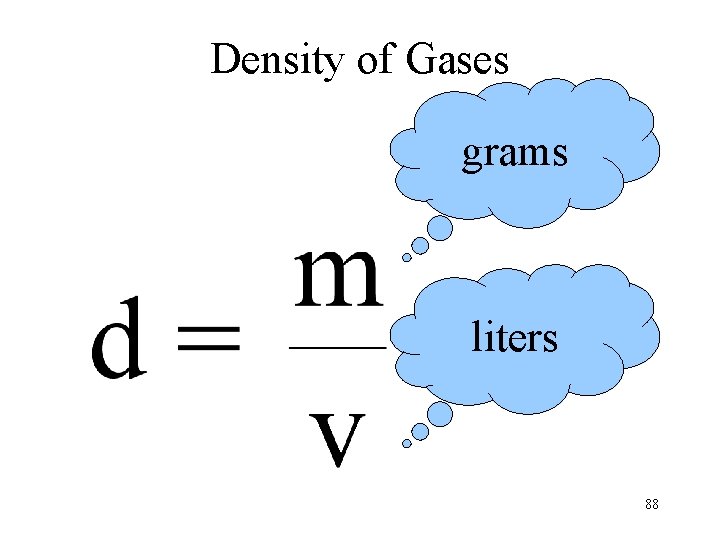
Density of Gases grams liters 88

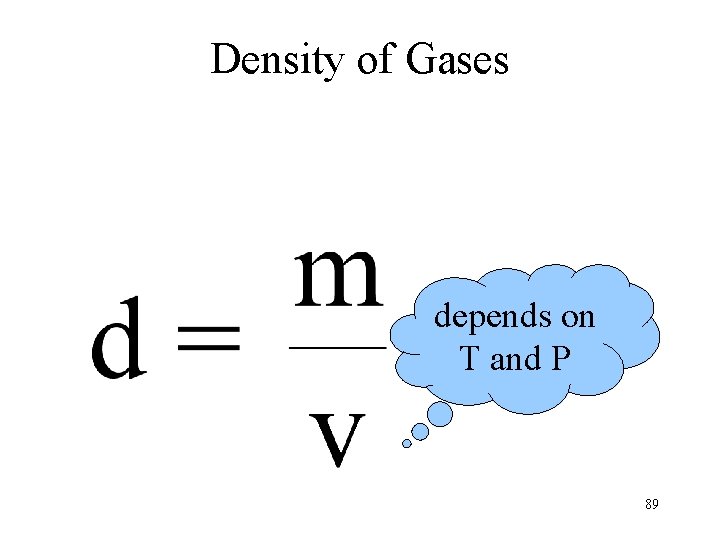
Density of Gases depends on T and P 89

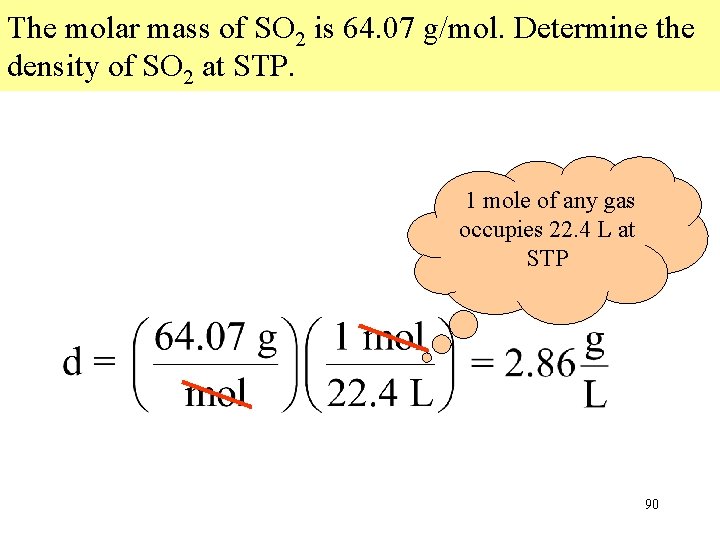
The molar mass of SO 2 is 64. 07 g/mol. Determine the density of SO 2 at STP. 1 mole of any gas occupies 22. 4 L at STP 90

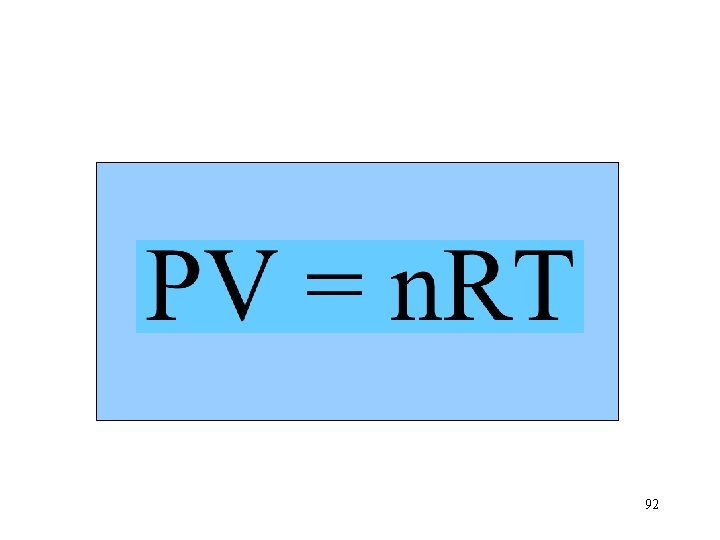
12. 13 Ideal Gas Law 91

n. T Va P 92

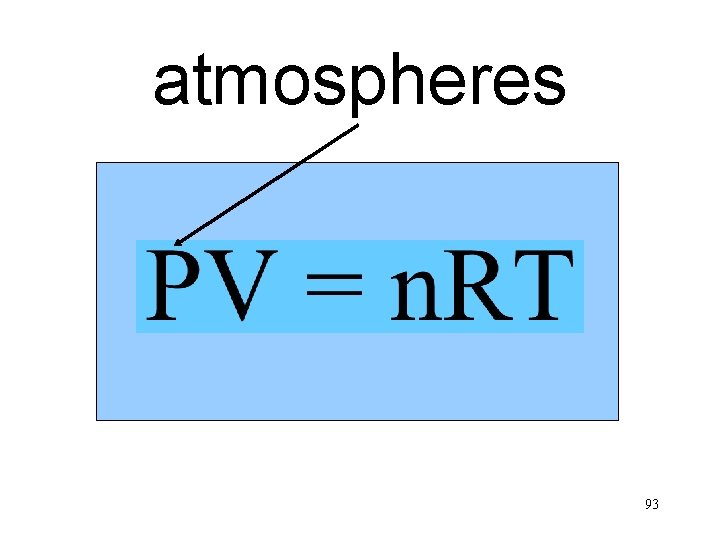
atmospheres n. T Va P 93

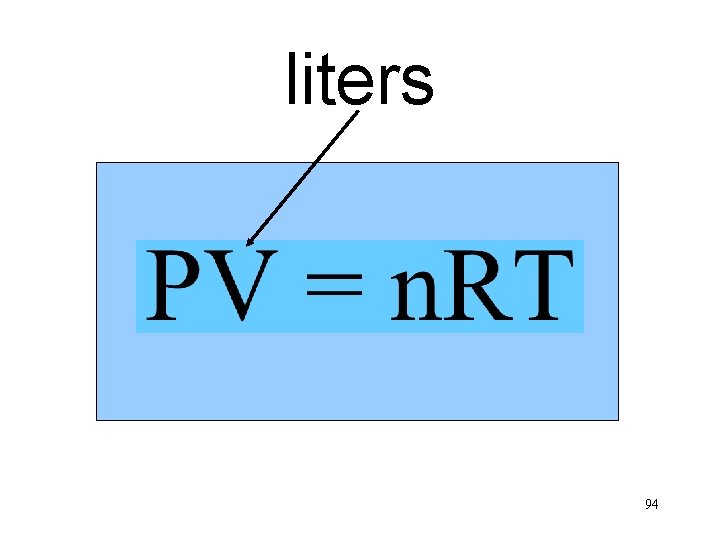
liters n. T Va P 94

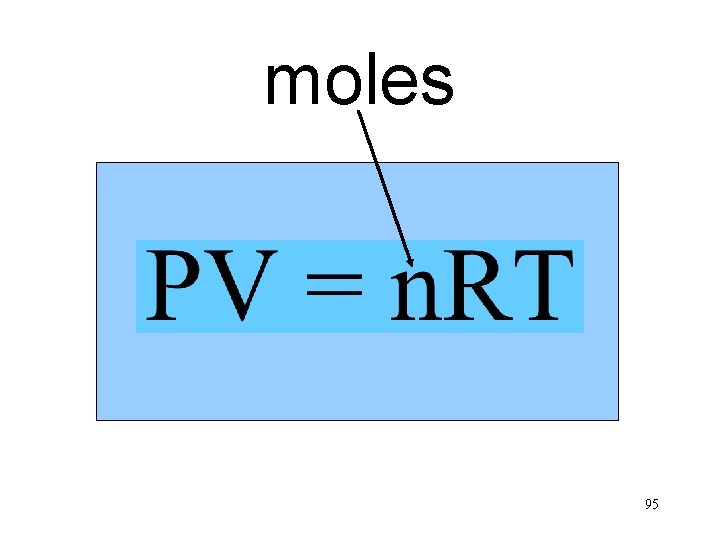
moles n. T Va P 95

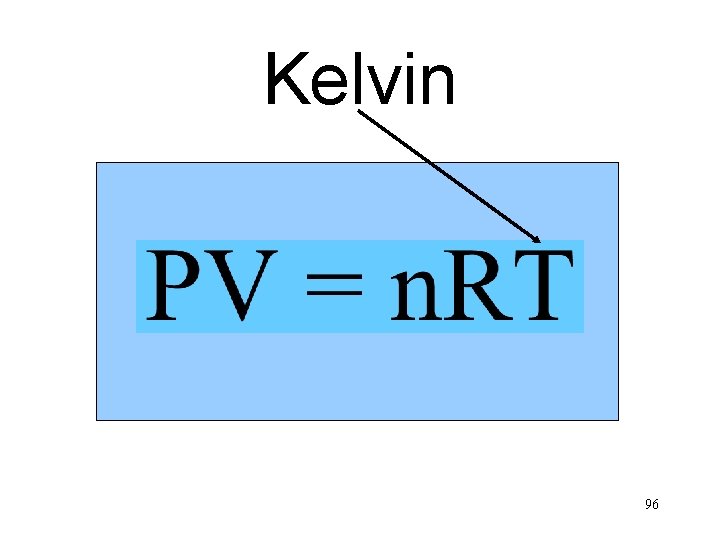
Kelvin n. T Va P 96

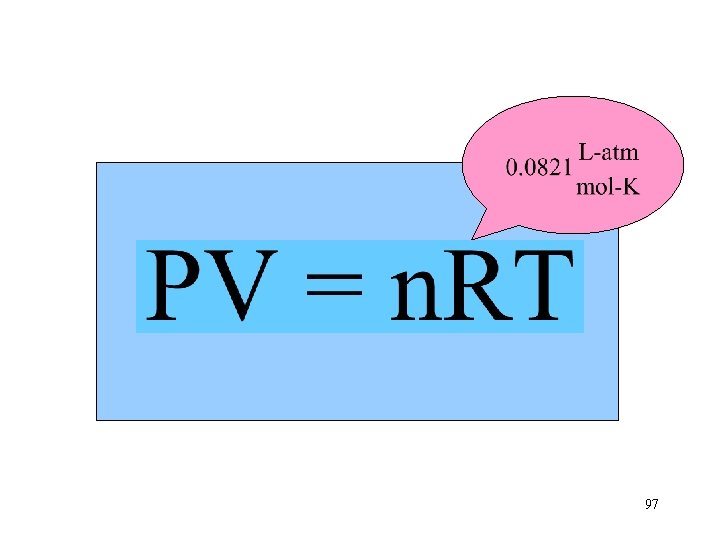
Ideal Gas Constant n. T Va P 97

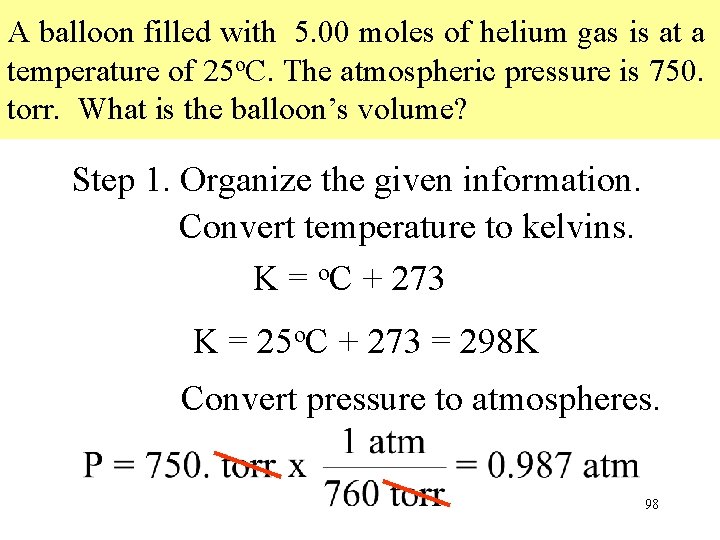
A balloon filled with 5. 00 moles of helium gas is at a temperature of 25 o. C. The atmospheric pressure is 750. torr. What is the balloon’s volume? Step 1. Organize the given information. Convert temperature to kelvins. K = o. C + 273 K = 25 o. C + 273 = 298 K Convert pressure to atmospheres. 98

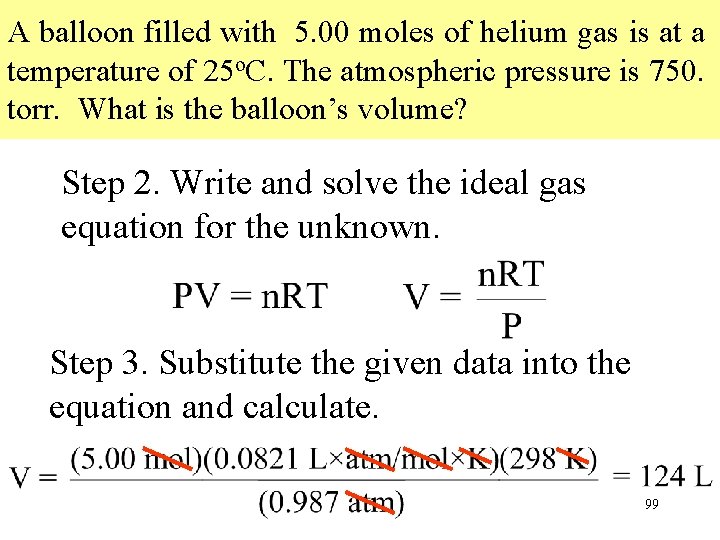
A balloon filled with 5. 00 moles of helium gas is at a temperature of 25 o. C. The atmospheric pressure is 750. torr. What is the balloon’s volume? Step 2. Write and solve the ideal gas equation for the unknown. Step 3. Substitute the given data into the equation and calculate. 99

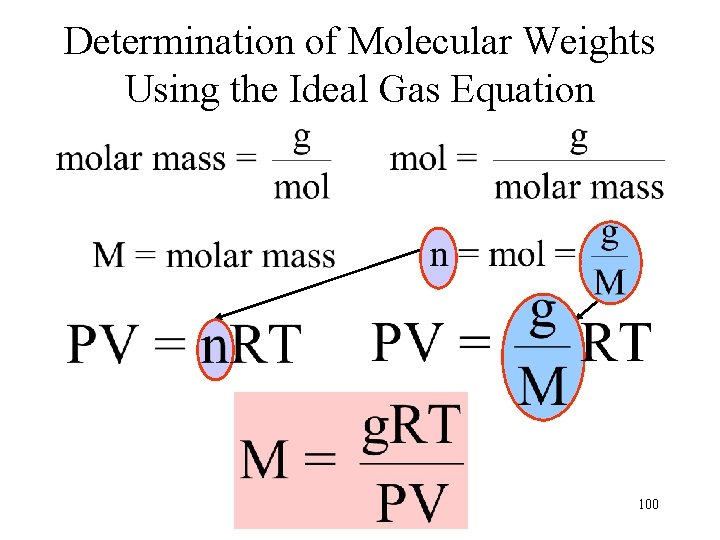
Determination of Molecular Weights Using the Ideal Gas Equation 100

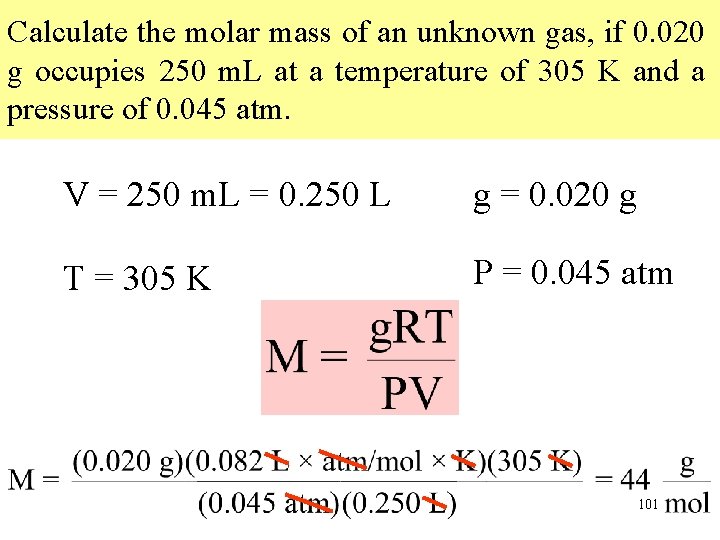
Calculate the molar mass of an unknown gas, if 0. 020 g occupies 250 m. L at a temperature of 305 K and a pressure of 0. 045 atm. V = 250 m. L = 0. 250 L g = 0. 020 g T = 305 K P = 0. 045 atm 101

12. 14 Gas Stoichiometry 102

• All calculations are done at STP. • Gases are assumed to behave as ideal gases. • A gas not at STP is converted to STP. 103

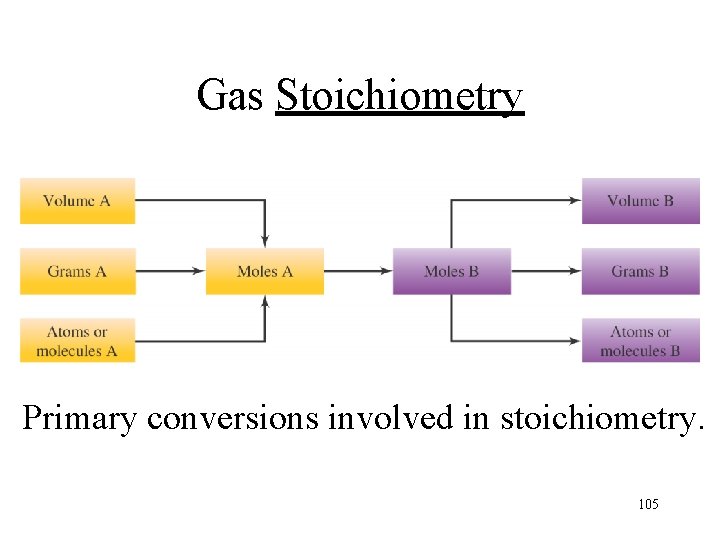
Gas Stoichiometry Primary conversions involved in stoichiometry. 105

Mole-Volume Calculations Mass-Volume Calculations 106

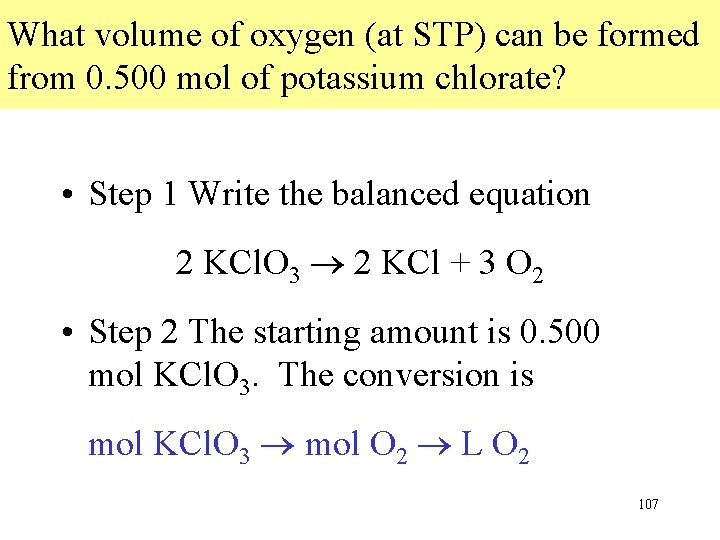
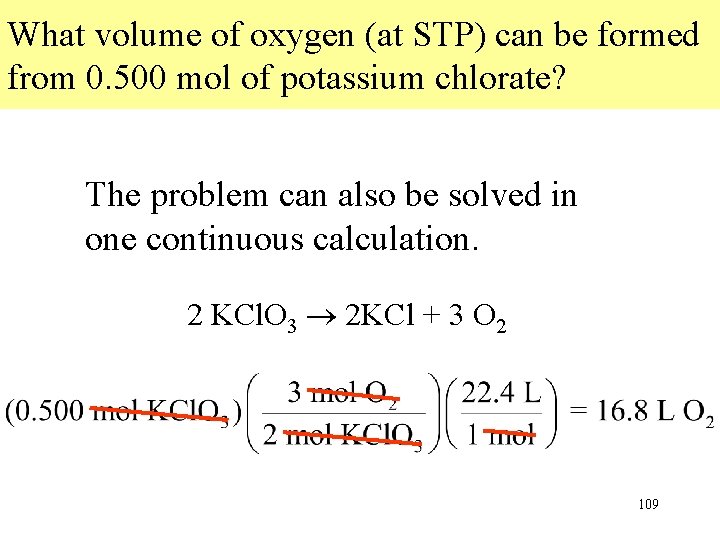
What volume of oxygen (at STP) can be formed from 0. 500 mol of potassium chlorate? • Step 1 Write the balanced equation 2 KCl. O 3 2 KCl + 3 O 2 • Step 2 The starting amount is 0. 500 mol KCl. O 3. The conversion is mol KCl. O 3 mol O 2 L O 2 107

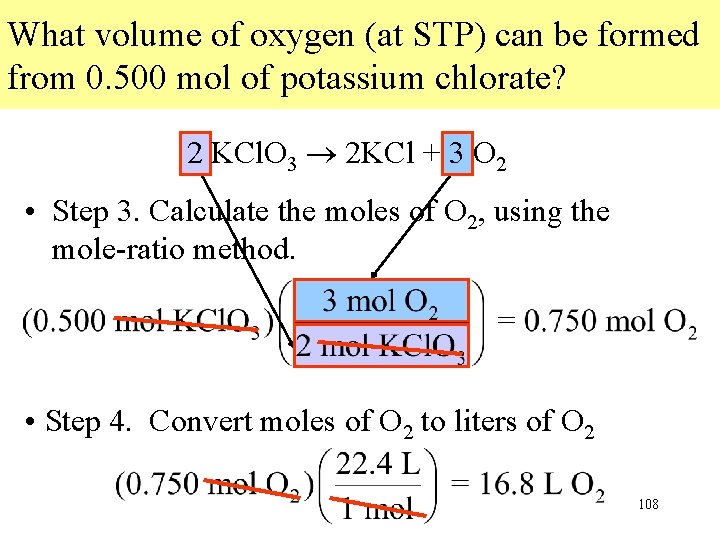
What volume of oxygen (at STP) can be formed from 0. 500 mol of potassium chlorate? 2 KCl. O 3 2 KCl + 3 O 2 • Step 3. Calculate the moles of O 2, using the mole-ratio method. • Step 4. Convert moles of O 2 to liters of O 2 108

What volume of oxygen (at STP) can be formed from 0. 500 mol of potassium chlorate? The problem can also be solved in one continuous calculation. 2 KCl. O 3 2 KCl + 3 O 2 109

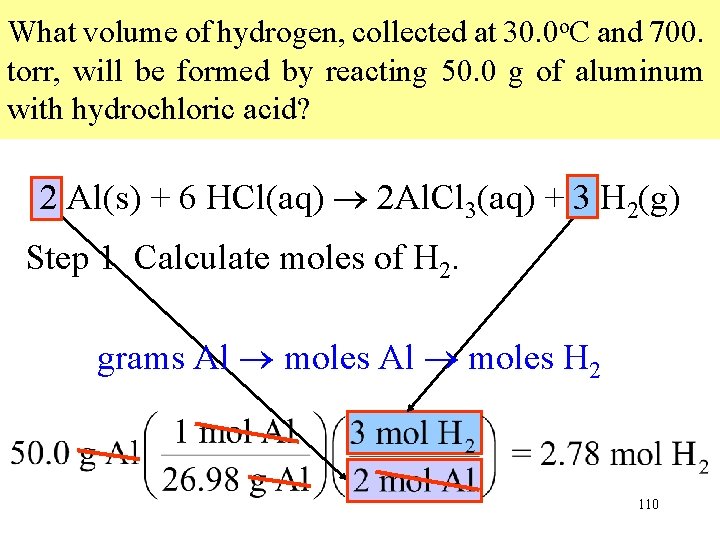
What volume of hydrogen, collected at 30. 0 o. C and 700. torr, will be formed by reacting 50. 0 g of aluminum with hydrochloric acid? 2 Al(s) + 6 HCl(aq) 2 Al. Cl 3(aq) + 3 H 2(g) Step 1 Calculate moles of H 2. grams Al moles H 2 110

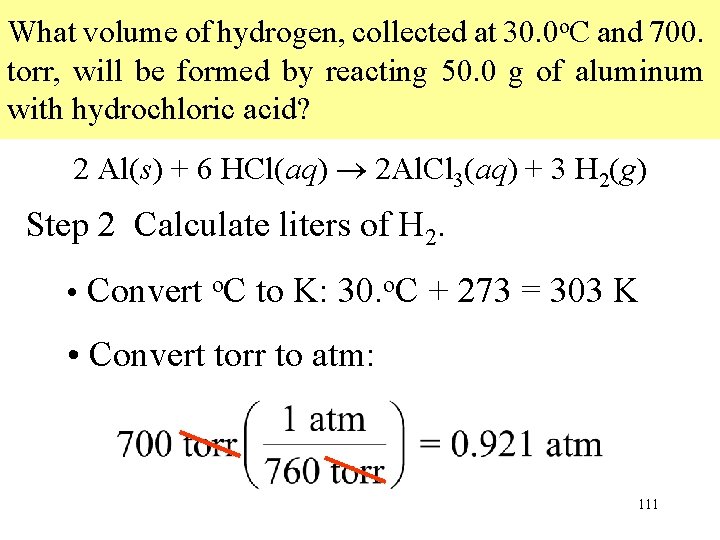
What volume of hydrogen, collected at 30. 0 o. C and 700. torr, will be formed by reacting 50. 0 g of aluminum with hydrochloric acid? 2 Al(s) + 6 HCl(aq) 2 Al. Cl 3(aq) + 3 H 2(g) Step 2 Calculate liters of H 2. • Convert o. C to K: 30. o. C + 273 = 303 K • Convert torr to atm: 111

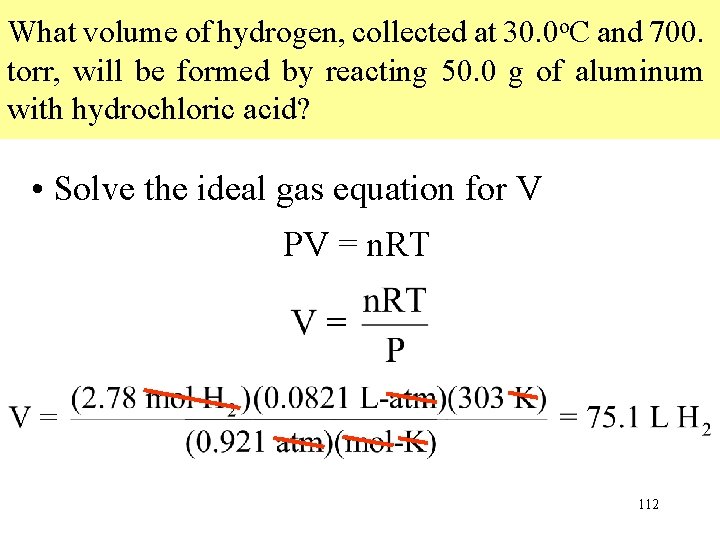
What volume of hydrogen, collected at 30. 0 o. C and 700. torr, will be formed by reacting 50. 0 g of aluminum with hydrochloric acid? • Solve the ideal gas equation for V PV = n. RT 112

Volume-Volume Calculations 113

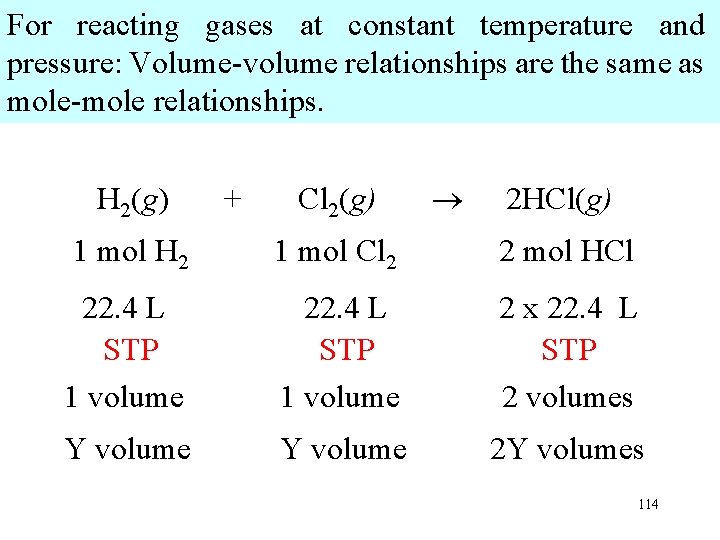
For reacting gases at constant temperature and pressure: Volume-volume relationships are the same as mole-mole relationships. H 2(g) + Cl 2(g) 2 HCl(g) 1 mol H 2 1 mol Cl 2 2 mol HCl 22. 4 L STP 1 volume 2 x 22. 4 L STP 2 volumes Y volume 2 Y volumes 114

What volume of nitrogen will react with 600. m. L of hydrogen to form ammonia? What volume of ammonia will be formed? N 2(g) + 3 H 2(g) 2 NH 3(g) 115

12. 15 Real Gases 116

Ideal Gas • An ideal gas obeys the gas laws. – The volume the molecules of an ideal gas occupy is negligible compared to the volume of the gas. This is true at all temperatures and pressures. – The intermolecular attractions between the molecules of an ideal gas are negligible at all temperatures and pressures. 117

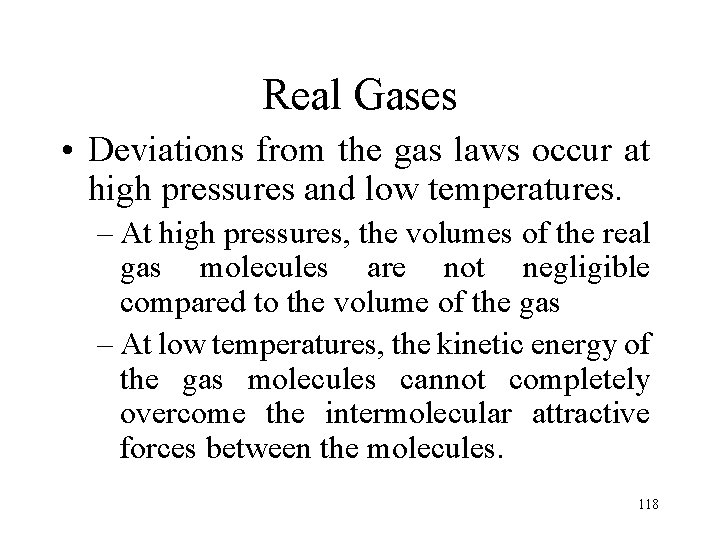
Real Gases • Deviations from the gas laws occur at high pressures and low temperatures. – At high pressures, the volumes of the real gas molecules are not negligible compared to the volume of the gas – At low temperatures, the kinetic energy of the gas molecules cannot completely overcome the intermolecular attractive forces between the molecules. 118

119
 Gaseous state chapter
Gaseous state chapter Chapter 2 matter section 1 classifying matter answer key
Chapter 2 matter section 1 classifying matter answer key Meetrekregeling deelnemingsvrijstelling
Meetrekregeling deelnemingsvrijstelling Kitten carousel
Kitten carousel Zica azar
Zica azar Arlene hein
Arlene hein Merike hein
Merike hein Merike hein
Merike hein Maarja hein
Maarja hein Hein tonnaer
Hein tonnaer Stalen gasleiding vervangen
Stalen gasleiding vervangen Duas formiguinhas partiram ao mesmo tempo hein
Duas formiguinhas partiram ao mesmo tempo hein Law of combining volumes
Law of combining volumes Hein stigum
Hein stigum Hein stigum
Hein stigum Tobias hein
Tobias hein My very excited mother planets
My very excited mother planets Inorganic gaseous pollutants of air
Inorganic gaseous pollutants of air Xeromorphic plants
Xeromorphic plants Inorganic gases
Inorganic gases Gaseous exchange in grasshopper
Gaseous exchange in grasshopper Granular porosity denture
Granular porosity denture Gaseous exchange in animals
Gaseous exchange in animals Inhalanda
Inhalanda Gaseous equilibrium
Gaseous equilibrium Gaseous envelope of the sun
Gaseous envelope of the sun Grade 11 practical gaseous exchange
Grade 11 practical gaseous exchange In earthworm
In earthworm Gaseous solution
Gaseous solution Phosphate buffer system equation
Phosphate buffer system equation Define dosage form
Define dosage form At 500 k one mole of gaseous oncl
At 500 k one mole of gaseous oncl Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Grey vs white matter
Grey vs white matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Gyrus and sulcus function
Gyrus and sulcus function Section 1 composition of matter chapter 15 answer key
Section 1 composition of matter chapter 15 answer key Gray matter and white matter
Gray matter and white matter What does grey matter do
What does grey matter do Flow of energy vs flow of matter
Flow of energy vs flow of matter Plasma and bose-einstein condensate
Plasma and bose-einstein condensate Properties of liquid state of matter
Properties of liquid state of matter States of matter thermal energy
States of matter thermal energy Sixth state of matter
Sixth state of matter Which is a gas
Which is a gas Is pepsi solid liquid or gas
Is pepsi solid liquid or gas Physical state of matter
Physical state of matter Change in state of matter
Change in state of matter Vaporization matter
Vaporization matter Venn diagram of solid and liquid
Venn diagram of solid and liquid Colloidal state of matter
Colloidal state of matter State of matter in chemical equations
State of matter in chemical equations Law of constancy of interfacial angle
Law of constancy of interfacial angle Compressible state of matter
Compressible state of matter State of matter
State of matter Liquid homogeneous mixture
Liquid homogeneous mixture 6th state of matter
6th state of matter Liquid particle diagram
Liquid particle diagram Concept map matter
Concept map matter Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Frameset trong html5
Frameset trong html5 Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Chụp tư thế worms-breton
Chụp tư thế worms-breton Hát lên người ơi alleluia
Hát lên người ơi alleluia Môn thể thao bắt đầu bằng từ chạy
Môn thể thao bắt đầu bằng từ chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính thế năng
Công thức tính thế năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân 101012 bằng
101012 bằng độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng xinh xinh thế chỉ nói điều hay thôi
Cái miệng xinh xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ V cc
V cc Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Fecboak
Fecboak Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Số nguyên tố là gì
Số nguyên tố là gì Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập Hệ hô hấp
Hệ hô hấp Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi State to state regionalism
State to state regionalism Excitation table for sr flip flops
Excitation table for sr flip flops Transition bugs in software testing
Transition bugs in software testing T state vs r state
T state vs r state Subshell orbital
Subshell orbital T state vs r state
T state vs r state Absorptive state vs postabsorptive state
Absorptive state vs postabsorptive state Glycogen synthesis
Glycogen synthesis Age of consent per state
Age of consent per state Salesforce 101: introduction to salesforce kurs
Salesforce 101: introduction to salesforce kurs Which two states are equivalent state
Which two states are equivalent state Duncker diagram
Duncker diagram Zczc state graph
Zczc state graph What is initial state + goal state in search terminology?
What is initial state + goal state in search terminology? Tasscc state of the state
Tasscc state of the state Submachine state in state diagram
Submachine state in state diagram